A Retrospective Analysis of the Prognostic Factors and Adverse Events in the Treatment of Mucosal Melanoma in a Single Centre
Abstract
1. Introduction
2. Methods
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawson, R.V.; Wilmott, J.S.; Scolyer, R.A. Mucosal Melanoma: A Review Emphasizing the Molecular Landscape and Implications for Diagnosis and Management. Surg. Pathol. Clin. 2021, 14, 293–307. [Google Scholar] [CrossRef]
- Yde, S.S.; Sjoegren, P.; Heje, M.; Stolle, L.B. Mucosal Melanoma: A Literature Review. Curr. Oncol. Rep. 2018, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef]
- Spencer, K.R.; Mehnert, J.M. Mucosal Melanoma: Epidemiology, Biology and Treatment. Cancer Treat. Res. 2016, 167, 295–320. [Google Scholar] [PubMed]
- Bobos, M. Histopathologic classification and prognostic factors of melanoma: A 2021 update. Ital. J. Dermatol. Venereol. 2021, 156, 300–321. [Google Scholar] [CrossRef] [PubMed]
- Furney, S.J.; Turajlic, S.; Stamp, G.; Nohadani, M.; Carlisle, A.; Thomas, J.M.; Hayes, A.; Strauss, D.; Gore, M.; van den Oord, J.; et al. Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J. Pathol. 2013, 230, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Pabla, S.; Conroy, J.M.; Nesline, M.K.; Glenn, S.T.; Dressman, D.; Papanicolau-Sengos, A.; Burgher, B.; Andreas, J.; Giamo, V.; et al. Predicting response to checkpoint inhibitors in melanoma beyond PD-L1 and mutational burden. J. Immunother. Cancer 2018, 6, 32. [Google Scholar] [CrossRef]
- Newell, F.; Johansson, P.A.; Wilmott, J.S.; Nones, K.; Lakis, V.; Pritchard, A.L.; Lo, S.N.; Rawson, R.V.; Kazakoff, S.H.; Colebatch, A.J.; et al. Comparative Genomics Provides Etiologic and Biological Insight into Melanoma Subtypes. Cancer Discov. 2022, 12, 2856–2879. [Google Scholar] [CrossRef]
- Klemen, N.D.; Wang, M.; Rubinstein, J.C.; Olino, K.; Clune, J.; Ariyan, S.; Cha, C.; Weiss, S.A.; Kluger, H.M.; Sznol, M. Survival after checkpoint inhibitors for metastatic acral, mucosal and uveal melanoma. J. Immunother. Cancer 2020, 8, e000341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, J.; Guo, J.; Si, L.; Bai, X. Evolving Treatment Approaches to Mucosal Melanoma. Curr. Oncol. Rep. 2022, 24, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Messersmith, H.; Kaur, V.; Kirkwood, J.M.; Kudchadkar, R.; McQuade, J.L.; Provenzano, A.; Swami, U.; Weber, J.; Alluri, K.C.; et al. Systemic Therapy for Melanoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 3947–3970. [Google Scholar] [CrossRef] [PubMed]
- Verkhovskaia, S.; Di Pietro, F.R.; Mastroeni, S.; Carbone, M.L.; Abeni, D.; Morese, R.; Morelli, F.M.; D’Atri, S.; Marchetti, P.; De Galitiis, F.; et al. Vitiligo-like leukoderma as an indicator of clinical response to immune checkpoint inhibitors in late-stage melanoma patients. J. Cancer Res. Clin. Oncol. 2022, 148, 2529–2538. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Boussemart, L.; Mateus, C.; Routier, E.; Boutros, C.; Cazenave, H.; Viollet, R.; Thomas, M.; Roy, S.; Benannoune, N.; et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016, 152, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Agarwala, S.S.; Messersmith, H.; Alluri, K.C.; Ascierto, P.A.; Atkins, M.B.; Bollin, K.; Chacon, M.; Davis, N.; Faries, M.B.; et al. Systemic Therapy for Melanoma: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 4794–4820. [Google Scholar] [CrossRef]
- Gao, D.; Ma, X. Serum lactate dehydrogenase is a predictor of poor survival in malignant melanoma. Panminerva Med. 2017, 59, 332–337. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Wang, J.; Sun, C.; Zhu, X. Prognostic value of lactate dehydrogenase for melanoma patients receiving anti-PD-1/PD-L1 therapy: A meta-analysis. Medicine 2021, 100, e25318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Kotenko, S.; Li, W. Prognostic value of neutrophil-lymphocyte ratio and lactate dehydrogenase in melanoma patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Medicine 2022, 101, e29536. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, S.; Xiao, Z. Prognostic value of lactate dehydrogenase in patients with uveal melanoma treated with immune checkpoint inhibition. Aging 2023, 15, 8770–8781. [Google Scholar] [CrossRef]
- Petrelli, F.; Ardito, R.; Merelli, B.; Lonati, V.; Cabiddu, M.; Seghezzi, S.; Barni, S.; Ghidini, A. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: A systematic review and meta-analysis. Melanoma Res. 2019, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kiniwa, Y.; Okuyama, R. Recent advances in molecular targeted therapy for unresectable and metastatic BRAF-mutated melanoma. Jpn. J. Clin. Oncol. 2021, 51, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, F.; Namikawa, K.; Reijers, I.L.M.; Buchbinder, E.I.; Soon, J.A.; Zaremba, A.; Teterycz, P.; Mooradian, M.J.; Armstrong, E.; Nakamura, Y.; et al. Single-agent anti-PD-1 or combined with ipilimumab in patients with mucosal melanoma: An international, retrospective, cohort study. Ann. Oncol. 2022, 33, 968–980. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, Y.; Wang, D.; Zhu, Z. Treatment-related adverse events as surrogate to response rate to immune checkpoint blockade. Medicine 2020, 99, e22153. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Wu, Q.; Chen, F.; Liu, J.; Xie, X. Immune-related adverse events: Promising predictors for efficacy of immune checkpoint inhibitors. Cancer Immunol. Immunother. 2021, 70, 2559–2576. [Google Scholar] [CrossRef] [PubMed]
- El Osta, B.; Hu, F.; Sadek, R.; Chintalapally, R.; Tang, S.C. Not all immune-checkpoint inhibitors are created equal: Meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit. Rev. Oncol. Hematol. 2017, 119, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Plazy, C.; Hannani, D.; Gobbini, E. Immune Checkpoint Inhibitor Rechallenge and Resumption: A Systematic Review. Curr. Oncol. Rep. 2022, 24, 1095–1106. [Google Scholar] [CrossRef]
- De Risi, I.; Sciacovelli, A.M.; Guida, M. Checkpoint Inhibitors Immunotherapy in Metastatic Melanoma: When to Stop Treatment? Biomedicines 2022, 10, 2424. [Google Scholar] [CrossRef]
- Li, M.; Sack, J.S.; Rahma, O.E.; Hodi, F.S.; Zucker, S.D.; Grover, S. Outcomes after resumption of immune checkpoint inhibitor therapy after high-grade immune-mediated hepatitis. Cancer 2020, 126, 5088–5097. [Google Scholar] [CrossRef]
- Mao, L.; Qi, Z.; Zhang, L.; Guo, J.; Si, L. Immunotherapy in Acral and Mucosal Melanoma: Current Status and Future Directions. Front. Immunol. 2021, 12, 680407. [Google Scholar] [CrossRef]
- Gebhardt, C.; Lichtenberger, R.; Utikal, J. Biomarker value and pitfalls of serum S100B in the follow-up of high-risk melanoma patients. J. Dtsch. Dermatol. Ges. 2016, 14, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Roesch, A.; Weide, B.; Gutzmer, R.; Meier, F.; Loquai, C.; Kahler, K.C.; Gesierich, A.; Meissner, M.; von Bubnoff, D.; et al. Prognostic factors and treatment outcomes in 444 patients with mucosal melanoma. Eur. J. Cancer 2017, 81, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Sarac, E.; Amaral, T.; Keim, U.; Leiter, U.; Forschner, A.; Eigentler, T.K.; Garbe, C. Prognostic factors in 161 patients with mucosal melanoma: A study of German Central Malignant Melanoma Registry. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2021–2025. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Mathew, A.; Abraham, E.K.; Ahamed, I.M.; Nair, K.M. Primary malignant melanoma of the mucous membranes. Eur. J. Surg. Oncol. 1998, 24, 303–307. [Google Scholar] [CrossRef] [PubMed]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.; Bajaj, S.; Yu, J.; Hsu, M.; Balar, A.; Pavlick, A.; Weber, J.; Osman, I.; Zhong, J. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J. Immunother. Cancer 2019, 7, 222. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Moirano, G.; Fava, P.; Maule, M.; Ribero, S.; Quaglino, P. Obesity and immune-checkpoint inhibitors in advanced melanoma: A meta-analysis of survival outcomes from clinical studies. Semin. Cancer Biol. 2023, 91, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Langan, E.A.; Gratz, V.; Billmann, F.; Zillikens, D.; Terheyden, P. Does the gastrointestinal microbiome contribute to the ‘obesity paradox’ in melanoma survival? Br. J. Dermatol. 2018, 179, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Lian, B.; Zhou, L.; Song, X.; Zhang, X.; Wu, D.; Chi, Z.; Si, L.; Sheng, X.; Kong, Y.; et al. Multifactorial Analysis of Prognostic Factors and Survival Rates Among 706 Mucosal Melanoma Patients. Ann. Surg. Oncol. 2018, 25, 2184–2192. [Google Scholar] [CrossRef]
- van Zeijl, M.C.T.; Boer, F.L.; van Poelgeest, M.I.E.; van den Eertwegh, A.J.M.; Wouters, M.; de Wreede, L.C.; Aarts, M.J.B.; van den Berkmortel, F.; de Groot, J.W.B.; Hospers, G.A.P.; et al. Survival outcomes of patients with advanced mucosal melanoma diagnosed from 2013 to 2017 in the Netherlands—A nationwide population-based study. Eur. J. Cancer 2020, 137, 127–135. [Google Scholar] [CrossRef]
- Ercelep, O.; Topcu, T.O.; Bayoglu, I.V.; Ekinci, A.S.; Koca, S.; Kavgaci, H.; Ozcelik, M.; Alacacioglu, A.; Uzunoglu, S.; Bozkurt, O.; et al. Retrospective multicenter evaluation of patients diagnosed with mucosal melanoma: A study of Anatolian Society of Medical Oncology. Tumour Biol. 2016, 37, 12033–12038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhao, Y.; Li, P.; Duan, J.; Fu, X.; Tang, Y.; Ma, Y.; Zhou, Q. Survival analysis of comprehensive treatment in Chinese patients with metastatic melanoma: A retrospective analysis. Skin. Res. Technol. 2024, 30, e13546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lian, B.; Si, L.; Chi, Z.; Sheng, X.; Wang, X.; Mao, L.; Tang, B.; Li, S.; Yan, X.; et al. Real-world analysis of clinicopathological characteristics, survival rates, and prognostic factors in patients with melanoma brain metastases in China. J. Cancer Res. Clin. Oncol. 2021, 147, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Bluth, M.J.; Goldman, D.A.; Bitas, C.; Lefkowitz, R.A.; Postow, M.A.; Munhoz, R.R.; Buchar, G.; Hester, R.H.; Romero, J.A.; et al. Clinical features and response to systemic therapy in a historical cohort of advanced or unresectable mucosal melanoma. Melanoma Res. 2017, 27, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.L.; Le, A.; Tang, H.; Brown, M.; Scherrer, E.; Han, J.; Jiang, R.; Diede, S.J.; Shui, I.M. Burden and Risk Factors of Brain Metastases in Melanoma: A Systematic Literature Review. Cancers 2022, 14, 6108. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.S.; Lee, J.Y.; Lee, J.W.; Park, J.Y.; Kim, S.M.; Lee, J.H. Survival of oral mucosal melanoma according to treatment, tumour resection margin, and metastases. Br. J. Oral. Maxillofac. Surg. 2020, 58, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.K.; McKeown, J.; Grover, P.; Johnson, D.B.; Zaremba, A.; Dimitriou, F.; Weiser, R.; Farid, M.; Namikawa, K.; Sullivan, R.J.; et al. Outcomes of patients with resected stage III/IV acral or mucosal melanoma, treated with adjuvant anti-PD-1 based therapy. Eur. J. Cancer 2024, 199, 113563. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Mattei, J.; Tetzlaff, M.; Williams, M.D.; Davies, M.A.; Diab, A.; Oliva, I.C.G.; McQuade, J.; Patel, S.P.; Tawbi, H.; et al. Neoadjuvant checkpoint inhibitor immunotherapy for resectable mucosal melanoma. Front. Oncol. 2022, 12, 1001150. [Google Scholar] [CrossRef]
- Tang, A.; Taori, S.; Dang, S.; Gardner, P.A.; Zenonos, G.A.; Davar, D.; Kuan, E.C.; Snyderman, C.H.; Wang, E.W.; Choby, G. Immunotherapy in the Management of Sinonasal Mucosal Melanoma: A Systematic Review. Otolaryngol. Head Neck Surg. 2024, 171, 368–380. [Google Scholar] [CrossRef]
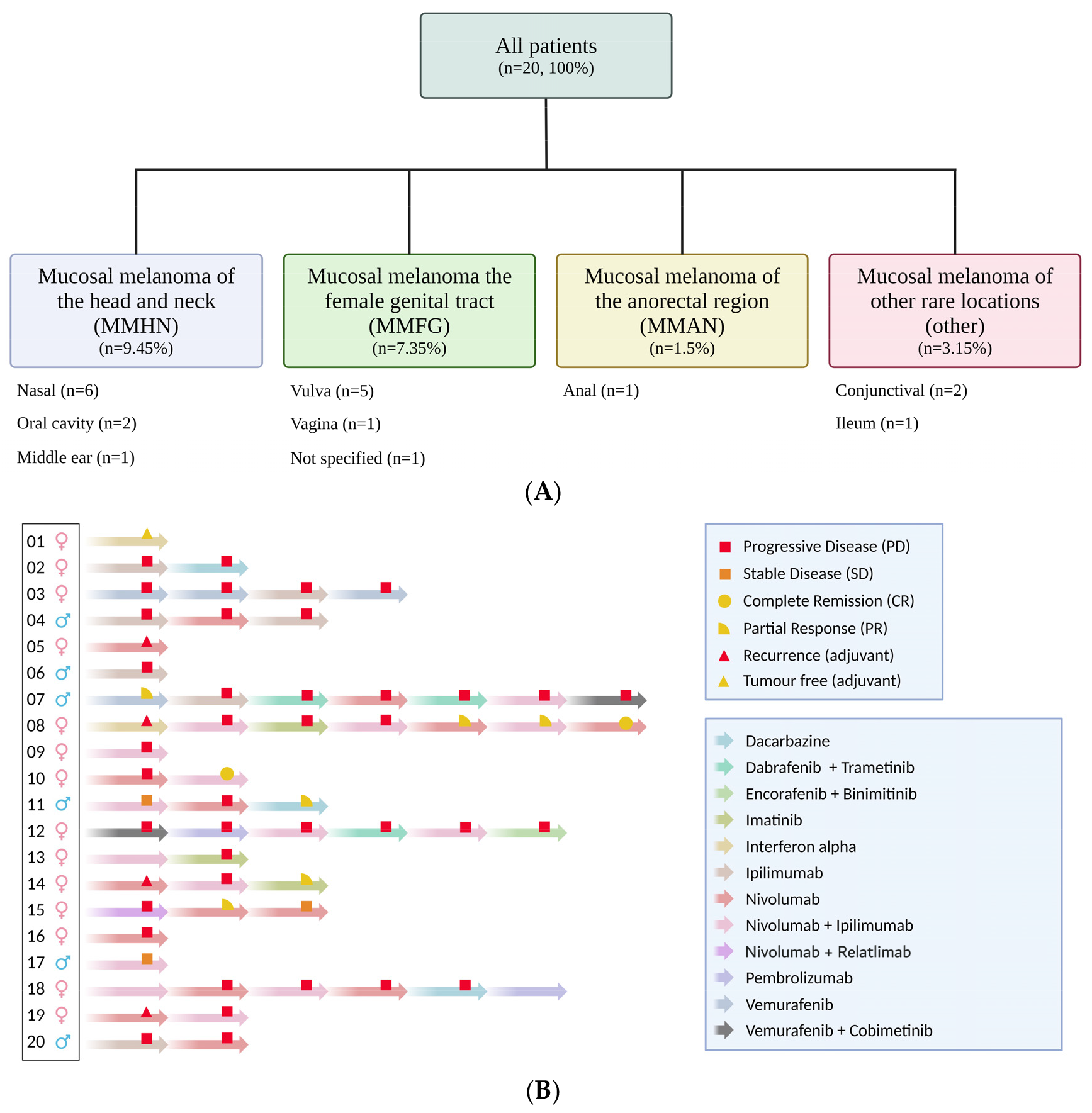
| Variable | Median OS (Years) | 95% Confidence Interval | Significant? p < 0.05 |
|---|---|---|---|
| Sex | |||
| Male | 2.3 | 0.7–3.8 | |
| Female | 1.3 | 0–2.5 | no |
| ECOG Status | |||
| 0 | 1.9 | 0.8–3.0 | |
| ≥1 | 4.3 | 0–10.5 | no |
| Comorbidities | |||
| CCI < 3 | 1.6 | 0.2–3.9 | |
| CCI ≥ 3 | 1.4 | 0–4.6 | no |
| Body mass index | |||
| ≤25 | 2.3 | 0–5.2 | |
| >25 | 1.6 | 0.6–2.7 | no |
| CNS metastases | |||
| Yes | 2.5 | 0–5.2 | |
| No | 1.9 | 0–3.2 | no |
| Variable | Median OS (Years) | 95% Confidence Interval | Significant? p < 0.05 |
|---|---|---|---|
| BRAF mutation | |||
| Positive | 2.3 | 1.0–3.5 | |
| Wild type | 1.6 | 0.7–2.5 | no |
| NRAS mutation | |||
| Positive | 0.2 | 0.22–0.24 | |
| Wild type | 1.9 | 0.7–3.0 | yes (p = 0.004) |
| cKit mutation | |||
| Positive | 4.2 | N/A | |
| Wild type | 1.6 | 0.8–2.4 | no |
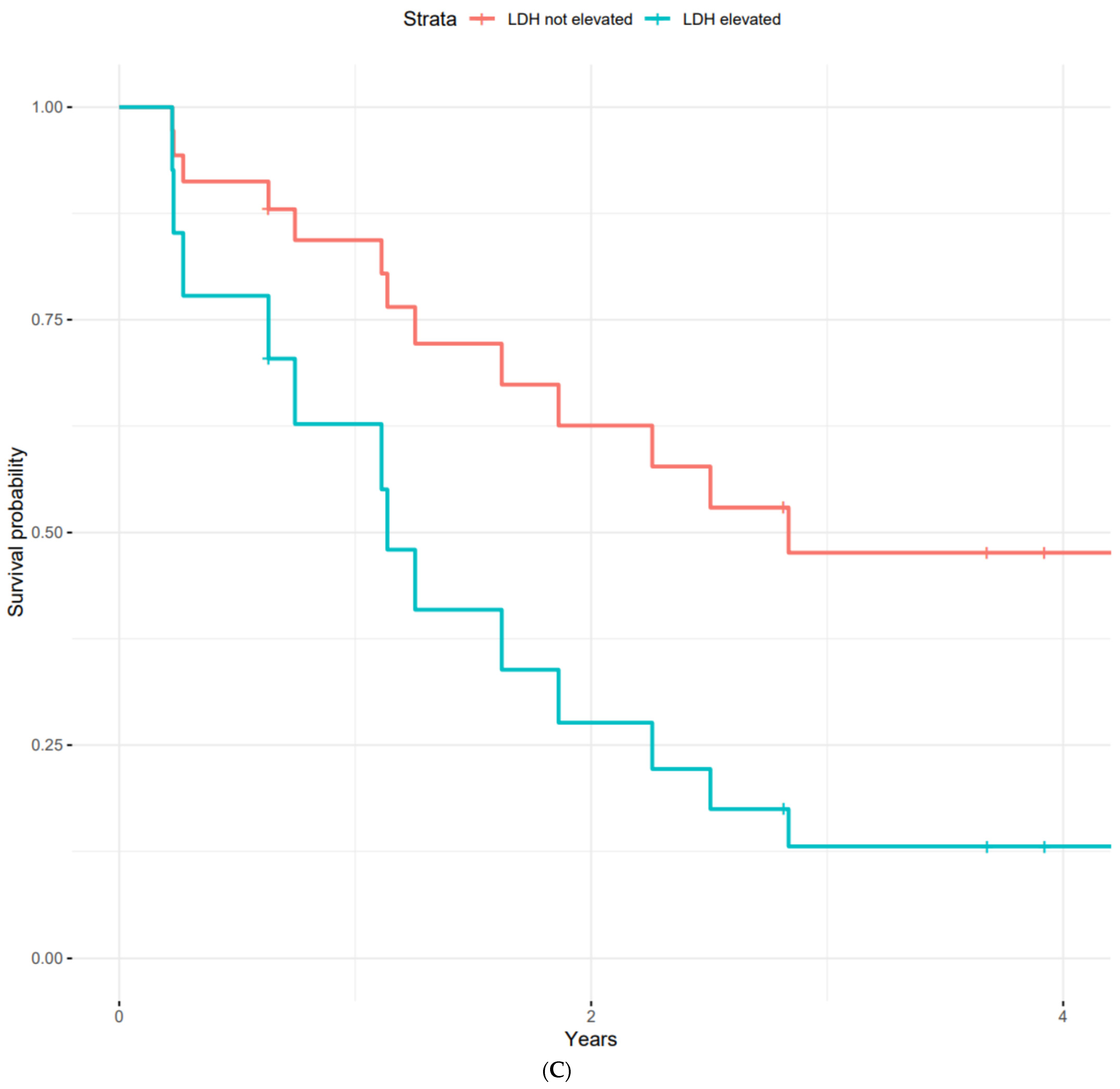
| LDH | |||
| Normal | 2.5 | 0.4–4.6 | |
| Elevated | 1.1 | 0–2.2 | no |
| Cobimetinib, Vermurafenib | Dabrafenib, Trametinib | Dacarbazine | Imatinib | Ipilimumab | Ipilimumab, Nivolumab | Nivolumab | Total | |
|---|---|---|---|---|---|---|---|---|
| Alanine aminotransferase increased (hepatitis) | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 4 |
| Alkaline phosphatase increased | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Alopecia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Anaemia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Arthritis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| CPK increased | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Creatinine increased (nephritis) | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Diarrhoea (colitis) | 0 | 0 | 0 | 0 | 2 | 6 | 2 | 10 |
| Facial nerve disorder | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Fever | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hyperglycemia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Hyperparathyroidism | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Hyperthyroidism | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| Hypophysitis | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Lipase increased | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 3 |
| Myocarditis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Nausea | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Peripheral motor neuropathy | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Platelet count decreased | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Pneumonitis | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Rash maculo-papular | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 3 |
| Serum amylase increased | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Skin hypopigmentation (Vitiligo) | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| Uveitis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| White blood cells decreased | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Gesamt | 7 | 1 | 2 | 3 | 4 | 23 | 7 | 47 |
| Immune-Related Adverse Event (irAE) | None | Grade 1–2 | Grade 3–4 | Grade 5 | Total |
|---|---|---|---|---|---|
| Alanine aminotransferase elevated (hepatitis) | 17 | 0 | 3 | 0 | 3 |
| Alkaline phosphatase elevated | 19 | 1 | 0 | 0 | 1 |
| Alopecia | 20 | 0 | 0 | 0 | 0 |
| Anaemia | 20 | 0 | 0 | 0 | 0 |
| Arthritis | 20 | 0 | 0 | 0 | 0 |
| Creatine phosphokinase elevated | 19 | 1 | 0 | 0 | 1 |
| Creatinine elevated (nephritis) | 18 | 2 | 0 | 0 | 2 |
| Diarrhoea | 15 | 4 | 1 | 0 | 5 |
| Facial nerve paralysis | 19 | 1 | 0 | 0 | 1 |
| Fever | 20 | 0 | 0 | 0 | 0 |
| Hyperglycaemia | 20 | 0 | 0 | 0 | 0 |
| Hyperparathyroidism | 20 | 0 | 0 | 0 | 0 |
| Hyperthyroidism | 19 | 0 | 0 | 0 | 1 |
| Hypophysitis | 20 | 0 | 0 | 0 | 0 |
| Lipase elevated | 18 | 0 | 2 | 0 | 2 |
| Myocarditis | 20 | 0 | 0 | 0 | 0 |
| Nausea | 20 | 0 | 0 | 0 | 0 |
| Peripheral motor neuropathy | 20 | 0 | 0 | 0 | 0 |
| Platelet count decreased | 20 | 0 | 0 | 0 | 0 |
| Pneumonitis | 20 | 0 | 0 | 0 | 0 |
| Maculo-papular rash | 19 | 1 | 0 | 0 | 1 |
| Serum amylase elevated | 19 | 1 | 0 | 0 | 1 |
| Skin hypopigmentation (vitiligo) | 20 | 0 | 0 | 0 | 0 |
| Uveitis | 20 | 0 | 0 | 0 | 0 |
| White blood cell count decreased | 20 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesener, L.; Hagelstein, V.; Terheyden, P.; Langan, E.A. A Retrospective Analysis of the Prognostic Factors and Adverse Events in the Treatment of Mucosal Melanoma in a Single Centre. J. Clin. Med. 2024, 13, 4741. https://doi.org/10.3390/jcm13164741
Wesener L, Hagelstein V, Terheyden P, Langan EA. A Retrospective Analysis of the Prognostic Factors and Adverse Events in the Treatment of Mucosal Melanoma in a Single Centre. Journal of Clinical Medicine. 2024; 13(16):4741. https://doi.org/10.3390/jcm13164741
Chicago/Turabian StyleWesener, Lambert, Victoria Hagelstein, Patrick Terheyden, and Ewan A. Langan. 2024. "A Retrospective Analysis of the Prognostic Factors and Adverse Events in the Treatment of Mucosal Melanoma in a Single Centre" Journal of Clinical Medicine 13, no. 16: 4741. https://doi.org/10.3390/jcm13164741
APA StyleWesener, L., Hagelstein, V., Terheyden, P., & Langan, E. A. (2024). A Retrospective Analysis of the Prognostic Factors and Adverse Events in the Treatment of Mucosal Melanoma in a Single Centre. Journal of Clinical Medicine, 13(16), 4741. https://doi.org/10.3390/jcm13164741




