Insights from a 7-Year Dementia Cohort (VALCODIS): ApoE Genotype Evaluation
Abstract
1. Introduction
2. Methods
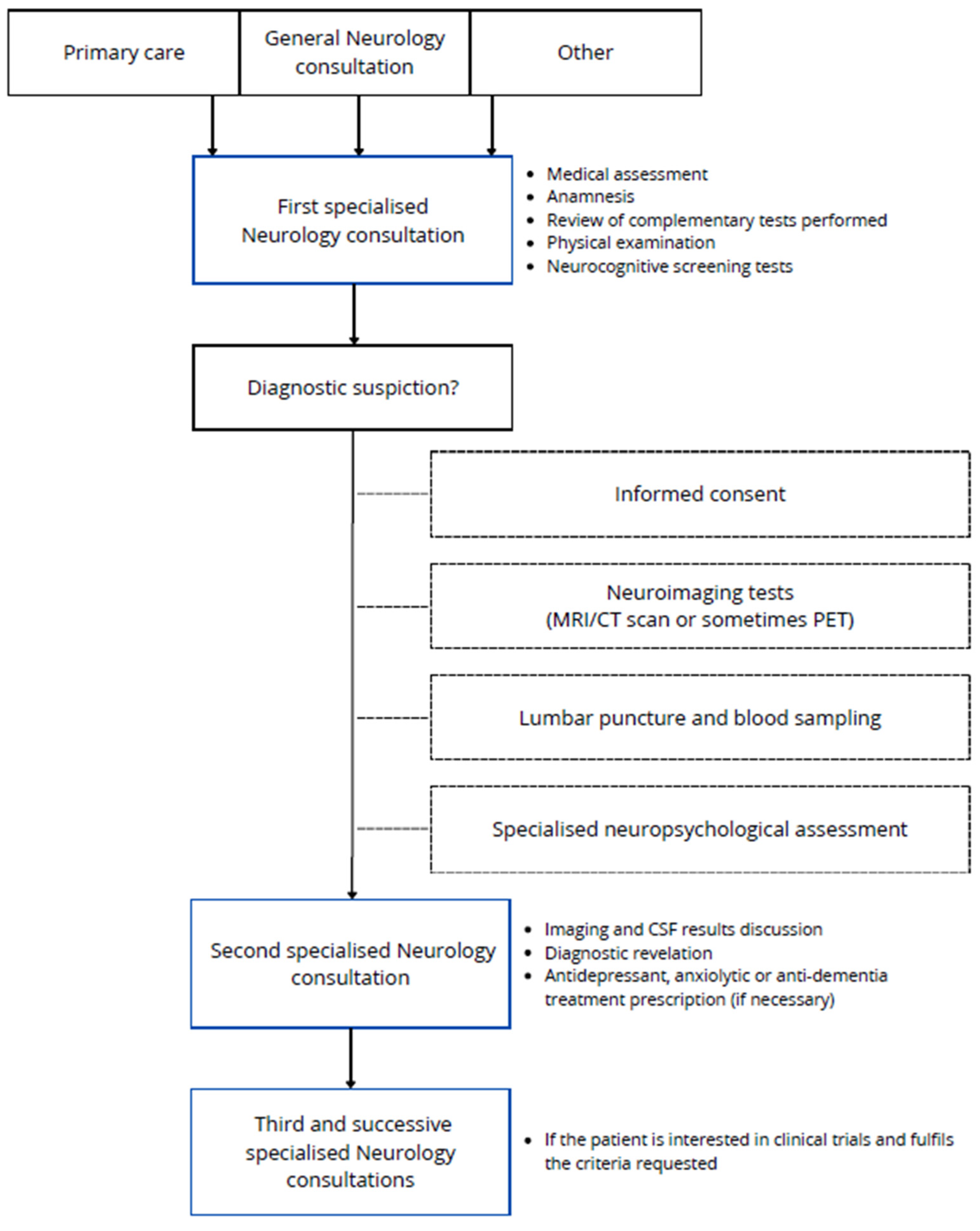
2.1. VALCODIS Cohort: General Protocol
2.2. Ethical Considerations
2.3. Sociodemographic and Anthropometric Data Collected
2.4. Clinical, Neuropsychological, Image, and Biomarker Data
2.4.1. Clinical Data
2.4.2. Neuropsychological Evaluation Data
2.4.3. Image Data
2.4.4. Biomarker Data
Cerebrospinal Fluid Samples
Blood and Plasma Samples
Other Biological Samples
2.5. Data Integration
2.6. Statistical Analyses
3. Results
3.1. Cohort Description
3.2. Relationship between ApoE Genotype and Clinical Variables
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Corrada, M.M.; Brookmeyer, R.; Paganini-Hill, A.; Berlau, D.; Kawas, C.H. Dementia incidence continues to increase with age in the oldest old: The 90+ study. Ann. Neurol. 2010, 67, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Garre-Olmo, J. Epidemiology of Alzheimer’s disease and other dementias. Rev. Neurol. 2018, 66, 377–386. [Google Scholar] [PubMed]
- Jia, L.; Quan, M.; Fu, Y.; Zhao, T.; Li, Y.; Wei, C.; Tang, Y.; Qin, Q.; Wang, F.; Qiao, Y.; et al. Dementia in China: Epidemiology, clinical management, and research advances. Lancet Neurol. 2020, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Duong, S.; Patel, T.; Chang, F. Dementia. Can. Pharm. J. Rev. Pharm. Can. 2017, 150, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Dementia in parkinsonism. Ann. Indian Acad. Neurol. 2011, 14, S21–S24. [CrossRef]
- Greene, P. Progressive Supranuclear Palsy, Corticobasal Degeneration, and Multiple System Atrophy. Contin. Lifelong Learn. Neurol. 2019, 25, 919–935. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Roca, M.; López-cuevas, R.; Baquero, M.; Vento, M.; Cháfer-pericás, C. Metabolomics study to identify plasma biomarkers in alzheimer disease: ApoE genotype effect. J. Pharm. Biomed. Anal. 2020, 180, 113088. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.M.; Heinsinger, N.M.; William Rebeck, G. Alzheimer’s Disease Genetic Risk Factor APOE-ε4 also Affects Normal Brain Function. Curr. Alzheimer Res. 2016, 13, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Bussy, A.; Snider, B.J.; Coble, D.; Xiong, C.; Fagan, A.M.; Cruchaga, C.; Benzinger, T.L.S.; Gordon, B.A.; Hassenstab, J.; Bateman, R.J.; et al. Effect of apolipoprotein E4 on clinical, neuroimaging, and biomarker measures in noncarrier participants in the Dominantly Inherited Alzheimer Network. Neurobiol. Aging 2019, 75, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Dong, L.; Huang, X.; Wang, T.; Mao, C.; Li, J.; Wang, J.; Liu, C.; Gao, J. Association of APOE ε4/ε4 with fluid biomarkers in patients from the PUMCH dementia cohort. Front. Aging Neurosci. 2023, 15, 1119070. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Uy, R.A.Z.; Vidoni, E.D.; Wilkins, H.M.; Archer, A.E.; Thyfault, J.P.; Miles, J.M.; Burns, J.M. Effect of APOE ε4 Genotype on Metabolic Biomarkers in Aging and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.; Kalaria, R.; O’Brien, J.; Skoog, I.; Alladi, S.; Black, S.E.; Blacker, D.; Blazer, D.G.; Chen, C.; Chui, H.; et al. Diagnostic Criteria for Vascular Cognitive Disorders. Alzheimer Dis. Assoc. Disord. 2014, 28, 206–218. [Google Scholar] [CrossRef]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.-P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Lacritz, L.H.; Hall, J.; Waring, S.C.; Chan, W.; Khodr, Z.G.; Massman, P.J.; Hobson, V.; Cullum, C.M. Validation of the New Interpretive Guidelines for the Clinical Dementia Rating Scale Sum of Boxes Score in the National Alzheimer’s Coordinating Center Database. Arch. Neurol. 2010, 67, 746–749. [Google Scholar] [CrossRef]
- Lee, J.; Ganguli, M.; Weerman, A.; Chien, S.; Lee, D.Y.; Varghese, M.; Dey, A.B. Online Clinical Consensus Diagnosis of Dementia: Development and Validation. J. Am. Geriatr. Soc. 2020, 68, S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.K.F.; Chan, J.Y.C.; Hirai, H.W.; Wong, S.Y.S.; Kwok, T.C.Y. Cognitive Tests to Detect Dementia. JAMA Intern. Med. 2015, 175, 1450. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Freilich, B.M.; Hyer, L.A. Relation of the Repeatable Battery for Assessment of Neuropsychological Status to Measures of Daily Functioning in Dementia. Psychol. Rep. 2007, 101, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Muntal Encinas, S.; Gramunt-Fombuena, N.; Badenes Guia, D.; Casas Hernanz, L.; Aguilar Barbera, M. Traducción y adaptación española de la batería neuropsicológica Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) forma A en una muestra piloto. Neurología 2012, 27, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.A.; Zoller, A.S.; Lorius, N.; Amariglio, R.E.; Locascio, J.J.; Johnson, K.A.; Sperling, R.A.; Rentz, D.M. Functional Activities Questionnaire Items that Best Discriminate and Predict Progression from Clinically Normal to Mild Cognitive Impairment. Curr. Alzheimer Res. 2015, 12, 493–502. [Google Scholar] [CrossRef]
- Galasko, D.; Bennett, D.; Sano, M.; Ernesto, C.; Thomas, R.; Grundman, M.; Ferris, S. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer’s Dis. Coop. Study. Alzheimer Dis. Assoc. Disord. 1997, 11 (Suppl. S2), S33–S39. [Google Scholar]
- Goldberg, T.E.; Koppel, J.; Keehlisen, L.; Christen, E.; Dreses-Werringloer, U.; Conejero-Goldberg, C.; Gordon, M.L.; Davies, P. Performance-Based Measures of Everyday Function in Mild Cognitive Impairment. Am. J. Psychiatry 2010, 167, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Bird, V.; Rizzo, M.; Meader, N. Diagnostic validity and added value of the geriatric depression scale for depression in primary care: A meta-analysis of GDS30 and GDS15. J. Affect. Disord. 2010, 125, 10–17. [Google Scholar] [CrossRef]
- Knopman, D.S.; DeKosky, S.T.; Cummings, J.L.; Chui, H.; Corey–Bloom, J.; Relkin, N.; Small, G.W.; Miller, B.; Stevens, J.C. Practice parameter: Diagnosis of dementia (an evidence-based review). Neurology 2001, 56, 1143–1153. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef]
- Johnson, K.A.; Minoshima, S.; Bohnen, N.I.; Donohoe, K.J.; Foster, N.L.; Herscovitch, P.; Karlawish, J.H.; Rowe, C.C.; Carrillo, M.C.; Hartley, D.M.; et al. Appropriate Use Criteria for Amyloid PET: A Report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. J. Nucl. Med. 2013, 54, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.; Koeppe, R.A.; Little, R.; An, H.; Junck, L.; Giordani, B.; Persad, C.; Heumann, M.; Wernette, K. Striatal monoamine terminals in Lewy body dementia and Alzheimer’s disease. Ann. Neurol. 2004, 55, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.S.; Okamura, N.; Arai, H.; Higuchi, M.; Matsui, T.; Tashiro, M.; Shinkawa, M.; Itoh, M.; Ido, T.; Sasaki, H. 18F-fluorodopa PET study of striatal dopamine uptake in the diagnosis of dementia with Lewy bodies. Neurology 2000, 55, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Varrone, A.; Asenbaum, S.; Vander Borght, T.; Booij, J.; Nobili, F.; Någren, K.; Darcourt, J.; Kapucu, Ö.L.; Tatsch, K.; Bartenstein, P.; et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.W.; Young, A.L.; Oxtoby, N.P.; Smith, R.; Ossenkoppele, R.; Strandberg, O.T.; La Joie, R.; Aksman, L.M.; Grothe, M.J.; Iturria-Medina, Y.; et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 2021, 27, 871–881. [Google Scholar] [CrossRef]
- Mattsson, N.; Palmqvist, S.; Stomrud, E.; Vogel, J.; Hansson, O. Staging β -Amyloid Pathology With Amyloid Positron Emission Tomography. JAMA Neurol. 2019, 76, 1319. [Google Scholar] [CrossRef] [PubMed]
- Ben Bouallègue, F.; Mariano-Goulart, D.; Payoux, P. Comparison of CSF markers and semi-quantitative amyloid PET in Alzheimer’s disease diagnosis and in cognitive impairment prognosis using the ADNI-2 database. Alzheimers. Res. Ther. 2017, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.-Y.; Kim, E.S.; Kim, H.-S.; Jeon, S.; Lee, M.J.; Pak, K.; Lee, J.-H.; Lee, Y.M.; Lee, K.; Shin, J.-H.; et al. Comparison of Diagnostic Performances Between Cerebrospinal Fluid Biomarkers and Amyloid PET in a Clinical Setting. J. Alzheimer’s Dis. 2020, 74, 473–490. [Google Scholar] [CrossRef]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870–881. [Google Scholar] [CrossRef]
- Zetterberg, H. Biofluid-based biomarkers for Alzheimer’s disease–related pathologies: An update and synthesis of the literature. Alzheimer’s Dement. 2022, 18, 1687–1693. [Google Scholar] [CrossRef]
- de-Almada, B.V.P.; De-Almeida, L.D.; Camporez, D.; De-Moraes, M.V.D.; Morelato, R.L.; Perrone, A.M.S.; Belcavello, L.; Louro, I.D.; De-Paula, F. Protective effect of the APOE-e3 allele in Alzheimer’s disease. Braz. J. Med. Biol. Res. 2012, 45, 8–12. [Google Scholar] [CrossRef] [PubMed]
- González, R.D.; Gomes, I.; Gomes, C.; Rocha, R.; Durães, L.; Sousa, P.; Figueruelo, M.; Rodríguez, M.; Pita, C.; Hornero, R.; et al. APOE Variants in an Iberian Alzheimer Cohort Detected through an Optimized Sanger Sequencing Protocol. Genes 2020, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Ibarreta, D.; Gómez-Isla, T.; Portera-Sánchez, A.; Parrilla, R.; Ayuso, M.S. Apolipoprotein E genotype in Spanish patients of Alzheimer’s or Parkinson’s disease. J. Neurol. Sci. 1995, 134, 146–149. [Google Scholar] [CrossRef] [PubMed]


| Variables | SMC (n = 165) | MCI AD (n = 302) | Mild Dementia AD (n = 215) | Moderate or Severe Dementia AD (n = 30) | DLB (n = 10) | FTLD (n = 61) | Other (VaD…) (n = 466) |
|---|---|---|---|---|---|---|---|
| Age (years, Median (IQR)) | 63 (59–68) | 71 (67–74) | 72 (68–74) | 70 (65–73) | 71 (69–74) | 67 (62–71) | 67 (61–73) |
| Gender (female n (%)) | 93 (56.4%) | 180 (59.6%) | 132 (61.4%) | 23 (76.7%) | 6 (60.0%) | 37 (60.7%) | 250 (53.6%) |
| ApoE (ε4 carrier n (%)) (n = 900) | 19 (11.5%) | 123 (40.7%) | 77 (35.8%) | 16 (53.3%) | 1 (10.0%) | 5 (8.2%) | 64 (13.7%) |
| Aβ42 (pg·mL−1, median (IQR)) | 1224 (985–1468) | 644 (496–783) | 600 (453–737) | 544 (452–659) | 841 (791–1238) | 1093 (858–1487) | 1087 (809–1420) |
| Aβ40 (pg·mL−1, median (IQR)) (n = 592) | 11,368 (9783–14,193) | 13,419 (10,599–16,012) | 12,572 (10,624–15,663) | 10,420 (7730–12,684) | 12,136 (7866–12,989) | 11,727 (8641–15,968) | 11,794 (8976–14,637) |
| t-tau (pg·mL−1, median (IQR)) | 225 (159–310) | 593 (399–806) | 640 (452–879) | 699 (410–1008) | 263 (156–388) | 317 (226–519) | 278 (187–394) |
| p-tau181 (pg·mL−1, median (IQR)) | 36 (28–47) | 96 (66–134) | 98 (73–141) | 101 (72–149) | 39 (24–58) | 40 (32–61) | 42 (30–58) |
| Aβ42/Aβ40 median (IQR) (n = 591) | 0.108 (0.099–0.116) | 0.050 (0.043–0.058) | 0.049 (0.040–0.055) | 0.051 (0.041–0.057) | 0.102 (0.062–0.111) | 0.106 (0.089–0.112) | 0.107 (0.082–0.114) |
| Aβ42/t-tau median (IQR) (n = 898) | 0.190 (0.150–0.240) | 0.890 (0.650–1.310) | 1.050 (0.710–1.645) | 1.180 (0.805–1.750) | 0.250 (0.163–0.467) | 0.300 (0.230–0.383) | 0.300 (0.230–0.383) |
| NfL (pg·mL−1, median (IQR)) (n = 595) | 558 (442–759) | 1010 (768–1359) | 1157 (885–1557) | 1112 (969–1560) | 795 (601–949) | 2029 (919–3976) | 782 (512–1236) |
| ADCS-ADL-MCI (score, median (IQR)) | 47 (44–50) | 43 (38–46) | 36 (31–40) | 26 (18–33) | 39 (33–44) | 31 (23–41) | 42 (35–46) |
| CDR (score, median (IQR)) | 0 (0–0.5) | 0.5 (0.5–0.5) | 0.5 (0.5–1) | 2 (2–2) | 0.5 (0.5–1) | 1 (0.5–1) | 0.5 (0–0.5) |
| FAQ (score, median (IQR)) | 1 (0–2) | 4 (1–6) | 14 (11–18) | 23 (18–25) | 7 (3–16) | 12 (7–22) | 5 (1–10) |
| MMSE (score, median (IQR)) | 29 (28–30) | 25 (22–27) | 21 (17–24) | 14 (10–18) | 23 (17–25) | 23 (18–25) | 25 (22–28) |
| RBANS.DM (score, median (IQR)) | 98 (92–102) | 56 (44–78) | 48 (40–56) | 40 (40–48) | 75 (42–83) | 56 (44–78) | 68 (52–84) |
| GDS (score, median (IQR)) | 9 (5–16) | 8 (5–13) | 10 (6–16) | 9 (6–14) | 18 (8–23) | 13 (6–18) | 12 (6–19) |
| Variables | Non-Carrier Participants (n = 378, 79.6%) | ε4 Heterozygous Participants (n = 83, 17.5%) | ε4 Homozygous Participants (n = 14, 2.9%) | p-Value a |
|---|---|---|---|---|
| Aβ42 (pg·mL−1, median (IQR)) | 1196 (925–1544) | 901 (673–1242) | 515 (370–1158) | <0.001 |
| Aβ40 (pg·mL−1, median (IQR)) | 11,727 (9187–14,883) | 12,232 (8806–14,168) | 10,637 (7325–15,474) | 0.695 |
| t-tau (pg·mL−1, median (IQR)) | 259 (182–361) | 269 (172–444) | 409 (236–652) | 0.051 |
| p-tau181 (pg·mL−1, median (IQR)) | 36 (27–49) | 42 (31–61) | 71 (36–96) | 0.002 |
| Aβ42/Aβ40 median (IQR) | 0.109 (0.100–0.116) | 0.087 (0.069–0.105) | 0.053 (0.035–0.102) | <0.001 |
| Aβ42/t-tau median (IQR) | 0.210 (0.170–0.290) | 0.285 (0.180–0.460) | 0.900 (0.270–1.505) | <0.001 |
| NfL (pg·mL−1, median (IQR)) | 760 (516–1205) | 694 (440–1074) | 899 (480–2395) | 0.151 |
| ADCS-ADL-MCI (score, median (IQR)) | 42 (35–47) | 44 (37–48) | 43 (37–47) | 0.203 |
| CDR (score, median (IQR)) | 0.5 (0.5–0.5) | 0.5 (0.5–0.5) | 0.5 (0.5–0.5) | 0.534 |
| FAQ (score, median (IQR)) | 4 (1–10) | 3 (0–7) | 1 (0–9) | 0.135 |
| MMSE (score, median (IQR)) | 26 (23–28) | 27 (24–29) | 24 (22–27) | 0.174 |
| RBANS.DM (score, median (IQR)) | 78 (56–95) | 78 (60–94) | 56 (40–92) | 0.288 |
| Variables | Non-Carrier Participants (n = 193, 45.4%) | ε4 Heterozygous Participants (n = 199, 46.8%) | ε4 Homozygous Participants (n = 33, 7.8%) | p-Value a |
|---|---|---|---|---|
| Aβ42 (pg·mL−1, median (IQR)) | 643 (482–796) | 632 (498–752) | 501 (390–606) | <0.001 |
| Aβ40 (pg·mL−1, median (IQR)) | 12,907 (10,349–16,115) | 13,145 (10,751–15,752) | 11,049 (9419–13,656) | 0.073 |
| t-tau (pg·mL−1, median (IQR)) | 623 (443–859) | 626 (403–867) | 587 (368–927) | 0.854 |
| p-tau181 (pg·mL−1, median (IQR)) | 103 (71–141) | 104 (70–143) | 86 (69–131) | 0.738 |
| Aβ42/Aβ40 median (IQR)) | 0.050 (0.042–0.059) | 0.050 (0.043–0.056) | 0.045 (0.036–0.055) | 0.098 |
| Aβ42/t-tau median (IQR)) | 0.930 (0.660–1.375) | 0.990 (0.685–1.420) | 1130 (0.700–1.685) | 0.290 |
| NfL (pg·mL−1, median (IQR)) | 1108 (882–1556) | 1051 (811–1409) | 901 (724–1242) | 0.084 |
| ADCS-ADL-MCI (score, median (IQR)) | 39 (33–45) | 39 (34–45) | 38 (33–42) | 0.493 |
| CDR (score, median (IQR)) | 0.5 (0.5–1) | 0.5 (0.5–1) | 0.5 (0.5–1) | 0.226 |
| FAQ (score, median (IQR)) | 7 (3–14) | 7 (2–13) | 8 (5–14) | 0.878 |
| MMSE (score, median (IQR)) | 22 (19–26) | 23 (19–26) | 24 (18–26) | 0.946 |
| RBANS.DM (score, median (IQR)) | 56 (44–71) | 48 (40–64) | 44 (40–56) | 0.001 |
| r a (p Value) | ADCS-ADL-MCI | CDR | FAQ | MMSE | RBANS.DM | |
|---|---|---|---|---|---|---|
| non-carrier | Aβ42 | 0.062 (0.262) | −0.020 (0.713) | −0.050 (0.364) | 0.066 (0.228) | 0.078 (0.163) |
| Aβ40 | 0.061 (0.294) | −0.043 (0.461) | −0.074 (0.209) | 0.015 (0.790) | 0.035 (0.555) | |
| t-tau | −0.199 (<0.001) | 0.171 (0.002) | 0.199 (<0.001) | −0.254 (<0.001) | −0.144 (0.010) | |
| p-tau | −0.139 (0.012) | 0.100 (0.069) | 0.139 (0.012) | −0.256 (<0.001) | −0.174 (0.002) | |
| Aβ42/Aβ40 | 0.040 (0.496) | −0.006 (0.924) | −0.063 (0.282) | 0.132 (0.022) | 0.103 (0.083) | |
| Aβ42/t-tau | −0.202 (<0.001) | 0.144 (0.010) | 0.202 (<0.001) | −0.288 (<0.001) | −0.196 (0.001) | |
| NfL | −0.318 (<0.001) | 0.313 (<0.001) | 0.350 (<0.001) | −0.285 (<0.001) | −0.287 (<0.001) | |
| ε4 heterozygous | Aβ42 | 0.224 (0.070) | −0.279 (0.024) | −0.213 (0.093) | 0.334 (0.005) | 0.191 (0.119) |
| Aβ40 | 0.067 (0.606) | −0.053 (0.684) | −0.059 (0.658) | 0.025 (0.841) | −0.019 (0.884) | |
| t-tau | −0.046 (0.713) | 0.170 (0.177) | 0.152 (0.234) | −0.415 (<0.001) | −0.313 (0.009) | |
| p-tau | −0.140 (0.261) | 0.207 (0.098) | 0.182 (0.153) | −0.440 (<0.001) | −0.381 (0.001) | |
| Aβ42/Aβ40 | 0.255 (0.045) | −0.425 (0.001) | −0.274 (0.036) | 0.458 (<0.001) | 0.252 (0.045) | |
| Aβ42/t-tau | −0.255 (0.046) | 0.372 (0.003) | 0.320 (0.013) | −0.579 (<0.001) | −0.372 (0.002) | |
| NfL | −0.336 (0.008) | 0.356 (0.005) | 0.367 (0.004) | −0.419 (0.001) | −0.255 (0.042) | |
| ε4 homozygous | Aβ42 | 0.251 (0.588) | −0.345 (0.449) | −0.626 (0.374) | 0.439 (0.325) | 0.718 (0.108) |
| Aβ40 | 0.234 (0.614) | −0.445 (0.316) | −0.448 (0.552) | 0.296 (0.519) | 0.307 (0.555) | |
| t-tau | −0.117 (0.802) | 0.012 (0.979) | −0.019 (0.981) | −0.263 (0.569) | −0.126 (0.812) | |
| p-tau | −0.138 (0.768) | −0.079 (0.867) | 0.436 (0.564) | −0.357 (0.431) | −0.602 (0.206) | |
| Aβ42/Aβ40 | 0.347 (0.445) | −0.242 (0.601) | −0.929 (0.071) | 0.607 (0.149) | 0.888 (0.018) | |
| Aβ42/t-tau | −0.370 (0.414) | 0.465 (0.294) | 0.999 (0.001) | −0.706 (0.076) | −0.754 (0.084) | |
| NfL | −0.676 (0.096) | 0.854 (0.014) | −0.252 (0.748) | −0.786 (0.036) | −0.453 (0.368) |
| r a (p Value) | ADCS-ADL-MCI | CDR | FAQ | MMSE | RBANS.DM | |
|---|---|---|---|---|---|---|
| non-carrier | Aβ42 | 0.201 (0.007) | −0.232 (0.001) | −0.226 (0.002) | 0.243 (0.001) | 0.315 (<0.001) |
| Aβ40 | 0.158 (0.049) | −0.201 (0.012) | −0.149 (0.065) | 0.109 (0.173) | 0.061 (0.458) | |
| t-tau | 0.037 (0.624) | 0.066 (0.371) | 0.073 (0.322) | −0.253 (0.001) | −0.306 (<0.001) | |
| p-tau | 0.008 (0.919) | 0.071 (0.339) | 0.093 (0.211) | −0.229 (0.002) | −0.334 (<0.001) | |
| Aβ42/Aβ40 | 0.069 (0.396) | −0.036 (0.653) | −0.102 (0.205) | 0.166 (0.037) | 0.333 (<0.001) | |
| Aβ42/t-tau | −0.015 (0.846) | 0.127 (0.085) | 0.148 (0.046) | −0.293 (<0.001) | −0.349 (<0.001) | |
| NfL | −0.170 (0.035) | 0.116 (0.150) | 0.206 (0.010) | −0.138 (0.087) | −0.136 (0.098) | |
| ε4 heterozygous | Aβ42 | 0.103 (0.169) | −0.099 (0.177) | −0.084 (0.253) | 0.106 (0.148) | 0.109 (0.145) |
| Aβ40 | 0.044 (0.596) | −0.168 (0.043) | −0.066 (0.427) | 0.032 (0.702) | −0.068 (0.426) | |
| t-tau | −0.108 (0.150) | 0.132 (0.071) | 0.120 (0.101) | −0.192 (0.008) | −0.297 (<0.001) | |
| p-tau | −0.086 (0.250) | 0.053 (0.466) | 0.055 (0.452) | −0.130 (0.073) | −0.260 (<0.001) | |
| Aβ42/Aβ40 | 0.061 (0.470) | 0.016 (0.846) | −0.036 (0.670) | 0.096 (0.250) | 0.188 (0.027) | |
| Aβ42/t-tau | −0.108 (0.151) | 0.117 (0.110) | 0.094 (0.197) | −0.162 (0.026) | −0.253 (0.001) | |
| NfL | −0.188 (0.023) | 0.123 (0.139) | 0.199 (0.016) | −0.118 (0.154) | −0.303 (<0.001) | |
| ε4 homozygous | Aβ42 | 0.265 (0.174) | −0.272 (0.146) | −0.127 (0.504) | 0.163 (0.389) | −0.018 (0.925) |
| Aβ40 | −0.022 (0.918) | −0.030 (0.889) | 0.175 (0.414) | −0.092 (0.670) | −0.011 (0.960) | |
| t-tau | −0.537 (0.003) | 0.356 (0.054) | 0.214 (0.255) | −0.283 (0.130) | −0.196 (0.307) | |
| p-tau | −0.426 (0.024) | 0.390 (0.033) | 0.294 (0.115) | −0.369 (0.045) | −0.205 (0.286) | |
| Aβ42/Aβ40 | 0.273 (0.197) | −0.272 (0.198) | −0.424 (0.039) | 0.245 (0.249) | −0.050 (0.819) | |
| Aβ42/t-tau | −0.591 (0.001) | 0.396 (0.030) | 0.215 (0.255) | −0.249 (0.185) | −0.149 (0.440) | |
| NfL | −0.168 (0.433) | 0.257 (0.226) | 0.184 (0.389) | −0.009 (0.965) | −0.078 (0.724) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baquero, M.; Ferré-González, L.; Álvarez-Sánchez, L.; Ferrer-Cairols, I.; García-Vallés, L.; Peretó, M.; Raga, L.; García-Lluch, G.; Peña-Bautista, C.; Muria, B.; et al. Insights from a 7-Year Dementia Cohort (VALCODIS): ApoE Genotype Evaluation. J. Clin. Med. 2024, 13, 4735. https://doi.org/10.3390/jcm13164735
Baquero M, Ferré-González L, Álvarez-Sánchez L, Ferrer-Cairols I, García-Vallés L, Peretó M, Raga L, García-Lluch G, Peña-Bautista C, Muria B, et al. Insights from a 7-Year Dementia Cohort (VALCODIS): ApoE Genotype Evaluation. Journal of Clinical Medicine. 2024; 13(16):4735. https://doi.org/10.3390/jcm13164735
Chicago/Turabian StyleBaquero, Miguel, Laura Ferré-González, Lourdes Álvarez-Sánchez, Inés Ferrer-Cairols, Lorena García-Vallés, Mar Peretó, Luis Raga, Gemma García-Lluch, Carmen Peña-Bautista, Beatriz Muria, and et al. 2024. "Insights from a 7-Year Dementia Cohort (VALCODIS): ApoE Genotype Evaluation" Journal of Clinical Medicine 13, no. 16: 4735. https://doi.org/10.3390/jcm13164735
APA StyleBaquero, M., Ferré-González, L., Álvarez-Sánchez, L., Ferrer-Cairols, I., García-Vallés, L., Peretó, M., Raga, L., García-Lluch, G., Peña-Bautista, C., Muria, B., Prieto, A., Jareño, I., & Cháfer-Pericás, C. (2024). Insights from a 7-Year Dementia Cohort (VALCODIS): ApoE Genotype Evaluation. Journal of Clinical Medicine, 13(16), 4735. https://doi.org/10.3390/jcm13164735






