Myocardial Involvement in Catastrophic Antiphospholipid Syndrome during Pregnancy or Puerperium: A Case of a Young Breastfeeding Woman and Literature Review
Abstract
1. Introduction
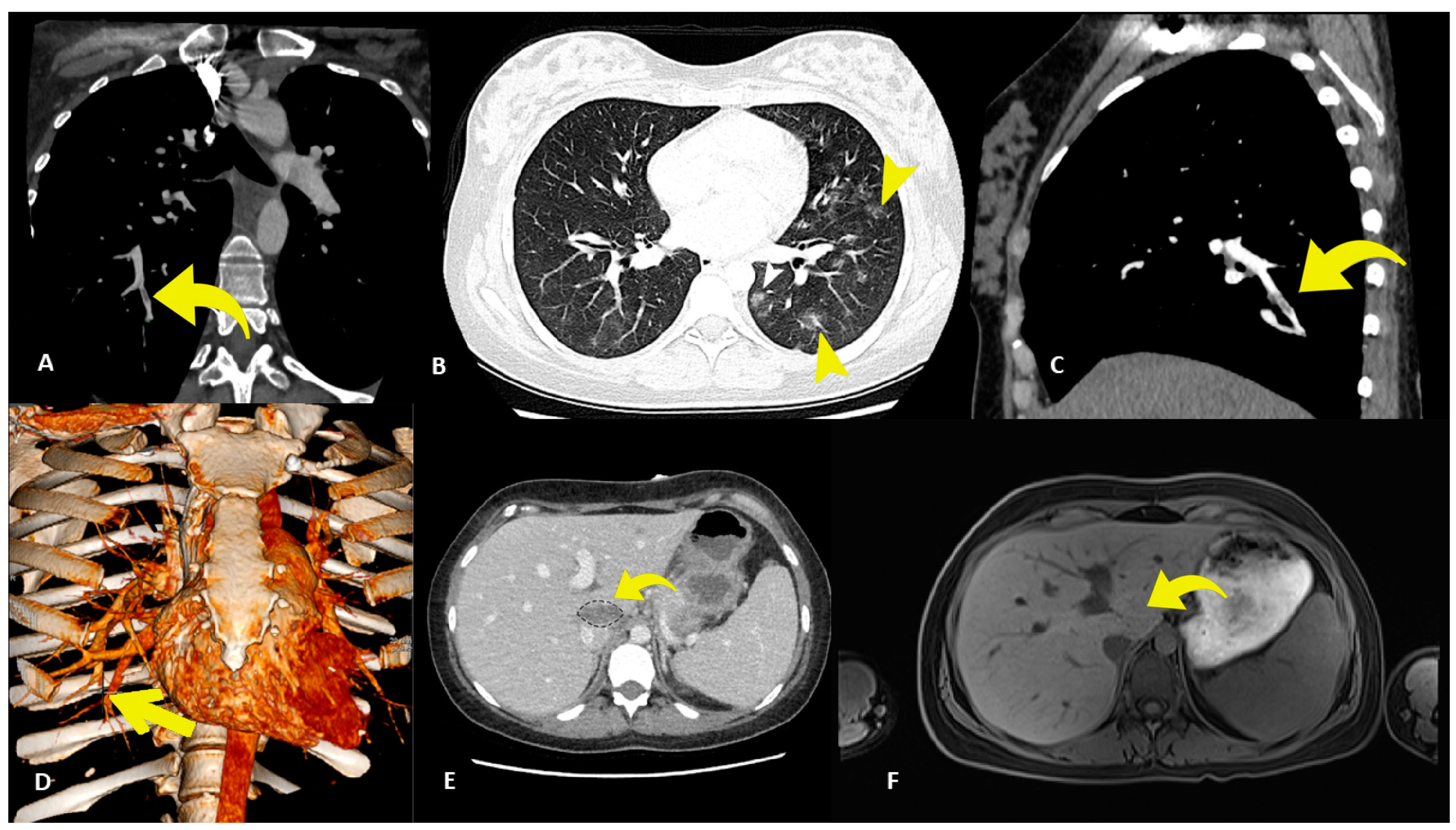
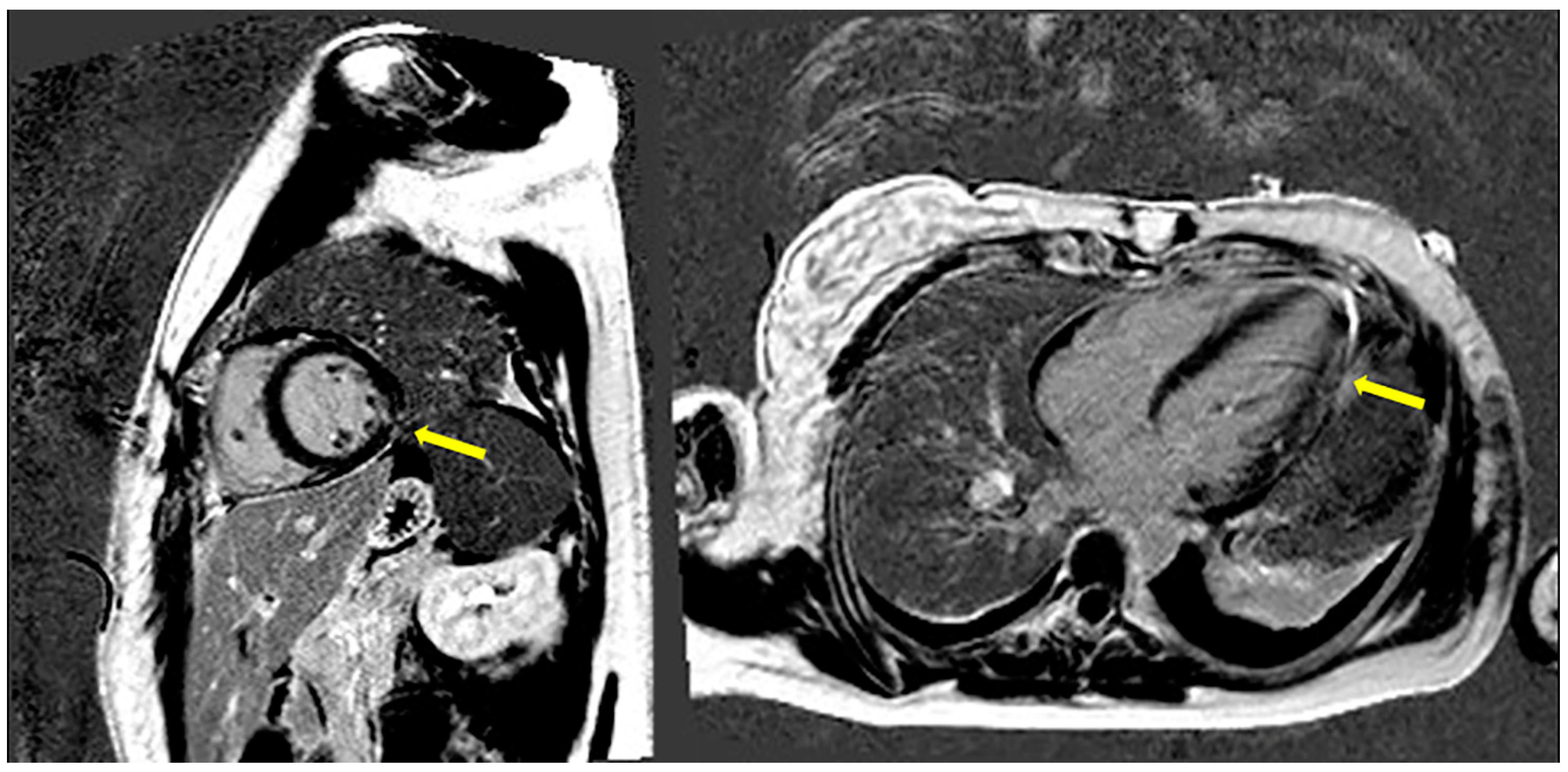
2. Case Presentation
3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cervera, R.; Piette, J.C.; Font, J.; Shoenfeld, Y.; Camps, M.T.; Jacobsen, S.; Lakos, G.; Tincani, A.; Kontopoulou-Griva, I.; Galeazzi, M.; et al. Antiphospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1000 patients. Arthritis Rheum. 2002, 46, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Hoayek, J.G.; Moussa, H.N.; Rehman, H.A.; Nasab, S.H.; Blackwell, S.C.; Sibai, B.M. Catastrophic antiphospholipid syndrome in pregnancy, a diagnosis that should not be missed. J. Matern. Fetal Neonatal Med. 2016, 29, 3950–3955. [Google Scholar] [CrossRef] [PubMed]
- Asherson, R.A.; Cervera, R.; De Groot, P.G.; Erkan, D.; Boffa, M.C.; Piette, J.C.; Khamashta, M.A.; Shoenfeld, Y. Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrophic antiphospholipid syndrome: International consensus statement on classification criteria and treatment guidelines. Lupus 2003, 12, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Rodriguez-Pintó, I.; Colafrancesco, S.; Conti, F.; Rosário, C.; Agmon-Levin, N.; Shoenfeld, Y.; Ferrão, C.; Faria, R.; Vasconcelos, C.; et al. 14th International Congress on Antiphospholipid Antibodies Task Force Report on Catastrophic Antiphospholipid Syndrome. Autoimmun. Rev. 2014, 13, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Pons, I.; Jeréz, A.; Espinosa, G.; Rodríguez-Pintó, I.; Erkan, D.; Shoenfeld, Y.; Cervera, R.; CAPS Registry Project Group/European Forum on Antiphospholipid Antibodies (Supplementary Material 1). Cardiac involvement in the catastrophic antiphospholipid syndrome (CAPS): Lessons from the “CAPS registry”. Semin. Arthritis Rheum. 2024, 66, 152439. [Google Scholar] [CrossRef] [PubMed]
- Delgado Alves, J.; Kumar, S.; Isenberg, D.A. Cross-reactivity between anti-cardiolipin, anti-high-density lipoprotein and anti-apolipoprotein A-I IgG antibodies in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Rheumatology 2003, 42, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Font, J.; Cervera, R. Cardiac manifestations in antiphospholipid syndrome. In Hughes Syndrome, 2nd ed.; Khamashta, M.A., Ed.; Springer: Singapore, 2006; pp. 41–53. [Google Scholar]
- Nazir, S.; Tachamo, N.; Lohani, S.; Hingorani, R.; Poudel, D.R.; Donato, A. Acute myocardial infarction and antiphospholipid antibody syndrome: A systematic review. Coron. Artery Dis. 2017, 28, 332–335. [Google Scholar] [CrossRef]
- Denas, G.; Jose, S.P.; Bracco, A.; Zoppellaro, G.; Pengo, V. Antiphospholipid syndrome and the heart: A case series and literature review. Autoimmun. Rev. 2015, 14, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Osula, S.; Bell, G.M.; Hornung, R.S. Acute myocardial infarction in young adults: Causes and management. Postgrad. Med. J. 2002, 78, 27–30. [Google Scholar] [CrossRef]
- Correia, A.F.; Oliveira, D.C.; Sanctos, M. Coronary artery thromboses, stent thrombosis and antiphospholipid antibody syndrome: Case report. Cardiol. Res. 2018, 9, 129–132. [Google Scholar] [CrossRef]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; de Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Braunstein, E.M.; Yuan, X.; Alexander, A.; Chen, H.; Gavriilaki, E.; Alluri, R.; Streiff, M.B.; Petri, M.; Crowther, M.A.; et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood 2020, 135, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Canaud, G.; Bienaimé, F.; Tabarin, F.; Bataillon, G.; Seilhean, D.; Noël, L.H.; Dragon-Durey, M.A.; Snanoudj, R.; Friedlander, G.; Halbwachs-Mecarelli, L.; et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N. Eng. J. Med. 2014, 371, 303–312. [Google Scholar] [CrossRef]
- Giannakopoulos, B.; Krilis, S.A. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 2013, 368, 1033–1044. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Gerli, R.; Doria, A.; Matsuura, E.; Cerinic, M.M.; Ronda, N.; Jara, L.J.; Abu-Shakra, M.; Meroni, P.L.; Sherer, Y. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation 2005, 112, 3337–3347. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O.; Manttari, M.; Manninen, V.; Tenkanen, L.; Puurunen, M.; Aho, K.; Palosuo, T. Anti-cardiolipin antibodies and risk of myocardial infarction in a prospective cohort of middle-aged men. Circulation 1995, 91, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pintó, I.; Moitinho, M.; Santacreu, I.; Shoenfeld, Y.; Erkan, D.; Espinosa, G.; Cervera, R.; CAPS Registry Project Group. Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of 500 patients from the international CAPS registry. Autoimmun. Rev. 2016, 15, 1120–1124. [Google Scholar] [CrossRef]
- Carmi, O.; Berla, M.; Shoenfeld, Y.; Levy, Y. Diagnosis and management of catastrophic antiphospholipid syndrome. Expert. Rev. Hematol. 2017, 10, 365–374. [Google Scholar] [CrossRef]
- Azoulay, L.-D.; Pineton de Chambrun, M.; Larcher, R.; Pène, F.; Argaud, L.; Mayaux, J.; Jamme, M.; Coudroy, R.; Mathian, A.; Gibelin, A.; et al. Prevalence, characteristics and outcome of cardiac manifestations in critically-ill antiphospholipid syndrome patients. J. Autoimmun. 2022, 133, 102908. [Google Scholar] [CrossRef]
- Repesse, X.; Freund, Y.; Mathian, A.; Hervier, B.; Amoura, Z.; Luyt, C.E. Successful extracorporeal membrane oxygenation for refractory cardiogenic shock due to the catastrophic antiphospholipid syndrome. Ann. Intern. Med. 2010, 153, 487–488. [Google Scholar] [CrossRef]
- Bucciarelli, S.; Cervera, R.; Espinosa, G.; Gómez-Puerta, J.A.; Ramos-Casals, M.; Font, J. Mortality in the catastrophic antiphospholipid syndrome: Causes of death and prognostic factors. Autoimmun. Rev. 2006, 6, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pintó, I.; Espinosa, G.; Erkan, D.; Shoenfeld, Y.; Cervera, R.; CAPS Registry Project Group. The effect of triple therapy on the mortality of catastrophic anti-phospholipid syndrome patients. Rheumatology 2018, 57, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Puerta, J.A.; Cervera, R.; Espinosa, G.; Asherson, R.A.; García-Carrasco, M.; da Costa, I.P.; Andrade, D.C.; Borba, E.F.; Makatsaria, A.; Bucciarelli, S.; et al. Catastrophic antiphospholipid syndrome during pregnancy and puerperium: Maternal and fetal characteristics of 15 cases. Ann. Rheum. Dis. 2007, 66, 740–746. [Google Scholar] [CrossRef]
- Girish, B.; Gainder, S.; Saha, S.C.; Krishnappa, D. Rare presentation of catastrophic antiphospholipid syndrome with myocarditis in post-partum period: Case report and review of literature. J. Obstet. Gynecol. India 2018, 68, 70–72. [Google Scholar] [CrossRef]
- Bendon, R.W.; Wilson, J.; Getahun, B.; van der Bel-Kahn, J. A maternal death due to thrombotic disease associated with anticardiolipin antibody. Arch. Pathol. Lab. Med. 1987, 111, 370–372. [Google Scholar] [PubMed]
- Hochfeld, M.; Druzin, M.L.; Maia, D.; Wright, J.; Lambert, R.E.; McGuire, J. Pregnancy complicated by primary an-tiphospholipid antibody syndrome. Obstet. Gynecol. 1994, 83, 804–805. [Google Scholar] [PubMed]
- Ortiz, P.; Castro, A.; Vallés, M.; Coll, E.; Casas, M.; Mauri, J.M. Catastrophic antiphospholipid syndrome in the immediate puerperium. Nefrologia 2003, 23, 459–462. [Google Scholar] [PubMed]
- Coward, L.J.; Kullmann, D.M.; Hirsch, N.P.; Howard, R.S.; Lucas, S.B. Catastrophic primary antiphospholipid syndrome presenting as status epilepticus. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1607–1608. [Google Scholar] [CrossRef]
- Ciołkiewicz, M.; Domysławska, I.; Kita, K.; Muklewicz, E.; Kowal-Bielecka, O.; Lewandowski, B.; Sierakowski, S. Secondary lethal catastrophic antiphospholipid syndrome in 24-years old female patient with overlap syndrome (systemic sclerosis and systemic lupus ery-thematosus). Pol. Merkur. Lekarski 2006, 20, 337–340. [Google Scholar] [PubMed]
- Zieba, B.; Wegrzyn, A.; Mital, A.; Szczepińska-Nowak, M.; Lewicki, L.; Chmielecki, M.; Puchalski, W.; Rynkiewicz, A. Catastrophic antiphospholipid syndrome complicated by cardiogenic shock—A case report. Kardiol. Pol. 2009, 67, 769–773. [Google Scholar] [PubMed]
- Hanouna, G.; Morel, N.; Thi Huong, D.L.; Josselin, L.; Vauthier-Brouzes, D.; Saadoun, D.; Kettaneh, A.; Levesque, K.; Le Guern, V.; Goffinet, F.; et al. Catastrophic antiphospholipid syndrome and pregnancy: An experience of 13 cases. Rheumatology 2013, 52, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Rijo, D.; Costa, S.S.; Monteiro, J.P.; Monteiro, J.P.; Oliveira, M.; Ferraz, R.; Neves, F.; Vouga, L.; Guerra, M. Papillary muscle rupture as a manifestation of SLE in the puerperium. Rev. Port. Cir. Cardiotorac. Vasc. 2017, 24, 63–65. [Google Scholar] [PubMed]
- Khizroeva, J.; Bitsadze, V.; Makatsariya, A. Catastrophic antiphospholipid syndrome and pregnancy. Clinical report. J. Matern. Fetal Neonatal Med. 2019, 32, 2091–2094. [Google Scholar] [CrossRef] [PubMed]
- Ruffatti, A.; Tarzia, V.; Fedrigo, M.; Calligaro, A.; Favaro, M.; Macor, P.; Tison, T.; Cucchini, U.; Cosmi, E.; Tedesco, F.; et al. Evidence of complement activation in the thrombotic small vessels of a patient with cat-astrophic antiphospholipid syndrome treated with eculizumab. Autoimmun. Rev. 2019, 18, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Wimberly, K.; Guglin, M. Systemic lupus and catastrophic antiphospholipid syndrome manifesting as cardiogenic shock. Lupus 2019, 28, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.; Ministro, A.; Carreira, N.R.; Cardoso, A.; Gonçalves, C.S.; Henriques, M.; Rato, J.; Silva, E.; Pedro, L.M. A cata-strophic seronegative anti-phospholipid syndrome: Case and literature review. Thromb. J. 2021, 19, 103. [Google Scholar] [CrossRef]
- Rato, I.R.; Barbosa, A.R.; Afonso, D.J.; Beça, S. Catastrophic antiphospholipid syndrome presented as ruptured papillary muscle during puerperium in a patient with systemic lupus erythematosus. Lupus 2021, 30, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Collict, M.; Sciberras Buhagiar, W.; Mercieca, C.; Thake, J. Catastrophic antiphospholipid syndrome in pregnancy: A life-threatening condition. BMJ Case Rep. 2019, 12, e230863. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.M. Catastrophic antiphospholipid syndrome and pregnancy. Semin. Perinatol. 2018, 42, 26–32. [Google Scholar] [CrossRef]
- Rovere-Querini, P.; Canti, V.; Erra, R.; Bianchi, E.; Slaviero, G.; D’Angelo, A.; Rosa, S.; Candiani, M.; Castiglioni, M.T. Eculizumab in a pregnant patient with laboratory onset of catastrophic antiphospholipid syndrome. Medicine 2018, 97, e12584. [Google Scholar] [CrossRef]
- Rosenbaum, A.N.; Anavekar, N.S.; Ernste, F.C.; Mankad, S.V.; Le, R.J.; Manocha, K.K.; Barsness, G.W. A case of catastrophic antiphospholipid syndrome: First report with advanced cardiac imaging using MRI. Lupus 2015, 24, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Sacré, K.; Brihaye, B.; Hyafil, F.; Serfaty, J.M.; Escoubet, B.; Zennaro, M.C.; Lidove, O.; Laissy, J.P.; Papo, T. Asymptomatic myocardial ischemic disease in antiphospholipid syndrome: A controlled cardiac magnetic resonance imaging study. Arthritis Rheum. 2010, 62, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Nayer, A.; Ortega, L.M. Catastrophic antiphospholipid syndrome: A clinical review. J. Nephropathol. 2014, 3, 9–17. [Google Scholar] [CrossRef]
- Asherson, R.A.; Cervera, R.; Piette, J.C.; Shoenfeld, Y.; Espinosa, G.; Petri, M.A.; Lim, E.; Lau, T.C.; Gurjal, A.; Jedryka-Góral, A.; et al. Catastrophic antiphospholipid syndrome: Clues to the pathogenesis from a series of 80 patients. Medicine 2001, 80, 355–377. [Google Scholar] [CrossRef]
- Mavrogeni, S.I.; Sfikakis, P.P.; Kitas, G.D.; Kolovou, G.; Tektonidou, M.G. Cardiac involvement in antiphospholipid syndrome: The diagnostic role of noninvasive cardiac imaging. Semin. Arthritis Rheum. 2016, 45, 611–616. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. WG on Cardiovascular Pharmacotherapy. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Kruchinova, S. Prevalence of thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries and cryptogenic stroke. Eur. Heart J. 2020, 41 (Suppl. S2), ehaa946.1531. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Wypasek, E.; Zalewski, J.; Undas, A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: Comparison with cryptogenic stroke. Int. J. Cardiol. 2019, 290, 1–6. [Google Scholar] [CrossRef]
- Mavrogeni, S.I.; Markousis-Mavrogenis, G.; Karapanagiotou, O.; Toutouzas, K.; Argyriou, P.; Velitsista, S.; Kanoupakis, G.; Apostolou, D.; Hautemann, D.; Sfikakis, P.P.; et al. Silent myocardial perfusion abnormalities detected by stress cardiovascular magnetic resonance in antiphospholipid syndrome: A case-control study. J. Clin. Med. 2019, 8, 1084. [Google Scholar] [CrossRef]
- Bernhardt, P.; Levenson, B.; Albrecht, A.; Engels, T.; Strohm, O. Detection of cardiac small vessel disease by adenosine-stress magnetic resonance. Int. J. Cardiol. 2007, 121, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Al-Saadi, N.; Nagel, E.; Gross, M.; Bornstedt, A.; Schnackenburg, B.; Klein, C.; Klimek, W.; Oswald, H.; Fleck, E. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation 2000, 101, 1379–1383. [Google Scholar] [CrossRef]
- Markousis-Mavrogenis, G.; Sfikakis, P.P.; Mavrogeni, S.I.; Tektonidou, M.G. Combined brain/heart magnetic resonance imaging in antiphospholipid syndrome-two sides of the same coin. Clin. Rheumatol. 2021, 40, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Markousis-Mavrogenis, G.; Mitsikostas, D.D.; Koutsogeorgopoulou, L.; Dimitroulas, T.; Katsifis, G.; Argyriou, P.; Apostolou, D.; Velitsista, S.; Vartela, V.; Manolopoulou, D.; et al. Combined brain-heart magnetic resonance imaging in autoimmune rheumatic disease patients with cardiac symptoms: Hypothesis generating insights from a cross-sectional study. J. Clin. Med. 2020, 9, 447. [Google Scholar] [CrossRef]
- American College of Obstetricians Gynecologists’ Committee on Obstetric Practice. Committee opinion no. 656: Guidelines for diagnostic imaging during pregnancy and lactation. Obstet. Gynecol. 2016, 127, e75–e80. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Vermeulen, M.J.; Bharatha, A.; Montanera, W.J.; Park, A.L. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 2016, 316, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Winterstein, A.G.; Thai, T.N.; Nduaguba, S.; Smolinski, N.E.; Wang, X.; Sahin, L.; Krefting, I.; Gelperin, K.; Bird, S.T.; Rasmussen, S.A. Risk of fetal or neonatal death or neonatal intensive care unit admission associated with gadolinium magnetic resonance imaging exposure during pregnancy. Am. J. Obstet. Gynecol. 2023, 228, 465.e1–465.e11. [Google Scholar] [CrossRef] [PubMed]
- Radin, M.; Schreiber, K.; Costanzo, P.; Cecchi, I.; Roccatello, D.; Baldovino, S.; Bazzan, M.; Cuadrado, M.J.; Sciascia, S. The adjusted Global AntiphosPholipid Syndrome Score (aGAPSS) for risk stratification in young APS patients with acute myocardial infarction. Int. J. Cardiol. 2017, 240, 72–77. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; DEGroot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Legault, K.; Schunemann, H.; Hillis, C.; Yeung, C.; Akl, E.A.; Carrier, M.; Cervera, R.; Crowther, M.; Dentali, F.; Erkan, D.; et al. McMaster RARE-Bestpractices clinical practice guideline on diagnosis and management of the catastrophic antiphospholipid syndrome. J. Thromb. Haemost. 2018, 16, 1656–1664. [Google Scholar] [CrossRef]
- Linnemann, B. Antiphospholipid syndrome—An update. Vasa Eur. J. Vasc. Med. 2018, 47, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M.; Greer, A.; Middeldorp, S.; Veenstra, D.L.; Prabulos, A.M.; Vandvik, P.O. VTE, thrombophilia, antithrombotic therapy, and pregnancy—Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012, 141 (Suppl. S2), e691S–e736S. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, C.L.; Erkan, D. Catastrophic antiphospholipid syndrome: How to diagnose a rare but highly fatal disease. Ther. Adv. Musculoskelet. Dis. 2013, 5, 305–314. [Google Scholar] [CrossRef] [PubMed]
| Criteria for CAPS | |
|---|---|
| 1. Evidence of involvement of three or more organs, systems and/or tissues. Usually, clinical evidence of vessel occlusions, confirmed by imaging techniques when appropriate. Renal involvement is defined by a 50% rise in serum creatinine, severe systemic hypertension (>180/100 mmHg) and/or proteinuria (>500 mg/24 h). | |
| 2. Development of manifestations simultaneously or in less than a week. | |
| 3. Confirmation by histopathology of small vessel occlusion in at least one organ or tissue. For histopathological confirmation, significant evidence of thrombosis must be present, although vasculitis may coexist occasionally. | |
| 4. Laboratory confirmation of the presence of antiphospholipid antibodies (lupus anticoagulant and/or anticardiolipin antibodies). Antiphospholipid antibodies must be detected on two or more occasions at least six weeks apart (not necessarily at the time of the event). | |
| Diagnosis of CAPS | |
| Definite CAPS | All 4 criteria. |
| Probable CAPS | All 4 criteria, except for only two organs, systems and/or tissues involvement. |
| All 4 criteria, except for the absence of laboratory confirmation at least six weeks apart due to the early death of a patient never tested for aPL before the catastrophic APS. | |
| 1, 2 and 4. | |
| 1, 3 and 4 and the development of a third event in more than a week but less than a month, despite anticoagulation. | |
| Organ Manifestation | Clinical Signs | Altered Laboratory Data | (Reference Range) | Normal Laboratory Tests |
|---|---|---|---|---|
| Lung Bilateral Pulmonary Embolism | Polypnea Dyspnea | Hemoglobin: 79 g/L WBC 16 × 109/L Platelet count: 75 × 109/L | 110–153 g/L 3.5–11.0 × 109/L 115–370 × 109/L | |
| Kidney | - | Proteinuria >500 mg/g Creat. Leukocyturia 596 cel/µL Erythrocyturia 343 cel/µL | <80 Negative Negative | Serum creatinine |
| Brain | Headache Blurred vision Neurosensory deficit Vomit attacks Comatose state | pH 7.47 pCO2 34 pO2 72 HCO3− 21 | 7.35–7.45 35–45 mmHg 80–100 mmHg 22–26 mmol/L | Absence of Leiden Factor V mutation Absence of prothrombin mutation PT20210A Protein C and protein S |
| Heart MINOCA | Epigastric pain Chest pain Hypotension Tachycardia | I-Troponin (HS) 4741 ng/L | <40 ng/L | NT-proBNP |
| Skin | Fever Livedo reticularis | Anti-mitochondria antibody (AMA) Anti-smooth muscle antibody (ASMA) | ||
| Liver Elevated liver enzymes | Right hypochondrium pain | AST 70 U/L ALT 88 U/L | 0–35 U/L 0–35 U/L | Bilirubin (total and direct) GammaGT Anti-Liver-Kidney Microsomial antibody |
| Pancreas | - | Pancreatic amylase 67 U/L LDH 365 U/L | 0–53 U/L 0–210 U/L | Anti-gastric parietal cells antibodies |
| Spleen | - | D-dimers 5850 μg/L | 0–500 μg/L FEU | |
| Thyroid | - | TSH | ||
| Placenta | Areas of necrosis Vascular congestion Small placental infarctions | Fibrinogen 574 mg/dL | 200–400 mg/dL | INR: 1.1 aPTT: 36 |
| Infections | VES 79 mm/h | 0–30 | Procalcitonin 0.22 µg/L (<0.50) | |
| C-Reactive Protein 12.3 mg/dL | 0–0.50 | Anti-HIV 1–2 (CHIV ag/Ab), Ag-HBs, Anti-HCV, SARS-CoV-2 | ||
| Blood culture (×8): negative | ||||
| Urine culture (×3): negative | ||||
| Rectal swab: negative | ||||
| Anti-HSV 1-2 IgG antibodies Anti-EBV antibodies Anti-OMV antibodies | Present Present Present | Toxoplasmosis, Varicella-Zoster virus, Cytomegalovirus, Measles antibodies, Paramyxovirus, Echovirus, Coxachievirus, Adenovirus, hRSV, Chlamydia pneumoniae, Q-Fever, Ab. Anti T. pallidum Ig | ||
| Immune system | Anti-Double-Stranded-DNA antibodies 65 UI/mL | ≤35 UI/mL | Rheumatoid factor | |
| LAC 1.36/2.35 | Ratio < 1.20 | Functional Antithrombin III | ||
| Anti-Cardiolipin IgG 851 U/mL Anti-Cardiolipin IgM 770 U/mL | <20 <20 | Complement 3 1.5 g/L (0.9–1.8) | ||
| aβ2GPI IgG 2761 U/mL aβ2GPI IgM >840 U/mL | <20 <20 | Complement 4 0.16 g/L (0.10–0–40) | ||
| ANA 1:160 | <1:80 | IgG, IgM, IgA (mg/dL): negative |
| Author/Year | DOI/PMID | Maternal Age | Gestational Age (Time of Onset) | CAPS Features | Treatment | Maternal Follow-Up | Fetal Outcome |
|---|---|---|---|---|---|---|---|
| Bendon RW et al., 1987 [26] | https://pubmed.ncbi.nlm.nih.gov/3827544 PMID: 3827544 | 22 years | 30 week of gestation | Cardiac, renal, placenta, gastrointestinal and myometrium TMA | Death | Intrauterine fetal death | |
| Hochfeld M et al., 1994 [27] | https://pubmed.ncbi.nlm.nih.gov/8159355/ PMID: 8159355 | 37 years | Second day after fetal death | Cardiac, neurological, renal failure, pulmonary, splenic, adrenal infarct, cerebral haemorrhage | Cyclophosphamide, S, plasma exchange | Death | Intrauterine fetal death |
| Ortiz P et al., 2003 [28] | https://pubmed.ncbi.nlm.nih.gov/14658174/ PMID: 14658174 | 32 years | 32 week of gestation | Cardiac (aortic valve lesions), renal, neurological, thrombocitopenia | A, H, S | 1 years | Healthy twins |
| Coward LJ et al., 2005 [29] | https://doi.org/10.1136/jnnp.2005.066746 PMID: 16227567 | 30 years | 3rd week of puerperium | Cardiac, cerebral, renal, pulmonary, hepatic, adrenal haemorrhage | Inotropic support, hemofiltration | Death | Healthy child |
| Ciolkiewicz M et al., 2006 [30] | https://pubmed.ncbi.nlm.nih.gov/16780270/ PMID: 16780270 | 24 years | 30th day of puerperium | Cardiac, pulmonary, renal, multiorgan failure | H, S, IVIG, plasma exchange | Death at 1 month | Fetal death |
| Zieba B et al., 2009 [31] | https://pubmed.ncbi.nlm.nih.gov/19650000/ PMID: 19650000 | 29 years | 18th day of puerperium | Cardiac, renal, cerebral, thrombocytopenia | A, S, IVIG, IABP, plasmapheresis | 3 months | Healthy child |
| Hanouna G et al., 2013 [32] | https://doi.org/10.1093/rheumatology/ket167 PMID: 23676524 | ||||||
| (Case 2) | 32 years | 8th day of puerperium | Cardiac, renal, hepatic, cutaneous, HA, thrombocytopenia | H, A, S, IVIG | 2.3 years | Healthy twins | |
| (Case 3) | 26 years | 25 week of gestation | Cardiac, neurological, renal, cutaneous, HA | H, S, IVIG, plasma exchange, dialysis | 5.8 years | Fetal death | |
| (Case 4) | 31 years | Third day of puerperium | Cardiac, renal, splenic, cutaneous, hepatic, thrombocytopenia, HA | H, A, S, IVIG, dialysis | 3.9 years | Fetal death | |
| (Case 7) | 32 years | Fourth week of puerperium | Cardiac, neurological, pulmonary, renal, hepatic, pancreatic, splenic, ocular, thrombocytopenia | H, S, IVIG, plasma exchange, dialysis | 8 years | Fetal death | |
| (Case 8) | 29 years | 15th day of puerperium | Cardiac, neurological, renal, cutaneous, hepatic, pancreatic, gastric, ocular, thrombocytopenia, HA | H, S, plasma exchange, dialysis | Sudden death at 6 years | Fetal death | |
| (Case 10) | 36 years | Third day of puerperium | Cardiac, neurological, renal, hepatic, pancreatic, cutaneous, thrombocytopenia, HA | H, S | 5 years | Healthy child | |
| (Case 12) | 27 years | On the day of delivery at 13 weeks gestation | Cardiac, cutaneous, hepatic, placenta, thrombocytopenia | H, S, IVIG | 5.2 years | Fetal death | |
| (Case 13) | 23 years | 31 week of gestation | Cardiac, renal, cutaneous, thrombocytopenia, HA | H, A, S, plasma exchange | Sudden death at 2.5 years | Child with developmental delay | |
| Rijo D et al., 2017 [33] | https://pubmed.ncbi.nlm.nih.gov/29898299 PMID: 29898299 | 32 years | 15th day of puerperium | Cardiac, cerebral, multiorgan failure | IVIG, S | N/A | N/A |
| Khizroeva J et al., 2019 [34] | https://doi.org/10.1080/14767058.2017.1422715 PMID: 29284338 | 24 years | 28 week of gestation | Cardiac, pulmonary, placenta, thrombocytopenia | H, S, ultrafiltration | 2 years | Prematurity |
| Ruffatti A et al., 2019 [35] | https://doi.org/10.1016/j.autrev.2019.03.015 PMID: 30844561 | 32 years | 29 week of gestation | Cardiac, pulmonary, renal | H, S, IVIG, plasma exchange, ECMO, ecolizumab, Bi-Vad | 1 years | Healthy child |
| Schultz M et al., 2019 [36] (case 2) | https://doi.org/10.1177/0961203319871099 PMID: 31451079 | 37 years | N/A | Cardiac, renal, small vessel thromboembolic disease. | H, S, plasmapheresis, rituximab, W | N/A | Fetal death |
| Pinto V et al., 2021 [37] | https://doi.org/10.1186/s12959-021-00356-w PMID: 34930339 | 38 years | 32 week of gestation | Cardiac, renal, pulmonary, hepatic, splenic, arterial-venous thrombosis | H, plasmapheresis, rituximab, vitamin K antagonist | Six months | Fetal death |
| Rato IR et al., 2021 [38] | https://doi.org/10.1177/09612033211002273 PMID: 33736518 | 32 years | 39th week of gestation | Cardiac, cerebral, placenta | S, H, IVIG, | 2 years | Healthy child |
| Collict M et al., 2021 [39] | https://doi.org/10.1136/bcr-2019-230863 PMID: 31527209 | 31 years | 28 week of gestation | Cardiac, cerebral, placenta, pulmonary, splenic | S, IVIG, H, A, W | 8 months | Healthy child |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varotto, L.; Spigolon, L.; Dotto, A.; Leonardi, D.; Bragantini, G.; Cerrito, L.F.; Deluca, C.; Hoxha, A. Myocardial Involvement in Catastrophic Antiphospholipid Syndrome during Pregnancy or Puerperium: A Case of a Young Breastfeeding Woman and Literature Review. J. Clin. Med. 2024, 13, 4732. https://doi.org/10.3390/jcm13164732
Varotto L, Spigolon L, Dotto A, Leonardi D, Bragantini G, Cerrito LF, Deluca C, Hoxha A. Myocardial Involvement in Catastrophic Antiphospholipid Syndrome during Pregnancy or Puerperium: A Case of a Young Breastfeeding Woman and Literature Review. Journal of Clinical Medicine. 2024; 13(16):4732. https://doi.org/10.3390/jcm13164732
Chicago/Turabian StyleVarotto, Leonardo, Luca Spigolon, Alberto Dotto, Denis Leonardi, Giulia Bragantini, Luca Felice Cerrito, Cristina Deluca, and Ariela Hoxha. 2024. "Myocardial Involvement in Catastrophic Antiphospholipid Syndrome during Pregnancy or Puerperium: A Case of a Young Breastfeeding Woman and Literature Review" Journal of Clinical Medicine 13, no. 16: 4732. https://doi.org/10.3390/jcm13164732
APA StyleVarotto, L., Spigolon, L., Dotto, A., Leonardi, D., Bragantini, G., Cerrito, L. F., Deluca, C., & Hoxha, A. (2024). Myocardial Involvement in Catastrophic Antiphospholipid Syndrome during Pregnancy or Puerperium: A Case of a Young Breastfeeding Woman and Literature Review. Journal of Clinical Medicine, 13(16), 4732. https://doi.org/10.3390/jcm13164732







