Harnessing Mirror Neurons: A New Frontier in Parkinson’s Disease Rehabilitation—A Scoping Review of the Literature
Abstract
:1. Introduction
- Action Observation (AO): Patients watch videos or live demonstrations of specific movements or tasks. These can include activities such as walking, reaching, or balancing exercises. The goal is to engage the mirror neuron system by closely observing the actions [20].
- Motor Imagery (MI): After observing the action, patients are guided to mentally rehearse the observed movements without physically performing them. This mental practice helps to reinforce the neural pathways involved in the action [21].
- Physical Execution: Patients then physically attempt to perform the observed and mentally rehearsed actions. This step helps to consolidate the motor learning that has been initiated through observation and imagery.
- Feedback and Adjustment: During the physical execution of the movements, patients receive feedback from therapists to correct and refine their movements. This iterative process ensures that the motor learning is accurate and effective.
- Integration into Daily Activities: The learned movements and exercises are gradually integrated into the patients’ daily routines to enhance functional mobility and independence.
2. Materials and Methods
2.1. Review Question
2.2. Eligibility Criteria
2.3. Exclusion Criteria
2.4. Search Strategy
2.5. Study Selection
2.6. Data Extraction and Data Synthesis
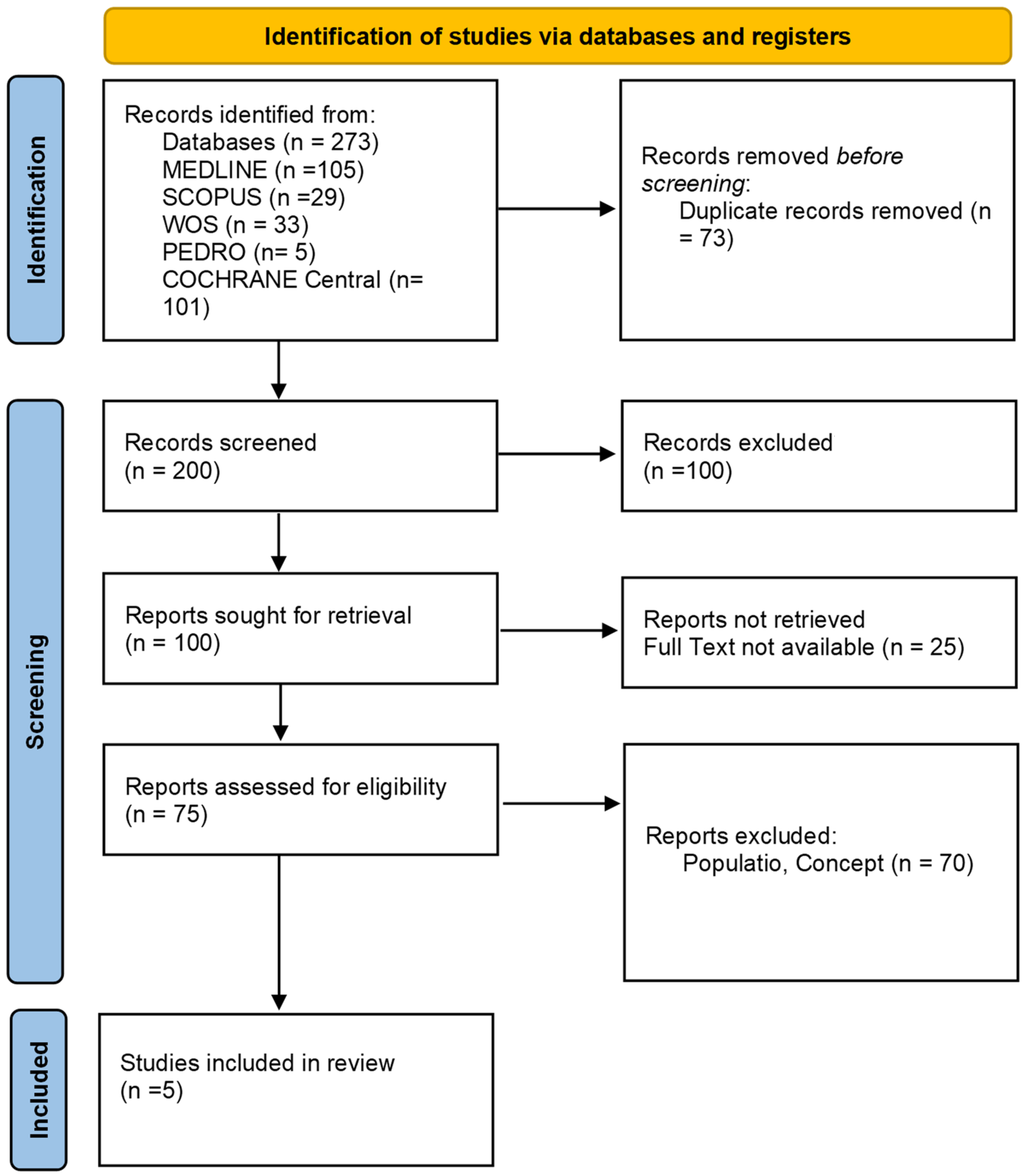
3. Results
4. Discussion
Clinical Practice Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ariz, M.; Martínez, M.; Alvarez, I.; Fernández-Seara, M.A.; Castellanos, G.; Catalonian Neuroimaging Parkinson’s Disease Consortium; Pastor, P.; Pastor, M.A.; Ortiz de Solórzano, C. Automatic Segmentation and Quantification of Nigrosome-1 Neuromelanin and Iron in MRI: A Candidate Biomarker for Parkinson’s Disease. J. Magn. Reson. Imaging 2024, 60, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Jokar, M.; Jin, Z.; Huang, P.; Wang, Y.; Zhang, Y.; Li, Y.; Cheng, Z.; Liu, Y.; Tang, R.; Shi, X.; et al. Diagnosing Parkinson’s Disease by Combining Neuromelanin and Iron Imaging Features Using an Automated Midbrain Template Approach. Neuroimage 2023, 266, 119814. [Google Scholar] [CrossRef] [PubMed]
- Sola Fraca, D.; Sánchez Garrigós, E.; de Francisco Moure, J.; Marín Gonzalez, B.; Badiola Díez, J.J.; Acín Tresaco, C. Sleep Disturbance in Clinical and Preclinical Scrapie-Infected Sheep Measured by Polysomnography. Vet. Q. 2024, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, D.G.; MacLeod, K.R.; Middleton, J.S.; Bozoki, A.C.; Galvin, J.E.; Irwin, D.J.; Lippa, C.F.; Litvan, I.; Lopez, O.L.; Berman, S.; et al. Association of CSF α-Synuclein Seeding Amplification Assay Results With Clinical Features of Possible and Probable Dementia With Lewy Bodies. Neurology 2024, 103, e209656. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.G.; Rodrigues, P.; Viero, F.T.; Kudsi, S.Q.; Frare, J.M.; Rech, C.T.; Graiczicki, G.; Trevisan, G. Prevalence of Radicular Neuropathic Pain in Idiopathic Parkinson’s Disease: A Systematic Review and Meta-Analysis. Aging Res. Rev. 2024, 99, 102374. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef]
- Allen, N.E.; Schwarzel, A.K.; Canning, C.G. Recurrent Falls in Parkinson’s Disease: A Systematic Review. Park. Dis. 2013, 2013, 906274. [Google Scholar] [CrossRef]
- Harrison, E.C.; Earhart, G.M. The Effect of Auditory Cues on Gait Variability in People with Parkinson’s Disease and Older Adults: A Systematic Review. Neurodegener. Dis. Manag. 2023, 13, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R. Can Beneficial Frequencies in Physiotherapy Help Treatment? Scoping Review. Rwanda Med. J. 2023, 80, 88–94. [Google Scholar] [CrossRef]
- Carroll, L.M.; Volpe, D.; Morris, M.E.; Saunders, J.; Clifford, A.M. Aquatic Exercise Therapy for People With Parkinson Disease: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Rios Romenets, S.; Anang, J.; Fereshtehnejad, S.-M.; Pelletier, A.; Postuma, R. Tango for Treatment of Motor and Non-Motor Manifestations in Parkinson’s Disease: A Randomized Control Study. Complement. Ther. Med. 2015, 23, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.-Y.; Gong, L.; Zhu, Y.-L.; Hao, Y.-L. Tai Chi for Improvement of Motor Function, Balance and Gait in Parkinson’s Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e102942. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.; Bird, G.; Catmur, C.; Press, C.; Heyes, C. Mirror Neurons: From Origin to Function. Behav. Brain Sci. 2014, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Bowman, T.; Pergolini, A.; Carrozza, M.C.; Lencioni, T.; Marzegan, A.; Meloni, M.; Vitiello, N.; Crea, S.; Cattaneo, D. Wearable Biofeedback Device to Assess Gait Features and Improve Gait Pattern in People with Parkinson’s Disease: A Case Series. J. Neuroeng. Rehabil. 2024, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, I.; Cattaneo, D.; Bonora, G.; Bowman, T.; Martina, L.; Montesano, A.; Ferrarin, M. Wearable Sensor-Based Biofeedback Training for Balance and Gait in Parkinson Disease: A Pilot Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 622–630.e3. [Google Scholar] [CrossRef] [PubMed]
- de Vignemont, F.; Haggard, P. Action Observation and Execution: What Is Shared? Soc. Neurosci. 2008, 3, 421–433. [Google Scholar] [CrossRef]
- Sarasso, E.; Agosta, F.; Piramide, N.; Gardoni, A.; Canu, E.; Leocadi, M.; Castelnovo, V.; Basaia, S.; Tettamanti, A.; Volontè, M.A.; et al. Action Observation and Motor Imagery Improve Dual Task in Parkinson’s Disease: A Clinical/fMRI Study. Mov. Disord. 2021, 36, 2569–2582. [Google Scholar] [CrossRef]
- Bowering, K.J.; Butler, D.S.; Fulton, I.J.; Moseley, G.L. Motor Imagery in People with a History of Back Pain, Current Back Pain, Both, or Neither. Clin. J. Pain. 2014, 30, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Ladda, A.M.; Lebon, F.; Lotze, M. Using Motor Imagery Practice for Improving Motor Performance—A Review. Brain Cogn. 2021, 150, 105705. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R. Unlocking the Power of Motor Imagery: A Comprehensive Review on Its Application in Alleviating Foot Pain. Acta Neurol. Belg. 2024. [Google Scholar] [CrossRef]
- Peters: Joanna Briggs Institute Reviewer’s Manual, JBI—Google Scholar. Available online: https://jbi-global-wiki.refined.site/space/MANUAL (accessed on 9 June 2022).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Lahuerta-Martín, S.; Llamas-Ramos, R.; Llamas-Ramos, I. Effectiveness of Therapies Based on Mirror Neuron System to Treat Gait in Patients with Parkinson’s Disease-A Systematic Review. J. Clin. Med. 2022, 11, 4236. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulos, I.; Karanika, P.; Papaxanthis, C.; Tsaklis, P. The Effects of Action Observation Therapy as a Rehabilitation Tool in Parkinson’s Disease Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 3311. [Google Scholar] [CrossRef] [PubMed]
- Pelosin, E.; Barella, R.; Bet, C.; Magioncalda, E.; Putzolu, M.; Di Biasio, F.; Cerulli, C.; Casaleggio, M.; Abbruzzese, G.; Avanzino, L. Effect of Group-Based Rehabilitation Combining Action Observation with Physiotherapy on Freezing of Gait in Parkinson’s Disease. Neural Plast. 2018, 2018, 4897276. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, P.T.; Santiago, L.M.; Silva, I.A.; Souza, A.A.; Pegado, C.L.; Damascena, C.M.; Ribeiro, T.S.; Lindquist, A.R. Action Observation and Motor Imagery Have No Effect on Balance and Freezing of Gait in Parkinson’s Disease: A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2022, 58, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Gnanalingham, K.K.; Byrne, E.J.; Thornton, A.; Sambrook, M.A.; Bannister, P. Motor and Cognitive Function in Lewy Body Dementia: Comparison with Alzheimer’s and Parkinson’s Diseases. J. Neurol. Neurosurg. Psychiatry 1997, 62, 243–252. [Google Scholar] [CrossRef]
- Tedeschi, R. Assessment of Postural Control and Proprioception Using the Delos Postural Proprioceptive System. Reabil. Moksl. Slauga Kineziter. Ergoter. 2023, 2, 93–109. [Google Scholar] [CrossRef]
- Tedeschi, R. Automated Mechanical Peripheral Stimulation for Gait Rehabilitation in Parkinson’s Disease: A Comprehensive Review. Clin. Park. Relat. Disord. 2023, 9, 100219. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Shima, A.; Kambe, D.; Furukawa, K.; Sakamaki-Tsukita, H.; Yoshimura, K.; Wada, I.; Sakato, Y.; Terada, Y.; Sawamura, M.; et al. Frontoparietal-Striatal Network and Nucleus Basalis Modulation in Patients With Parkinson Disease and Gait Disturbance. Neurology 2024, 103, e209606. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Leyva, G.; Cubo, E. Freezing of Gait: Pharmacological and Surgical Options. Curr. Opin. Neurol. 2024, 37, 394–399. [Google Scholar] [CrossRef]
- Nascimento, L.R.; Boening, A.; Rocha, R.J.; do Carmo, W.A.; Ada, L. Walking Training with Auditory Cueing Improves Walking Speed More than Walking Training Alone in Ambulatory People with Parkinson’s Disease: A Systematic Review. J. Physiother. 2024, 70, 208–215. [Google Scholar] [CrossRef] [PubMed]
| Study | Objective | Participants | Intervention | Outcome | Results | Frequency/Number of Sessions and Follow-Up |
|---|---|---|---|---|---|---|
| Lahuerta-Martín et al., 2022 [26] | Evaluate the effectiveness of AO and MI in PD patients | 156 participants in 6 studies | Experimental: shown videos with strategies to avoid freezing during walking; control: shown videos of landscapes | Disease severity, quality of life, balance, and gait | AO therapy reduced disease severity and improved quality of life, balance, and gait. AO combined with MI and dual-task exercises was the most effective. | 3 sessions/week for 8 weeks; follow-up at 3 months |
| Giannakopoulos et al., 2022 [27] | Assess AO-based therapies’ impact on freezing and gait | 194 participants in 7 studies | Experimental: shown videos with strategies to avoid freezing or functional movements with auditory cues; control: shown videos of landscapes or auditory cues | Disease severity, quality of life, balance, and gait | AO improved motor control and clinical aspects. Effectiveness influenced by training amount/frequency and visual stimulus characteristics. | 2 sessions/week for 10 weeks; follow-up at 6 months |
| Pelosin et al., 2018 [28] | Investigate AO combined with physiotherapy on mobility | 64 participants | Experimental: shown videos with strategies to avoid freezing during walking; control: shown videos of landscapes | Freezing (FoG-Q), balance (BBS), and gait (TUG and 10 M-WT) | AO effective in reducing gait freezing and improving balance and gait. Safe and feasible as an additional physiotherapy strategy. | 2 sessions/week for 5 weeks; follow-up at 4 weeks |
| Sarasso et al., 2021 [20] | Evaluate AO and MI effects on mobility, balance, and the brain | 25 participants | Experimental: dual-task exercises with AO and MI; control: dual-task exercises alone | Functional movements, balance, and gait (TUG, 10 MWT, and ABC scale) | Both dual-task and dual-task + AO-MI improved clinical health and brain reorganization. Dual-task + AO-MI group showed greater improvements. | 3 sessions/week for 6 weeks; follow-up at 2 months |
| Bezerra et al., 2022 [29] | Determine AO and MI effects on gait, balance, and freezing | 39 participants | Experimental: shown videos of gait training and kinesthetic modality; control: shown educational videos about PD | Freezing (FOG-Q), balance (mini BESTest), and gait | The experimental group did not show significant improvements in balance, gait, and freezing compared to the control. | 2 sessions/week for 4 weeks; no follow-up spec |
| Study | AMSTAR 2 Scale | RoB-2 Scale | Quality Assessment |
|---|---|---|---|
| Systematic Reviews | |||
| Lahuerta-Martín et al., 2022 [26] | High quality | N/A | Comprehensive literature search, the inclusion of high-quality RCTs, proper randomization, adequate follow-up, and clear reporting of findings. |
| Giannakopoulos et al., 2022 [27] | Moderate quality | N/A | Adequate literature search, the inclusion of RCTs, some issues with blinding, and heterogeneity of the included studies. |
| Randomized Controlled Trials | |||
| Pelosin et al., 2018 [28] | N/A | Low risk of bias | PEDro Scale: 8/10. High methodological quality with random allocation; baseline comparability; the blinding of subjects, therapists, and assessors; adequate follow-up; and intention-to-treat analysis. |
| Sarasso et al., 2021 [20] | N/A | Low risk of bias | PEDro Scale: 7/10. High methodological quality with random allocation, baseline comparability, the blinding of subjects and assessors, adequate follow-up, and intention-to-treat analysis. |
| Bezerra et al., 2022 [29] | N/A | Moderate risk of bias | PEDro Scale: 6/10. Moderate methodological quality with random allocation and baseline comparability, but lacking sufficient blinding and intention-to-treat analysis. |
| Study | Rehabilitation Program | Complementary Therapies Included |
|---|---|---|
| Lahuerta-Martín et al., 2022 [26] | Conventional physiotherapy exercises | Gait training and balance training |
| Giannakopoulos et al., 2022 [27] | Physical therapy focused on motor function improvement | Gait training, auditory cues, and balance training |
| Pelosin et al., 2018 [28] | Group-based physiotherapy combined with AO | Gait training and balance training |
| Sarasso et al., 2021 [20] | Dual-task exercises combined with AO and MI | Cognitive tasks during motor exercises |
| Bezerra et al., 2022 [29] | Gait training in a kinesthetic modality combined with AO and MI | Educational videos about Parkinson’s disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, R.; Platano, D.; Donati, D.; Giorgi, F. Harnessing Mirror Neurons: A New Frontier in Parkinson’s Disease Rehabilitation—A Scoping Review of the Literature. J. Clin. Med. 2024, 13, 4539. https://doi.org/10.3390/jcm13154539
Tedeschi R, Platano D, Donati D, Giorgi F. Harnessing Mirror Neurons: A New Frontier in Parkinson’s Disease Rehabilitation—A Scoping Review of the Literature. Journal of Clinical Medicine. 2024; 13(15):4539. https://doi.org/10.3390/jcm13154539
Chicago/Turabian StyleTedeschi, Roberto, Daniela Platano, Danilo Donati, and Federica Giorgi. 2024. "Harnessing Mirror Neurons: A New Frontier in Parkinson’s Disease Rehabilitation—A Scoping Review of the Literature" Journal of Clinical Medicine 13, no. 15: 4539. https://doi.org/10.3390/jcm13154539
APA StyleTedeschi, R., Platano, D., Donati, D., & Giorgi, F. (2024). Harnessing Mirror Neurons: A New Frontier in Parkinson’s Disease Rehabilitation—A Scoping Review of the Literature. Journal of Clinical Medicine, 13(15), 4539. https://doi.org/10.3390/jcm13154539






