Clinical Management of Postoperative Growth Hormone Deficiency in Hypothalamic-Pituitary Tumors
Abstract
1. Introduction
2. Postoperative Growth Hormone Deficiency
3. Consequences of GH Deficiency and Benefits of rhGH Treatment
3.1. Growth and Development
3.2. Quality of Life
3.3. Body Composition
3.4. Bone Health and Fracture Risk
3.5. Liver Function
3.6. Metabolic and Cardiovascular Profiles
3.7. Cardiovascular Disease and Mortality
4. Risks, Side Effects, and Contraindications of rhGH Treatment
4.1. Risk of Tumor Progression and Recurrence and Neoplasia
4.1.1. Craniopharyngioma
4.1.2. Non-Functioning Pituitary Adenoma
4.1.3. Prolactinoma
4.1.4. Acromegaly
4.2. Side Effects
4.3. Contraindications
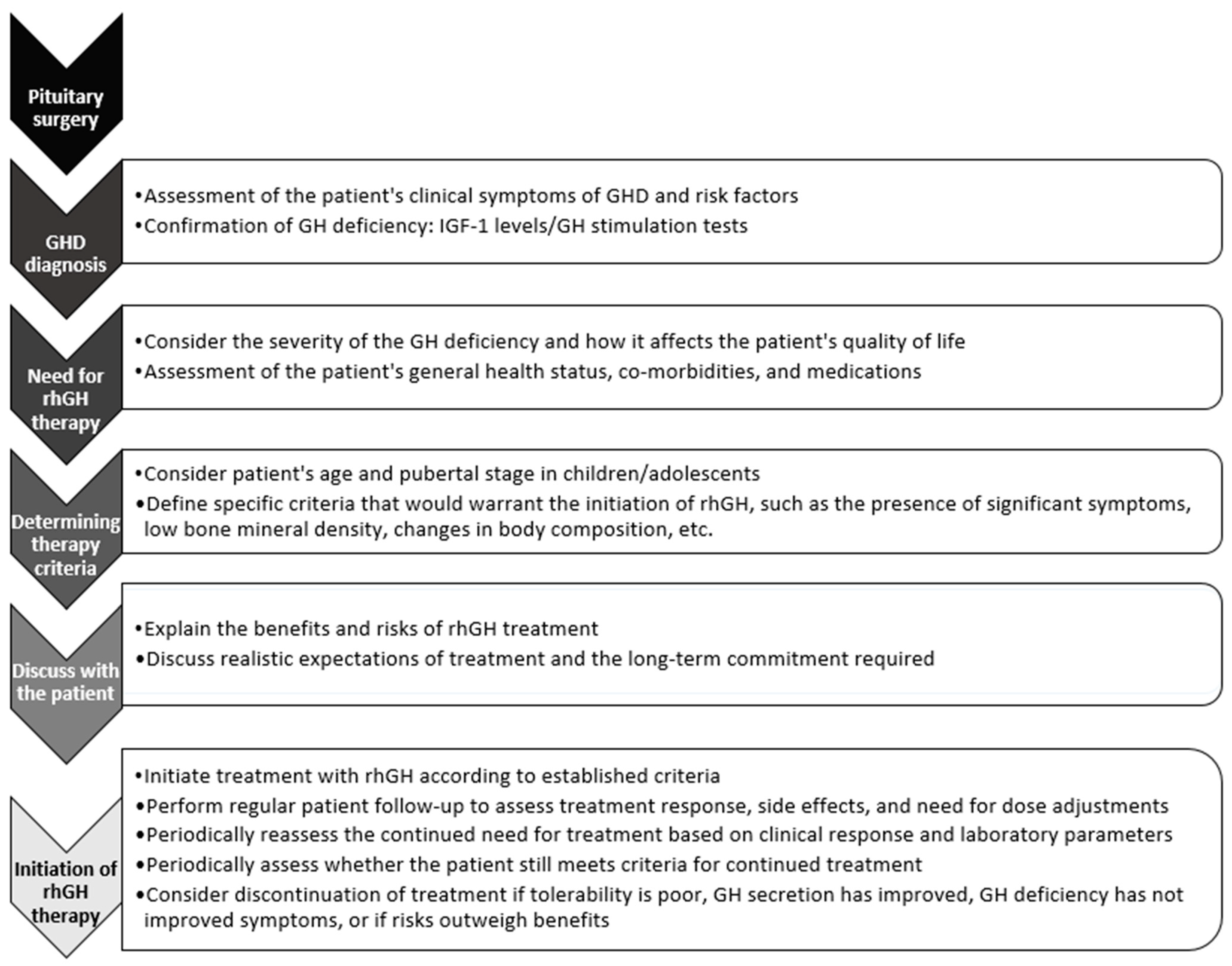
5. Diagnosis of Post-Surgical Growth Hormone Deficiency
6. Therapeutic Management: When to Start rhGH Treatment after Surgery and for How Long?
7. Conclusions
Funding
Conflicts of Interest
References
- Cuneo, R.C.; Salomon, F.; McGauley, G.A.; Sönksen, P.H. The Growth Hormone Deficiency Syndrome in Adults. Clin. Endocrinol. 1992, 37, 387–397. [Google Scholar] [CrossRef] [PubMed]
- de Boer, H.; Blok, G.J.; Van der Veen, E.A. Clinical Aspects of Growth Hormone Deficiency in Adults. Endocr. Rev. 1995, 16, 63–86. [Google Scholar] [CrossRef]
- Salomon, F.; Cuneo, R.C.; Hesp, R.; Sönksen, P.H. The Effects of Treatment with Recombinant Human Growth Hormone on Body Composition and Metabolism in Adults with Growth Hormone Deficiency. N. Engl. J. Med. 1989, 321, 1797–1803. [Google Scholar] [CrossRef]
- Yuen, K.C.J.; Biller, B.M.K.; Radovick, S.; Carmichael, J.D.; Jasim, S.; Pantalone, K.M.; Hoffman, A.R. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Growth Hormone Deficiency in Adults and Patients Transitioning from Pediatric to Adult Care. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2019, 25, 1191–1232. [Google Scholar] [CrossRef]
- Rosén, T.; Johannsson, G.; Johansson, J.O.; Bengtsson, B.A. Consequences of Growth Hormone Deficiency in Adults and the Benefits and Risks of Recombinant Human Growth Hormone Treatment. A Review Paper. Horm. Res. 1995, 43, 93–99. [Google Scholar] [CrossRef]
- Díez, J.J.; Cordido, F. Benefits and risks of growth hormone in adults with growth hormone deficiency. Med. Clin. 2014, 143, 354–359. [Google Scholar] [CrossRef][Green Version]
- Thomas, M.; Massa, G.; Craen, M.; de Zegher, F.; Bourguignon, J.P.; Heinrichs, C.; De Schepper, J.; Du Caju, M.; Thiry-Counson, G.; Maes, M. Prevalence and Demographic Features of Childhood Growth Hormone Deficiency in Belgium during the Period 1986–2001. Eur. J. Endocrinol. 2004, 151, 67–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abs, R.; Bengtsson, B.A.; Hernberg-Stâhl, E.; Monson, J.P.; Tauber, J.P.; Wilton, P.; Wüster, C. GH Replacement in 1034 Growth Hormone Deficient Hypopituitary Adults: Demographic and Clinical Characteristics, Dosing and Safety. Clin. Endocrinol. 1999, 50, 703–713. [Google Scholar] [CrossRef]
- Baldelli, R.; Bianchi, A.; Diacono, F.; Passeri, M.; Fusco, A.; Valle, D.; Poggi, M.; Terlini, M.; Toscano, V.; Tamburrano, G.; et al. Characteristics of Adult Patients with Growth Hormone Deficiency Who Underwent Neurosurgery for Functioning and Non-Functioning Pituitary Adenomas and Craniopharyngiomas. J. Endocrinol. Investig. 2005, 28, 157–161. [Google Scholar] [CrossRef]
- Mameli, C.; Guadagni, L.; Orso, M.; Calcaterra, V.; Wasniewska, M.G.; Aversa, T.; Granato, S.; Bruschini, P.; d’Angela, D.; Spandonaro, F.; et al. Epidemiology of Growth Hormone Deficiency in Children and Adolescents: A Systematic Review. Endocrine 2024. [Google Scholar] [CrossRef]
- Lebrethon, M.C.; Grossman, A.B.; Afshar, F.; Plowman, P.N.; Besser, G.M.; Savage, M.O. Linear Growth and Final Height after Treatment for Cushing’s Disease in Childhood. J. Clin. Endocrinol. Metab. 2000, 85, 3262–3265. [Google Scholar] [CrossRef][Green Version]
- Ranke, M.B. Short and Long-Term Effects of Growth Hormone in Children and Adolescents With GH Deficiency. Front. Endocrinol. 2021, 12, 720419. [Google Scholar] [CrossRef]
- Rosén, T.; Wirén, L.; Wilhelmsen, L.; Wiklund, I.; Bengtsson, B.A. Decreased Psychological Well-Being in Adult Patients with Growth Hormone Deficiency. Clin. Endocrinol. 1994, 40, 111–116. [Google Scholar] [CrossRef]
- Geisler, A.; Lass, N.; Reinsch, N.; Uysal, Y.; Singer, V.; Ravens-Sieberer, U.; Reinehr, T. Quality of Life in Children and Adolescents with Growth Hormone Deficiency: Association with Growth Hormone Treatment. Horm. Res. Paediatr. 2012, 78, 94–99. [Google Scholar] [CrossRef]
- Rosilio, M.; Blum, W.F.; Edwards, D.J.; Shavrikova, E.P.; Valle, D.; Lamberts, S.W.J.; Erfurth, E.M.; Webb, S.M.; Ross, R.J.; Chihara, K.; et al. Long-Term Improvement of Quality of Life during Growth Hormone (GH) Replacement Therapy in Adults with GH Deficiency, as Measured by Questions on Life Satisfaction-Hypopituitarism (QLS-H). J. Clin. Endocrinol. Metab. 2004, 89, 1684–1693. [Google Scholar] [CrossRef][Green Version]
- Mo, D.; Blum, W.F.; Rosilio, M.; Webb, S.M.; Qi, R.; Strasburger, C.J. Ten-Year Change in Quality of Life in Adults on Growth Hormone Replacement for Growth Hormone Deficiency: An Analysis of the Hypopituitary Control and Complications Study. J. Clin. Endocrinol. Metab. 2014, 99, 4581–4588. [Google Scholar] [CrossRef][Green Version]
- Elbornsson, M.; Horvath, A.; Götherström, G.; Bengtsson, B.-Å.; Johannsson, G.; Svensson, J. Seven Years of Growth Hormone (GH) Replacement Improves Quality of Life in Hypopituitary Patients with Adult-Onset GH Deficiency. Eur. J. Endocrinol. 2017, 176, 99–109. [Google Scholar] [CrossRef]
- Loftus, J.; Quitmann, J.; Valluri, S.R. Health-Related Quality of Life in Pre-Pubertal Children with Pediatric Growth Hormone Deficiency: 12-Month Results from a Phase 3 Clinical Trial of Once-Weekly Somatrogon versus Once-Daily Somatropin. Curr. Med. Res. Opin. 2024, 40, 175–184. [Google Scholar] [CrossRef]
- Newman, C.B.; Carmichael, J.D.; Kleinberg, D.L. Effects of Low Dose versus High Dose Human Growth Hormone on Body Composition and Lipids in Adults with GH Deficiency: A Meta-Analysis of Placebo-Controlled Randomized Trials. Pituitary 2015, 18, 297–305. [Google Scholar] [CrossRef]
- Ferruzzi, A.; Vrech, M.; Pietrobelli, A.; Cavarzere, P.; Zerman, N.; Guzzo, A.; Flodmark, C.-E.; Piacentini, G.; Antoniazzi, F. The Influence of Growth Hormone on Pediatric Body Composition: A Systematic Review. Front. Endocrinol. 2023, 14, 1093691. [Google Scholar] [CrossRef]
- Wydra, A.; Czajka-Oraniec, I.; Wydra, J.; Zgliczyński, W. The Influence of Growth Hormone Deficiency on Bone Health and Metabolisms. Reumatologia 2023, 61, 239–247. [Google Scholar] [CrossRef]
- Bouillon, R.; Koledova, E.; Bezlepkina, O.; Nijs, J.; Shavrikhova, E.; Nagaeva, E.; Chikulaeva, O.; Peterkova, V.; Dedov, I.; Bakulin, A.; et al. Bone Status and Fracture Prevalence in Russian Adults with Childhood-Onset Growth Hormone Deficiency. J. Clin. Endocrinol. Metab. 2004, 89, 4993–4998. [Google Scholar] [CrossRef]
- Elbornsson, M.; Götherström, G.; Bosæus, I.; Bengtsson, B.-Å.; Johannsson, G.; Svensson, J. Fifteen Years of GH Replacement Increases Bone Mineral Density in Hypopituitary Patients with Adult-Onset GH Deficiency. Eur. J. Endocrinol. 2012, 166, 787–795. [Google Scholar] [CrossRef]
- Appelman-Dijkstra, N.M.; Claessen, K.M.J.A.; Hamdy, N.A.T.; Pereira, A.M.; Biermasz, N.R. Effects of up to 15 Years of Recombinant Human GH (rhGH) Replacement on Bone Metabolism in Adults with Growth Hormone Deficiency (GHD): The Leiden Cohort Study. Clin. Endocrinol. 2014, 81, 727–735. [Google Scholar] [CrossRef]
- Kong, T.; Gu, Y.; Sun, L.; Zhou, R.; Li, J.; Shi, J. Association of Nonalcoholic Fatty Liver Disease and Growth Hormone Deficiency: A Systematic Review and Meta-Analysis. Endocr. J. 2023, 70, 959–967. [Google Scholar] [CrossRef]
- Dichtel, L.E.; Corey, K.E.; Haines, M.S.; Chicote, M.L.; Lee, H.; Kimball, A.; Colling, C.; Simon, T.G.; Long, M.T.; Husseini, J.; et al. Growth Hormone Administration Improves Nonalcoholic Fatty Liver Disease in Overweight/Obesity: A Randomized Trial. J. Clin. Endocrinol. Metab. 2023, 108, e1542–e1550. [Google Scholar] [CrossRef]
- Bartsch, L.; Bredella, M.; Chicote, M.L.; Colling, C.; Corey, K.; Drescher, H.; Haines, M.; Husseini, J.; Kimball, A.; Lauer, G.; et al. OR27-2 Growth Hormone Reduces Hepatic Steatosis, Inflammation and Fibrosis in Adults with Overweight/Obesity and Nonalcoholic Fatty Liver Disease. J. Endocr. Soc. 2022, 6, A525. [Google Scholar] [CrossRef]
- Abs, R.; Feldt-Rasmussen, U.; Mattsson, A.F.; Monson, J.P.; Bengtsson, B.-A.; Góth, M.I.; Wilton, P.; Koltowska-Häggström, M. Determinants of Cardiovascular Risk in 2589 Hypopituitary GH-Deficient Adults—A KIMS Database Analysis. Eur. J. Endocrinol. 2006, 155, 79–90. [Google Scholar] [CrossRef]
- Gola, M.; Bonadonna, S.; Doga, M.; Giustina, A. Clinical Review: Growth Hormone and Cardiovascular Risk Factors. J. Clin. Endocrinol. Metab. 2005, 90, 1864–1870. [Google Scholar] [CrossRef]
- Gazzaruso, C.; Gola, M.; Karamouzis, I.; Giubbini, R.; Giustina, A. Cardiovascular Risk in Adult Patients with Growth Hormone (GH) Deficiency and Following Substitution with GH—An Update. J. Clin. Endocrinol. Metab. 2014, 99, 18–29. [Google Scholar] [CrossRef]
- Di Somma, C.; Scarano, E.; Savastano, S.; Savanelli, M.C.; Pivonello, R.; Colao, A. Cardiovascular Alterations in Adult GH Deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 25–34. [Google Scholar] [CrossRef]
- Chen, M.; Gan, D.; Luo, Y.; Rampersad, S.; Xu, L.; Yang, S.; Li, N.; Li, H. Effect of Recombinant Human Growth Hormone Therapy on Blood Lipid and Carotid Intima-Media Thickness in Children with Growth Hormone Deficiency. Pediatr. Res. 2018, 83, 954–960. [Google Scholar] [CrossRef]
- Liang, S.; Xue, J.; Li, G. Effects of Recombinant Human Growth Hormone Administration on Cardiovascular Risk Factors in Obese Children with Relative Growth Hormone Deficiency. Lipids Health Dis. 2018, 17, 66. [Google Scholar] [CrossRef]
- Colao, A.; Di Somma, C.; Rota, F.; Pivonello, R.; Savanelli, M.C.; Spiezia, S.; Lombardi, G. Short-Term Effects of Growth Hormone (GH) Treatment or Deprivation on Cardiovascular Risk Parameters and Intima-Media Thickness at Carotid Arteries in Patients with Severe GH Deficiency. J. Clin. Endocrinol. Metab. 2005, 90, 2056–2062. [Google Scholar] [CrossRef]
- Borson-Chazot, F.; Serusclat, A.; Kalfallah, Y.; Ducottet, X.; Sassolas, G.; Bernard, S.; Labrousse, F.; Pastene, J.; Sassolas, A.; Roux, Y.; et al. Decrease in Carotid Intima-Media Thickness after One Year Growth Hormone (GH) Treatment in Adults with GH Deficiency. J. Clin. Endocrinol. Metab. 1999, 84, 1329–1333. [Google Scholar] [CrossRef]
- Maison, P.; Chanson, P. Cardiac Effects of Growth Hormone in Adults with Growth Hormone Deficiency: A Meta-Analysis. Circulation 2003, 108, 2648–2652. [Google Scholar] [CrossRef]
- Höybye, C.; Biller, B.M.K.; Ferran, J.-M.; Gordon, M.B.; Kelepouris, N.; Nedjatian, N.; Olsen, A.H.; Weber, M.M. Reduced CV Risk with Long-Term GH Replacement in AGHD: Data from Two Large Observational Studies. Endocr. Connect. 2023, 12, e220267. [Google Scholar] [CrossRef]
- Weber, M.M.; Gordon, M.B.; Höybye, C.; Jørgensen, J.O.L.; Puras, G.; Popovic-Brkic, V.; Molitch, M.E.; Ostrow, V.; Holot, N.; Pietropoli, A.; et al. Growth Hormone Replacement in Adults: Real-World Data from Two Large Studies in US and Europe. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. 2020, 50, 71–82. [Google Scholar] [CrossRef]
- Biller, B.M.K.; Höybye, C.; Ferran, J.-M.; Kelepouris, N.; Nedjatian, N.; Olsen, A.H.; Weber, M.M.; Gordon, M.B. Long-Term Effectiveness and Safety of GH Replacement Therapy in Adults ≥60 Years: Data From NordiNet® IOS and ANSWER. J. Endocr. Soc. 2023, 7, bvad054. [Google Scholar] [CrossRef]
- Miller, K.K.; Wexler, T.; Fazeli, P.; Gunnell, L.; Graham, G.J.; Beauregard, C.; Hemphill, L.; Nachtigall, L.; Loeffler, J.; Swearingen, B.; et al. Growth Hormone Deficiency after Treatment of Acromegaly: A Randomized, Placebo-Controlled Study of Growth Hormone Replacement. J. Clin. Endocrinol. Metab. 2010, 95, 567–577. [Google Scholar] [CrossRef]
- Höybye, C.; Ragnarsson, O.; Jönsson, P.J.; Koltowska-Häggström, M.; Trainer, P.; Feldt-Rasmussen, U.; Biller, B.M.K. Clinical Features of GH Deficiency and Effects of 3 Years of GH Replacement in Adults with Controlled Cushing’s Disease. Eur. J. Endocrinol. 2010, 162, 677–684. [Google Scholar] [CrossRef][Green Version]
- Tomlinson, J.W.; Holden, N.; Hills, R.K.; Wheatley, K.; Clayton, R.N.; Bates, A.S.; Sheppard, M.C.; Stewart, P.M. Association between Premature Mortality and Hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet Lond. Engl. 2001, 357, 425–431. [Google Scholar] [CrossRef]
- Svensson, J.; Bengtsson, B.-A.; Rosén, T.; Odén, A.; Johannsson, G. Malignant Disease and Cardiovascular Morbidity in Hypopituitary Adults with or without Growth Hormone Replacement Therapy. J. Clin. Endocrinol. Metab. 2004, 89, 3306–3312. [Google Scholar] [CrossRef]
- Jørgensen, J.O.L.; Juul, A. THERAPY OF ENDOCRINE DISEASE: Growth Hormone Replacement Therapy in Adults: 30 Years of Personal Clinical Experience. Eur. J. Endocrinol. 2018, 179, R47–R56. [Google Scholar] [CrossRef]
- Stochholm, K.; Berglund, A.; Juul, S.; Gravholt, C.H.; Christiansen, J.S. Socioeconomic Factors Do Not but GH Treatment Does Affect Mortality in Adult-Onset Growth Hormone Deficiency. J. Clin. Endocrinol. Metab. 2014, 99, 4141–4148. [Google Scholar] [CrossRef]
- Pappachan, J.M.; Raskauskiene, D.; Kutty, V.R.; Clayton, R.N. Excess Mortality Associated with Hypopituitarism in Adults: A Meta-Analysis of Observational Studies. J. Clin. Endocrinol. Metab. 2015, 100, 1405–1411. [Google Scholar] [CrossRef]
- Berglund, A.; Gravholt, C.H.; Olsen, M.S.; Christiansen, J.S.; Stochholm, K. Growth Hormone Replacement Does Not Increase Mortality in Patients with Childhood-Onset Growth Hormone Deficiency. Clin. Endocrinol. 2015, 83, 677–683. [Google Scholar] [CrossRef]
- Olsson, D.S.; Trimpou, P.; Hallén, T.; Bryngelsson, I.-L.; Andersson, E.; Skoglund, T.; Bengtsson, B.-Å.; Johannsson, G.; Nilsson, A.G. Life Expectancy in Patients with Pituitary Adenoma Receiving Growth Hormone Replacement. Eur. J. Endocrinol. 2017, 176, 67–75. [Google Scholar] [CrossRef]
- Al-Samerria, S.; Radovick, S. The Role of Insulin-like Growth Factor-1 (IGF-1) in the Control of Neuroendocrine Regulation of Growth. Cells 2021, 10, 2664. [Google Scholar] [CrossRef]
- Werner, H.; Laron, Z. Role of the GH-IGF1 System in Progression of Cancer. Mol. Cell. Endocrinol. 2020, 518, 111003. [Google Scholar] [CrossRef]
- Boguszewski, C.L.; da Silva Boguszewski, M.C. Growth Hormone’s Links to Cancer. Endocr. Rev. 2019, 40, 558–574. [Google Scholar] [CrossRef]
- Boguszewski, M.C.S.; Boguszewski, C.L.; Chemaitilly, W.; Cohen, L.E.; Gebauer, J.; Higham, C.; Hoffman, A.R.; Polak, M.; Yuen, K.C.J.; Alos, N.; et al. Safety of Growth Hormone Replacement in Survivors of Cancer and Intracranial and Pituitary Tumours: A Consensus Statement. Eur. J. Endocrinol. 2022, 186, P35–P52. [Google Scholar] [CrossRef] [PubMed]
- Karavitaki, N.; Warner, J.T.; Marland, A.; Shine, B.; Ryan, F.; Arnold, J.; Turner, H.E.; Wass, J.A.H. GH Replacement Does Not Increase the Risk of Recurrence in Patients with Craniopharyngioma. Clin. Endocrinol. 2006, 64, 556–560. [Google Scholar] [CrossRef]
- Olsson, D.S.; Buchfelder, M.; Wiendieck, K.; Kremenevskaja, N.; Bengtsson, B.-Å.; Jakobsson, K.-E.; Jarfelt, M.; Johannsson, G.; Nilsson, A.G. Tumour Recurrence and Enlargement in Patients with Craniopharyngioma with and without GH Replacement Therapy during More than 10 Years of Follow-Up. Eur. J. Endocrinol. 2012, 166, 1061–1068. [Google Scholar] [CrossRef]
- Alotaibi, N.M.; Noormohamed, N.; Cote, D.J.; Alharthi, S.; Doucette, J.; Zaidi, H.A.; Mekary, R.A.; Smith, T.R. Physiologic Growth Hormone-Replacement Therapy and Craniopharyngioma Recurrence in Pediatric Patients: A Meta-Analysis. World Neurosurg. 2018, 109, 487–496.e1. [Google Scholar] [CrossRef] [PubMed]
- van Schaik, J.; Kormelink, E.; Kabak, E.; van Dalen, E.C.; Schouten-van Meeteren, A.Y.N.; de Vos-Kerkhof, E.; Bakker, B.; Fiocco, M.; Hoving, E.W.; Tissing, W.J.E.; et al. Safety of Growth Hormone Replacement Therapy in Childhood-Onset Craniopharyngioma: A Systematic Review and Cohort Study. Neuroendocrinology 2023, 113, 987–1007. [Google Scholar] [CrossRef]
- Losa, M.; Castellino, L.; Pagnano, A.; Rossini, A.; Mortini, P.; Lanzi, R. Growth Hormone Therapy Does Not Increase the Risk of Craniopharyngioma and Nonfunctioning Pituitary Adenoma Recurrence. J. Clin. Endocrinol. Metab. 2020, 105, dgaa089. [Google Scholar] [CrossRef]
- Bolier, M.; van der Lelij, A.-J.; Janssens, G.O.; van den Heuvel-Eibrink, M.M.; Neggers, S.J.C.M.M. Long-Term Safety of Growth Hormone Replacement Therapy in Survivors of Cancer and Tumors of the Pituitary Region. Endocr. Relat. Cancer 2023, 30, e230026. [Google Scholar] [CrossRef]
- van Varsseveld, N.C.; van Bunderen, C.C.; Franken, A.A.M.; Koppeschaar, H.P.F.; van der Lely, A.J.; Drent, M.L. Tumor Recurrence or Regrowth in Adults with Nonfunctioning Pituitary Adenomas Using GH Replacement Therapy. J. Clin. Endocrinol. Metab. 2015, 100, 3132–3139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buchfelder, M.; Kann, P.H.; Wüster, C.; Tuschy, U.; Saller, B.; Brabant, G.; Kleindienst, A.; Nomikos, P. German KIMS Board Influence of GH Substitution Therapy in Deficient Adults on the Recurrence Rate of Hormonally Inactive Pituitary Adenomas: A Case Control Study. Eur. J. Endocrinol. 2007, 157, 149–156. [Google Scholar] [CrossRef][Green Version]
- Arnold, J.R.; Arnold, D.F.; Marland, A.; Karavitaki, N.; Wass, J.A.H. GH Replacement in Patients with Non-Functioning Pituitary Adenoma (NFA) Treated Solely by Surgery Is Not Associated with Increased Risk of Tumour Recurrence. Clin. Endocrinol. 2009, 70, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Olsson, D.S.; Buchfelder, M.; Schlaffer, S.; Bengtsson, B.-A.; Jakobsson, K.-E.; Johannsson, G.; Nilsson, A.G. Comparing Progression of Non-Functioning Pituitary Adenomas in Hypopituitarism Patients with and without Long-Term GH Replacement Therapy. Eur. J. Endocrinol. 2009, 161, 663–669. [Google Scholar] [CrossRef] [PubMed]
- van Bunderen, C.C.; van Varsseveld, N.C.; Erfurth, E.M.; Ket, J.C.F.; Drent, M.L. Efficacy and Safety of Growth Hormone Treatment in Adults with Growth Hormone Deficiency: A Systematic Review of Studies on Morbidity. Clin. Endocrinol. 2014, 81, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Petersenn, S.; Fleseriu, M.; Casanueva, F.F.; Giustina, A.; Biermasz, N.; Biller, B.M.K.; Bronstein, M.; Chanson, P.; Fukuoka, H.; Gadelha, M.; et al. Diagnosis and Management of Prolactin-Secreting Pituitary Adenomas: A Pituitary Society International Consensus Statement. Nat. Rev. Endocrinol. 2023, 19, 722–740. [Google Scholar] [CrossRef] [PubMed]
- Tritos, N.A. Growth Hormone Replacement in Adults with Cured Acromegaly: Efficacy and Safety. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101790. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Mahendran, B.; Reddy, K.S.; Ahluwalia, J.; Vaiphei, K.; Kochhar, R.K.; Gupta, P.; Srinivasan, A.; Prakash, M.; Mukherjee, K.K.; et al. Short-Term Efficacy of Recombinant Human GH Therapy in Cured Acromegaly Patients with GH Deficiency: A Single-Center Experience. Endocr. Connect. 2015, 4, 65–75. [Google Scholar] [CrossRef][Green Version]
- Luger, A.; Mattsson, A.F.; Koltowska-Häggström, M.; Thunander, M.; Góth, M.; Verhelst, J.; Abs, R. Incidence of Diabetes Mellitus and Evolution of Glucose Parameters in Growth Hormone-Deficient Subjects during Growth Hormone Replacement Therapy: A Long-Term Observational Study. Diabetes Care 2012, 35, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Møller, N.; Jørgensen, J.O.L. Effects of Growth Hormone on Glucose, Lipid, and Protein Metabolism in Human Subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.J.; Shalet, S.M. Which Adults Develop Side-Effects of Growth Hormone Replacement? Clin. Endocrinol. 1995, 43, 143–149. [Google Scholar] [CrossRef]
- Kim, J.H.; Chae, H.W.; Chin, S.O.; Ku, C.R.; Park, K.H.; Lim, D.J.; Kim, K.J.; Lim, J.S.; Kim, G.; Choi, Y.M.; et al. Diagnosis and Treatment of Growth Hormone Deficiency: A Position Statement from Korean Endocrine Society and Korean Society of Pediatric Endocrinology. Endocrinol. Metab. Seoul Korea 2020, 35, 272–287. [Google Scholar] [CrossRef]
- Grimberg, A.; DiVall, S.A.; Polychronakos, C.; Allen, D.B.; Cohen, L.E.; Quintos, J.B.; Rossi, W.C.; Feudtner, C.; Murad, M.H. Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society Guidelines for Growth Hormone and Insulin-Like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-Like Growth Factor-I Deficiency. Horm. Res. Paediatr. 2016, 86, 361–397. [Google Scholar] [CrossRef] [PubMed]
- Collett-Solberg, P.F.; Ambler, G.; Backeljauw, P.F.; Bidlingmaier, M.; Biller, B.M.K.; Boguszewski, M.C.S.; Cheung, P.T.; Choong, C.S.Y.; Cohen, L.E.; Cohen, P.; et al. Diagnosis, Genetics, and Therapy of Short Stature in Children: A Growth Hormone Research Society International Perspective. Horm. Res. Paediatr. 2019, 92, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.C.J.; Johannsson, G.; Ho, K.K.Y.; Miller, B.S.; Bergada, I.; Rogol, A.D. Diagnosis and Testing for Growth Hormone Deficiency across the Ages: A Global View of the Accuracy, Caveats, and Cut-Offs for Diagnosis. Endocr. Connect. 2023, 12, e220504. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, C.; Ibba, A.; Pilia, S.; Beltrami, N.; Di Iorgi, N.; Rollo, A.; Fratangeli, N.; Radetti, G.; Zucchini, S.; Maghnie, M.; et al. Cut-off Limits of the Peak GH Response to Stimulation Tests for the Diagnosis of GH Deficiency in Children and Adolescents: Study in Patients with Organic GHD. Eur. J. Endocrinol. 2016, 175, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hoeck, H.C.; Vestergaard, P.; Jakobsen, P.E.; Falhof, J.; Laurberg, P. Diagnosis of Growth Hormone (GH) Deficiency in Adults with Hypothalamic-Pituitary Disorders: Comparison of Test Results Using Pyridostigmine plus GH-Releasing Hormone (GHRH), Clonidine plus GHRH, and Insulin-Induced Hypoglycemia as GH Secretagogues. J. Clin. Endocrinol. Metab. 2000, 85, 1467–1472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dichtel, L.E.; Yuen, K.C.J.; Bredella, M.A.; Gerweck, A.V.; Russell, B.M.; Riccio, A.D.; Gurel, M.H.; Sluss, P.M.; Biller, B.M.K.; Miller, K.K. Overweight/Obese Adults with Pituitary Disorders Require Lower Peak Growth Hormone Cutoff Values on Glucagon Stimulation Testing to Avoid Overdiagnosis of Growth Hormone Deficiency. J. Clin. Endocrinol. Metab. 2014, 99, 4712–4719. [Google Scholar] [CrossRef]
- Aimaretti, G.; Corneli, G.; Razzore, P.; Bellone, S.; Baffoni, C.; Arvat, E.; Camanni, F.; Ghigo, E. Comparison between Insulin-Induced Hypoglycemia and Growth Hormone (GH)-Releasing Hormone + Arginine as Provocative Tests for the Diagnosis of GH Deficiency in Adults. J. Clin. Endocrinol. Metab. 1998, 83, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef] [PubMed]
- Sklar, C.A.; Antal, Z.; Chemaitilly, W.; Cohen, L.E.; Follin, C.; Meacham, L.R.; Murad, M.H. Hypothalamic-Pituitary and Growth Disorders in Survivors of Childhood Cancer: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 2761–2784. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Quoc, A.; Beccaria, K.; González Briceño, L.; Pinto, G.; Samara-Boustani, D.; Stoupa, A.; Beltrand, J.; Besançon, A.; Thalassinos, C.; Puget, S.; et al. GH and Childhood-Onset Craniopharyngioma: When to Initiate GH Replacement Therapy? J. Clin. Endocrinol. Metab. 2023, 108, 1929–1936. [Google Scholar] [CrossRef]
- Spaziani, M.; Tarantino, C.; Tahani, N.; Gianfrilli, D.; Sbardella, E.; Isidori, A.M.; Lenzi, A.; Radicioni, A.F. Clinical, Diagnostic, and Therapeutic Aspects of Growth Hormone Deficiency During the Transition Period: Review of the Literature. Front. Endocrinol. 2021, 12, 634288. [Google Scholar] [CrossRef]
- Wexler, T.L.; Reifschneider, K.; Backeljauw, P.; Cárdenas, J.F.; Hoffman, A.R.; Miller, B.S.; Yuen, K.C.J. Growth Hormone Deficiency Following Traumatic Brain Injury in Pediatric and Adolescent Patients: Presentation, Treatment, and Challenges of Transitioning from Pediatric to Adult Services. J. Neurotrauma 2023, 40, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Doknic, M.; Stojanovic, M.; Soldatovic, I.; Milenkovic, T.; Zdravkovic, V.; Jesic, M.; Todorovic, S.; Mitrovic, K.; Vukovic, R.; Miljic, D.; et al. Mapping the Journey of Transition: A Single-Center Study of 170 Childhood-Onset GH Deficiency Patients. Endocr. Connect. 2021, 10, 935–946. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, Y.; Yoo, H.-W.; Lee, Y.A.; Shin, C.H.; Choi, H.S.; Kim, H.-S.; Kim, J.H.; Moon, J.E.; Ko, C.W.; et al. Metabolic Impacts of Discontinuation and Resumption of Recombinant Human Growth Hormone Treatment during the Transition Period in Patients with Childhood-Onset Growth Hormone Deficiency. Endocrinol. Metab. Seoul Korea 2022, 37, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Leví, A.M.; Marazuela, M. Treatment of Adult Growth Hormone Deficiency with Human Recombinant Growth Hormone: An Update on Current Evidence and Critical Review of Advantages and Pitfalls. Endocrine 2018, 60, 203–218. [Google Scholar] [CrossRef]
- Melmed, S. Idiopathic Adult Growth Hormone Deficiency. J. Clin. Endocrinol. Metab. 2013, 98, 2187–2197. [Google Scholar] [CrossRef]
- Mazziotti, G.; Marzullo, P.; Doga, M.; Aimaretti, G.; Giustina, A. Growth Hormone Deficiency in Treated Acromegaly. Trends Endocrinol. Metab. TEM 2015, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Crowe, B.J.; Biller, B.M.K.; Ho, K.K.Y.; Clemmons, D.R.; Chipman, J.J.; HypoCCS Advisory Board and the US HypoCCS Study Group. Which Patients Do Not Require a GH Stimulation Test for the Diagnosis of Adult GH Deficiency? J. Clin. Endocrinol. Metab. 2002, 87, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Garrahy, A.; Sherlock, M.; Thompson, C.J. Management of Endocrine Disease: Neuroendocrine Surveillance and Management of Neurosurgical Patients. Eur. J. Endocrinol. 2017, 176, R217–R233. [Google Scholar] [CrossRef]
| Consequences of GH Deficiency | Benefits of rhGH Therapy |
|---|---|
| Growth and development | |
| Growth retardation and delayed bone age | Stimulates linear growth in children |
| Short stature in adulthood | Increases final height in children |
| Health-related QoL | |
| Lower score in QoL questionnaires | Greater benefit in patients with low QoL |
| Body composition | |
| Increased fat mass | Reduces body fat accumulation |
| Decreased lean mass | Increases muscle mass |
| Bone health and fracture risk | |
| Reduction in bone formation | Increases bone mineral content |
| Loss of BMD | Increases BMD in the lumbar spine |
| Increased risk of osteoporosis | Decreases the risk of vertebral and non-vertebral fractures |
| Liver function | |
| Increased prevalence of NAFLD and NASH | Reduces hepatic steatosis, inflammation, and fibrosis in overweight/obese individuals with NAFLD |
| Metabolic and CV profile | |
| Elevated total and LDL cholesterol | Reduces total and LDL cholesterol |
| High triglycerides levels | Lowers diastolic blood pressure |
| Decreased HDL cholesterol | Increases HDL cholesterol |
| IR and impaired glucose metabolism | Reduces carotid intimamedia thickness |
| Elevated pro-inflammatory cytokines (CRP) | Reduces CRP level |
| Improves endothelial and cardiac function | |
| CV and global mortality | |
| Increased risk of CV mortality | Reduces incidence rate of myocardial infarction |
| Tendency to decrease global mortality | |
| Potential Risks |
| Concerns about the possibility of tumor progression and recurrence |
| Potential increased risk of malignancies in certain medical conditions * |
| Side effects |
| Fluid retention |
| Injection site discomfort |
| Edema |
| Sensory disturbances |
| Joint pain |
| Carpal tunnel syndrome |
| Hyperglycemia/diabetes mellitus |
| Contraindications |
| Active malignancy |
| Acute decompensated heart failure |
| Severe liver disease |
| Severe renal insufficiency |
| Severe uncontrolled arterial hypertension |
| Active and untreated preproliferative or proliferative retinopathy (diabetic or other) |
| Intracranial hypertension |
| Critically ill patients |
| Pregnancy or lactation |
| Hypersensitivity to GH or any of the excipients of the preparation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, P. Clinical Management of Postoperative Growth Hormone Deficiency in Hypothalamic-Pituitary Tumors. J. Clin. Med. 2024, 13, 4307. https://doi.org/10.3390/jcm13154307
Iglesias P. Clinical Management of Postoperative Growth Hormone Deficiency in Hypothalamic-Pituitary Tumors. Journal of Clinical Medicine. 2024; 13(15):4307. https://doi.org/10.3390/jcm13154307
Chicago/Turabian StyleIglesias, Pedro. 2024. "Clinical Management of Postoperative Growth Hormone Deficiency in Hypothalamic-Pituitary Tumors" Journal of Clinical Medicine 13, no. 15: 4307. https://doi.org/10.3390/jcm13154307
APA StyleIglesias, P. (2024). Clinical Management of Postoperative Growth Hormone Deficiency in Hypothalamic-Pituitary Tumors. Journal of Clinical Medicine, 13(15), 4307. https://doi.org/10.3390/jcm13154307






