Epithelial Remodeling and Epithelial Wavefront Aberrometry after Spherical vs. Cylindrical Myopic Small Incision Lenticule Extraction (SMILE)
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion/Exclusion Criteria
2.2. Pre- and Postoperative Measurements
2.3. Surgical Procedure and Postoperative Therapy Regimen
2.4. Data Management and Statistical Analysis
3. Results
3.1. Patient Demographics and Baseline Parameters
3.2. Refractive Outcome
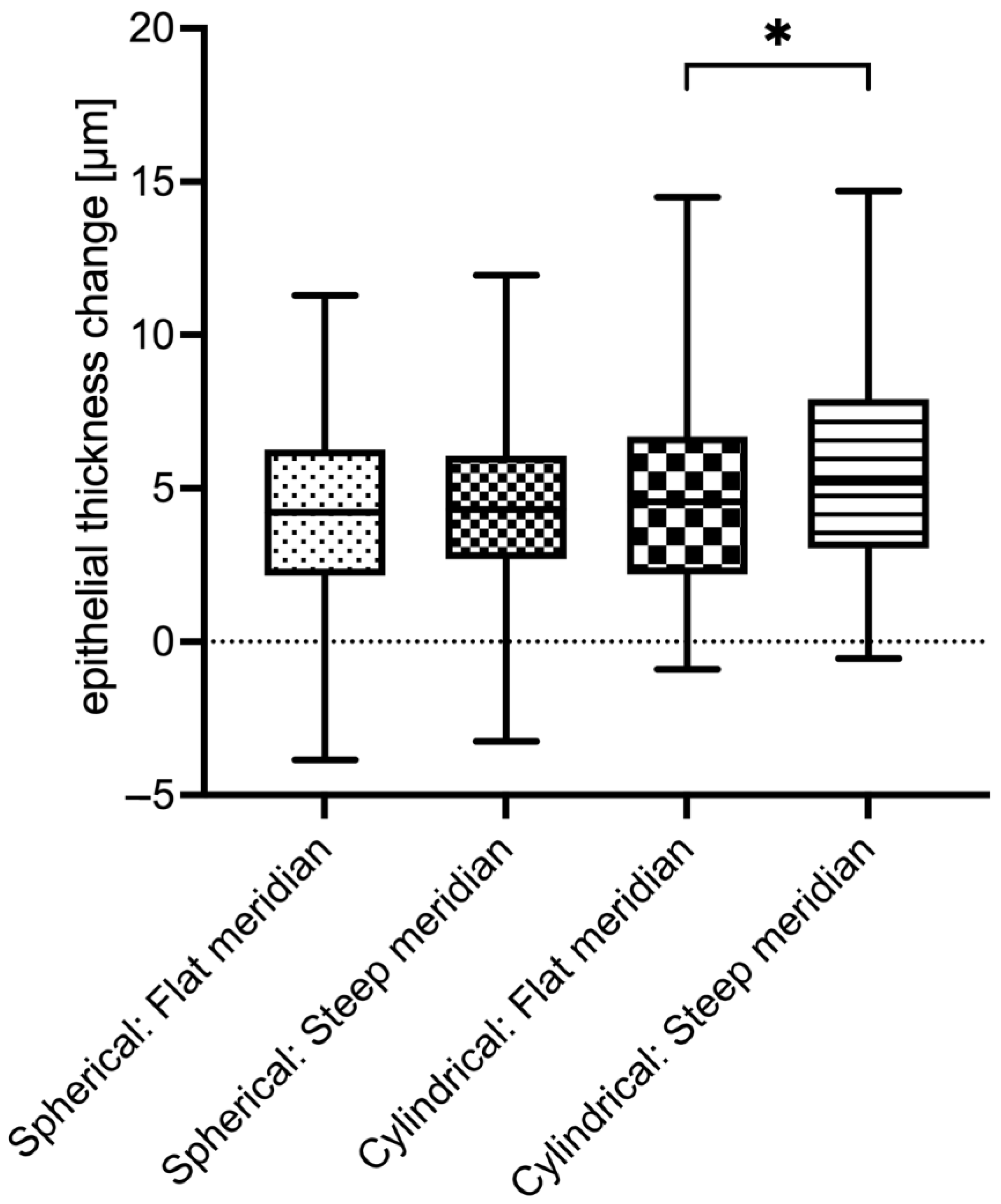
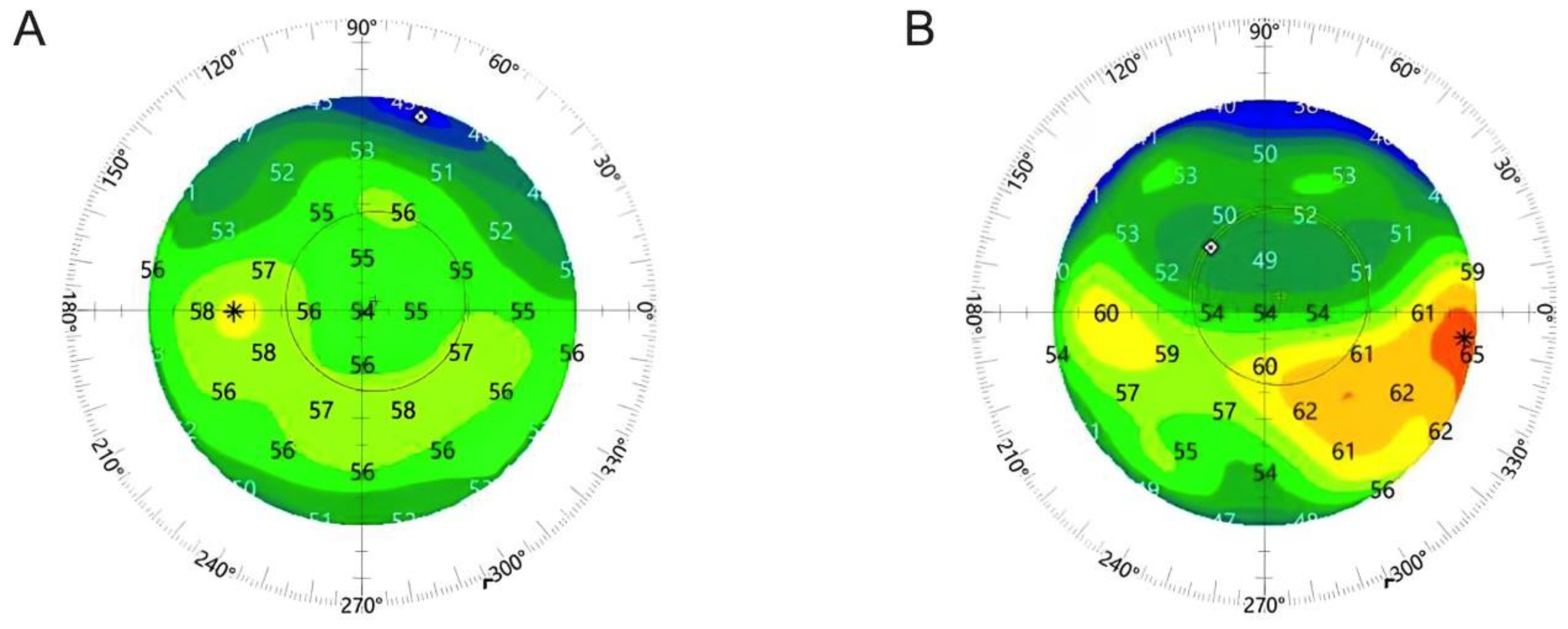
3.3. Epithelial Thickness Changes (Remodeling)
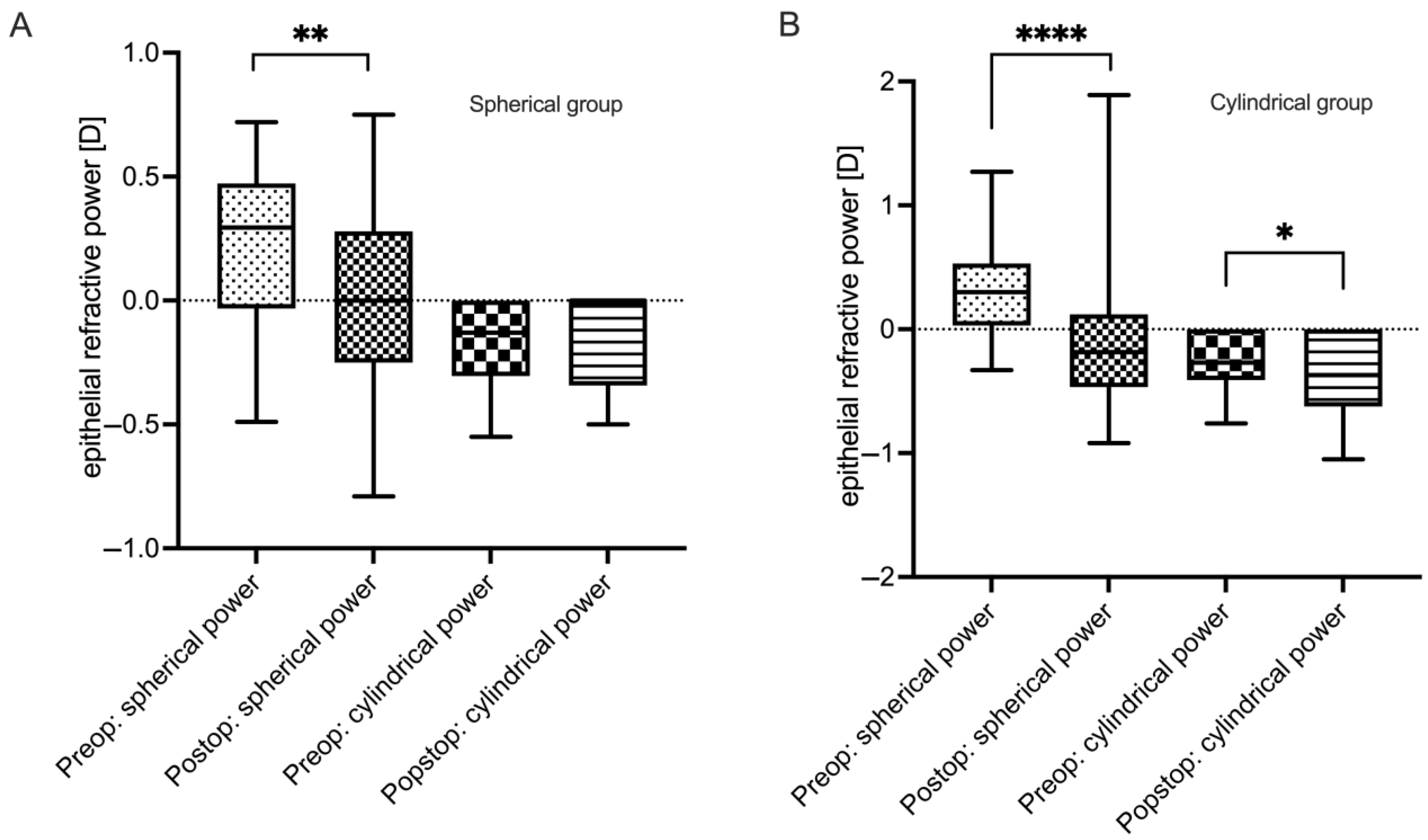
3.4. Epithelial Refractive Power Changes
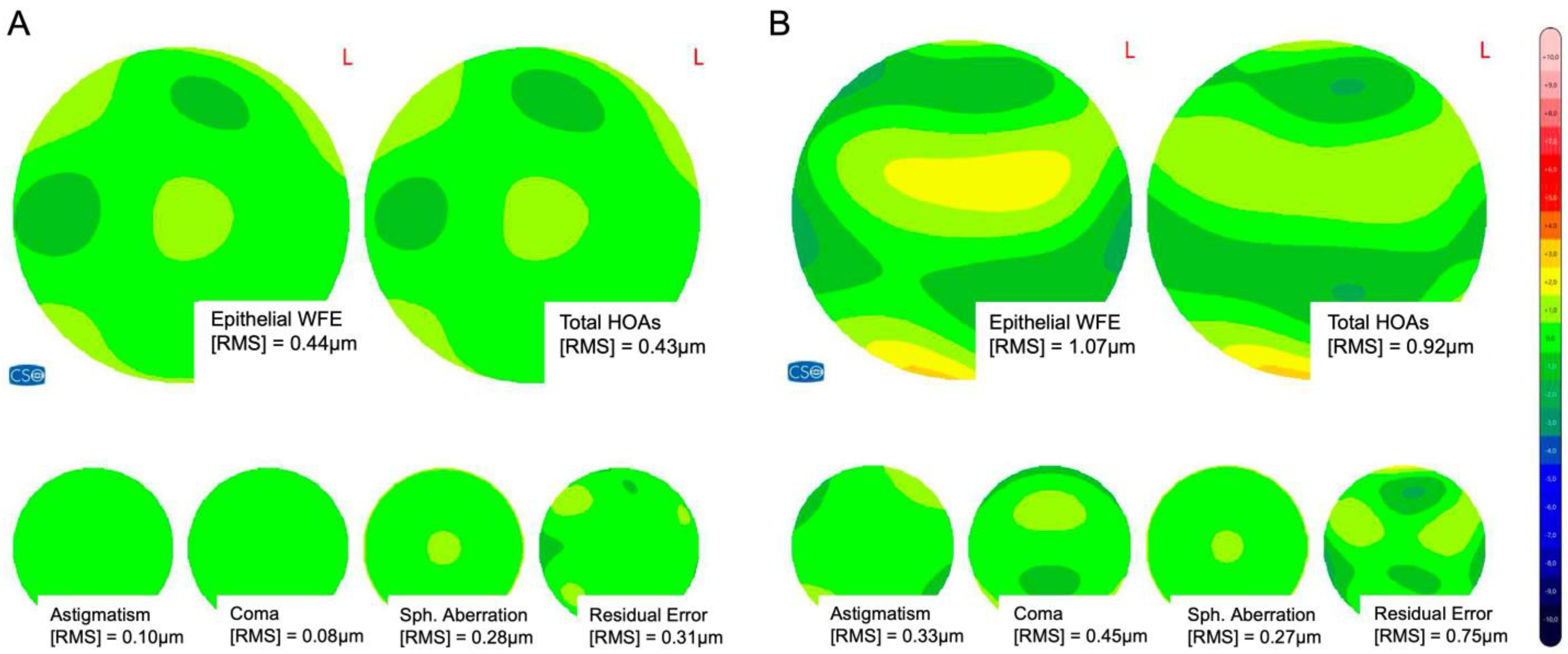
3.5. Epithelial Wavefront Error and Higher-Order Aberrations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogt, A. Atlas of the Slitlamp-Microscopy of the Living Eye; Springer: Berlin/Heidelberg, Germany, 1921; p. VII, 154. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Vida, R.S. Applications of epithelial thickness mapping in corneal refractive surgery. Saudi J. Ophthalmol. 2022, 36, 25–35. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Srivannaboon, S.; Gobbe, M.; Archer, T.J.; Silverman, R.H.; Sutton, H.; Coleman, D.J. Epithelial thickness profile changes induced by myopic LASIK as measured by Artemis very high-frequency digital ultrasound. J. Refract. Surg. 2009, 25, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Luft, N.; Ring, M.H.; Dirisamer, M.; Mursch-Edlmayr, A.S.; Kreutzer, T.C.; Pretzl, J.; Bolz, M.; Priglinger, S.G. Corneal Epithelial Remodeling Induced by Small Incision Lenticule Extraction (SMILE). Invest. Ophthalmol. Vis. Sci. 2016, 57, OCT176-183. [Google Scholar] [CrossRef]
- Ganesh, S.; Brar, S.; Relekar, K.J. Epithelial Thickness Profile Changes Following Small Incision Refractive Lenticule Extraction (SMILE) for Myopia and Myopic Astigmatism. J. Refract. Surg. 2016, 32, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Canto-Cerdan, M.; El Bahrawy, M.; Alió, J.L.; Casanova, L.; García, M.J.; Al-Amri, S.A.J.; Cavas, F.; Alió Del Barrio, J.L. Corneal Epithelium Thickness and Refractive Changes After Myopic Laser Corneal Refractive Surgery. J. Refract. Surg. 2022, 38, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gu, W.; Gao, Y.; Wang, W.; Zhang, F. Survival analysis of myopic regression after small incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis for low to moderate myopia. Eye Vis. 2022, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Ye, Y.; Chen, P.; Yang, Y.; Zhuang, J.; Yu, K. Corneal Epithelial Thickness Changes Following SMILE for Myopia with High Astigmatism. J. Refract. Surg. 2021, 37, 224–230. [Google Scholar] [CrossRef]
- Pedersen, I.B.; Ivarsen, A.; Hjortdal, J. Changes in Astigmatism, Densitometry, and Aberrations After SMILE for Low to High Myopic Astigmatism: A 12-Month Prospective Study. J. Refract. Surg. 2017, 33, 11–17. [Google Scholar] [CrossRef]
- Ivarsen, A.; Hjortdal, J. Correction of myopic astigmatism with small incision lenticule extraction. J. Refract. Surg. 2014, 30, 240–247. [Google Scholar] [CrossRef]
- Savini, G.; Schiano-Lomoriello, D.; Hoffer, K.J. Repeatability of automatic measurements by a new anterior segment optical coherence tomographer combined with Placido topography and agreement with 2 Scheimpflug cameras. J. Cataract. Refract. Surg. 2018, 44, 471–478. [Google Scholar] [CrossRef]
- Kommission Refraktive Chirurgie der DOG und des BVA. Bewertung und Qualitätssicherung refraktiv-chirurgischer Eingriffe durch die DOG und den BVA—KRC-Empfehlungen. Available online: http://bva.dog/krc/qualit.pdf (accessed on 22 January 2024).
- Liu, M.; Jin, C.; Lu, L.; Yuan, Y.; Chen, C.; Zhao, T.; Ke, B. The Impact of Corneal Epithelial Thickening and Inhomogeneity on Corneal Aberrations After Small Incision Lenticule Extraction. J. Refract. Surg. 2023, 39, 23–32. [Google Scholar] [CrossRef]
- Patel, S.; Marshall, J.; Fitzke, F.W., 3rd. Refractive index of the human corneal epithelium and stroma. J. Refract. Surg. 1995, 11, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulos, A.J.; Asimellis, G. Longitudinal postoperative lasik epithelial thickness profile changes in correlation with degree of myopia correction. J. Refract. Surg. 2014, 30, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M. Change in epithelial thickness profile 24 hours and longitudinally for 1 year after myopic LASIK: Three-dimensional display with Artemis very high-frequency digital ultrasound. J. Refract. Surg. 2012, 28, 195–201. [Google Scholar] [CrossRef]
- Zhu, M.; Xin, Y.; Vinciguerra, R.; Wang, Z.; Warsame, A.M.; Wang, C.; Zhu, D.; Qu, Z.; Wang, P.; Zheng, X.; et al. Corneal Epithelial Remodeling in a 6-Month Follow-up Period in Myopic Corneal Refractive Surgeries. J. Refract. Surg. 2023, 39, 187–196. [Google Scholar] [CrossRef]
- Vinciguerra, P.; Roberts, C.J.; Albé, E.; Romano, M.R.; Mahmoud, A.; Trazza, S.; Vinciguerra, R. Corneal curvature gradient map: A new corneal topography map to predict the corneal healing process. J. Refract. Surg. 2014, 30, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, Z.; Zhao, S.; Huang, Y. To Investigate the Changes in Corneal Curvature and Its Correlation with Corneal Epithelial Remodeling After Trans-PRK and FS-LASIK. Curr. Eye Res. 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gatinel, D.; Hoang-Xuan, T.; Azar, D.T. Three-dimensional representation and qualitative comparisons of the amount of tissue ablation to treat mixed and compound astigmatism. J. Cataract. Refract. Surg. 2002, 28, 2026–2034. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, P.; Yu, N.; Wan, L.; Lan, M.; Zheng, H.; Yu, K. Evaluation of Wide Corneal Epithelial Remodeling after Small Incision Lenticule Extraction (SMILE) with Wide-Field Optical Coherence Tomography. J. Ophthalmol. 2022, 2022, 8764103. [Google Scholar] [CrossRef]
- Li, A.; Liu, Z.; Lin, M.; Gong, Q.; Wei, L.; Lu, F.; Hu, L. Changes in Corneal Epithelial Thickness in Different Areas After Femtosecond Laser-Assisted LASIK in Patients with High Astigmatism. J. Refract. Surg. 2024, 40, e239–e244. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, J.; Schmelter, V.; Mayer, W.J.; Schworm, B.; Priglinger, S.G.; Dirisamer, M.; Luft, N. SMILE Versus Implantable Collamer Lens Implantation for High Myopia: A Matched Comparative Study. J. Refract. Surg. 2020, 36, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.S.W.; Chow, L.L.W.; Lee, C.Z.; Chan, T.C.Y. Astigmatism Correction Using SMILE. Asia Pac. J. Ophthalmol. 2019, 8, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Kim, D.; Kim, J.S.; Kim, H.S.; Ryu, I.H.; Lee, I.S.; Kim, J.K.; Na, K.H. Comparison of early visual outcomes after SMILE using VISUMAX 800 and VISUMAX 500 for myopia: A retrospective matched case-control study. Sci. Rep. 2024, 14, 11989. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Spherical Group | Cylindrical Group | p-Value |
|---|---|---|---|
| Age [years] | 32.9 ± 6.5 [19; 55] | 33.2 ± 6.9 [21; 48] | 0.82 |
| Eyes [n] | 43 | 43 | |
| Patients [n] | 43 | 43 | |
| Sex [m:f] | 20:23 | 20:23 | >0.99 (FE) |
| UCDVA pre-SMILE [logMAR] | 1.05 ± 0.30 [1.40; 0.40] | 0.95 ± 0.37 [1.40; 0.40] | 0.44 |
| BCDVA pre-SMILE [logMAR] | −0.11 ± 0.08 [0; −0.20] | −0.06 ± 0.10 [0.40; −0.20] | <0.01 ** |
| Refractive sphere [D] | −3.73 ± 1.52 [−1.50; −8.5] | −2.82 ± 1.64 [0.25; −6.50] | 0.02 * |
| Refractive cylinder [D] | −0.17 ± 0.18 [0; −0.50] | −1.99 ± 0.78 [−1.25; −5.00] | <0.0001 **** |
| Spherical equivalent [D] | −3.81 ± 1.48 [−1.50; −8.50] | −3.81 ± 1.55 [−0.88; −7.63] | 0.85 |
| Stromal thickness pre-SMILE [µm] | 490 ± 31 [434; 546] | 480 ± 32 [432; 532] | 0.20 |
| Total pachymetry preop. [µm] | 545 ± 31 [486; 599] | 534 ± 33 [483; 590] | 0.19 |
| K1 preoperative | 43.82 ± 1.38 [41.26; 47.80] | 42.22 ± 1.46 [39.11; 45.98] | <0.0001 **** |
| K2 preoperative | 44.49 ± 1.37 [41.73; 48.33] | 43.73 ± 1.29 [40.16; 47.55] | 0.0079 ** |
| KMean preoperative | 44.17 ± 1.38 [41.56; 48.07] | 42.96 ± 1.35 [39.63; 46.75] | 0.0003 *** |
| Spherical Group (n = 43) | Cylindrical Group (n = 43) | |||||
|---|---|---|---|---|---|---|
| Parameter | Preop. | Postop. | p-Value | Preop. | Postop. | p-Value |
| Epi-WFE [RMS, µm] | 0.60 ± 0.25 [0.26; 1.48] | 0.74 ± 0.35 [0.28; 2.39] | 0.02 * | 0.62 ± 0.22 [0.28; 1.19] | 0.92 ± 0.35 [0.40; 2.43] | <0.0001 **** |
| Epi-HOAs [RMS; µm] | 0.37 ± 0.12 [0.20; 0.77] | 0.50 ± 0.18 [0.20; 1.26] | <0.0001 **** | 0.35 ± 0.12 [0.16; 0.60] | 0.53 ± 0.20 [0.26; 1.01] | <0.0001 **** |
| Epi-SA [RMS; µm] | 0.12 ± 0.08 [0.01; 0.32] | 0.25 ± 0.15 [0.01; 0.64] | <0.0001 **** | 0.12 ± 0.10 [0.00; 0.33] | 0.24 ± 0.13 [0.06; 0.71] | <0.0001 **** |
| Epi-Coma [RMS; µm] | 0.12 ± 0.10 [0.00; 0.62] | 0.25 ± 0.19 [0.01; 1.10] | <0.0001 **** | 0.15 ± 0.10 [0.03; 0.38] | 0.23 ± 0.17 [0.01; 0.65] | 0.01 * |
| Epi-Trefoil [RMS; µm] | 0.14 ± 0.09 [0.02; 0.36] | 0.14 ± 0.07 [0.02; 0.29] | 0.83 | 0.11 ± 0.07 [0.01; 0.27] | 0.18 ± 0.14 [0.02; 0.61] | 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunner, B.S.; Feldhaus, L.; Mayer, W.J.; Siedlecki, J.; Dirisamer, M.; Priglinger, S.G.; Kassumeh, S.; Luft, N. Epithelial Remodeling and Epithelial Wavefront Aberrometry after Spherical vs. Cylindrical Myopic Small Incision Lenticule Extraction (SMILE). J. Clin. Med. 2024, 13, 3970. https://doi.org/10.3390/jcm13133970
Brunner BS, Feldhaus L, Mayer WJ, Siedlecki J, Dirisamer M, Priglinger SG, Kassumeh S, Luft N. Epithelial Remodeling and Epithelial Wavefront Aberrometry after Spherical vs. Cylindrical Myopic Small Incision Lenticule Extraction (SMILE). Journal of Clinical Medicine. 2024; 13(13):3970. https://doi.org/10.3390/jcm13133970
Chicago/Turabian StyleBrunner, Barbara S., Lukas Feldhaus, Wolfgang J. Mayer, Jakob Siedlecki, Martin Dirisamer, Siegfried G. Priglinger, Stefan Kassumeh, and Nikolaus Luft. 2024. "Epithelial Remodeling and Epithelial Wavefront Aberrometry after Spherical vs. Cylindrical Myopic Small Incision Lenticule Extraction (SMILE)" Journal of Clinical Medicine 13, no. 13: 3970. https://doi.org/10.3390/jcm13133970
APA StyleBrunner, B. S., Feldhaus, L., Mayer, W. J., Siedlecki, J., Dirisamer, M., Priglinger, S. G., Kassumeh, S., & Luft, N. (2024). Epithelial Remodeling and Epithelial Wavefront Aberrometry after Spherical vs. Cylindrical Myopic Small Incision Lenticule Extraction (SMILE). Journal of Clinical Medicine, 13(13), 3970. https://doi.org/10.3390/jcm13133970






