Effects of Remimazolam versus Sevoflurane on Hemodynamics in Patients Undergoing Coil Embolization of Cerebral Aneurysm: A Prospective Randomized Controlled Trial
Abstract
1. Introduction
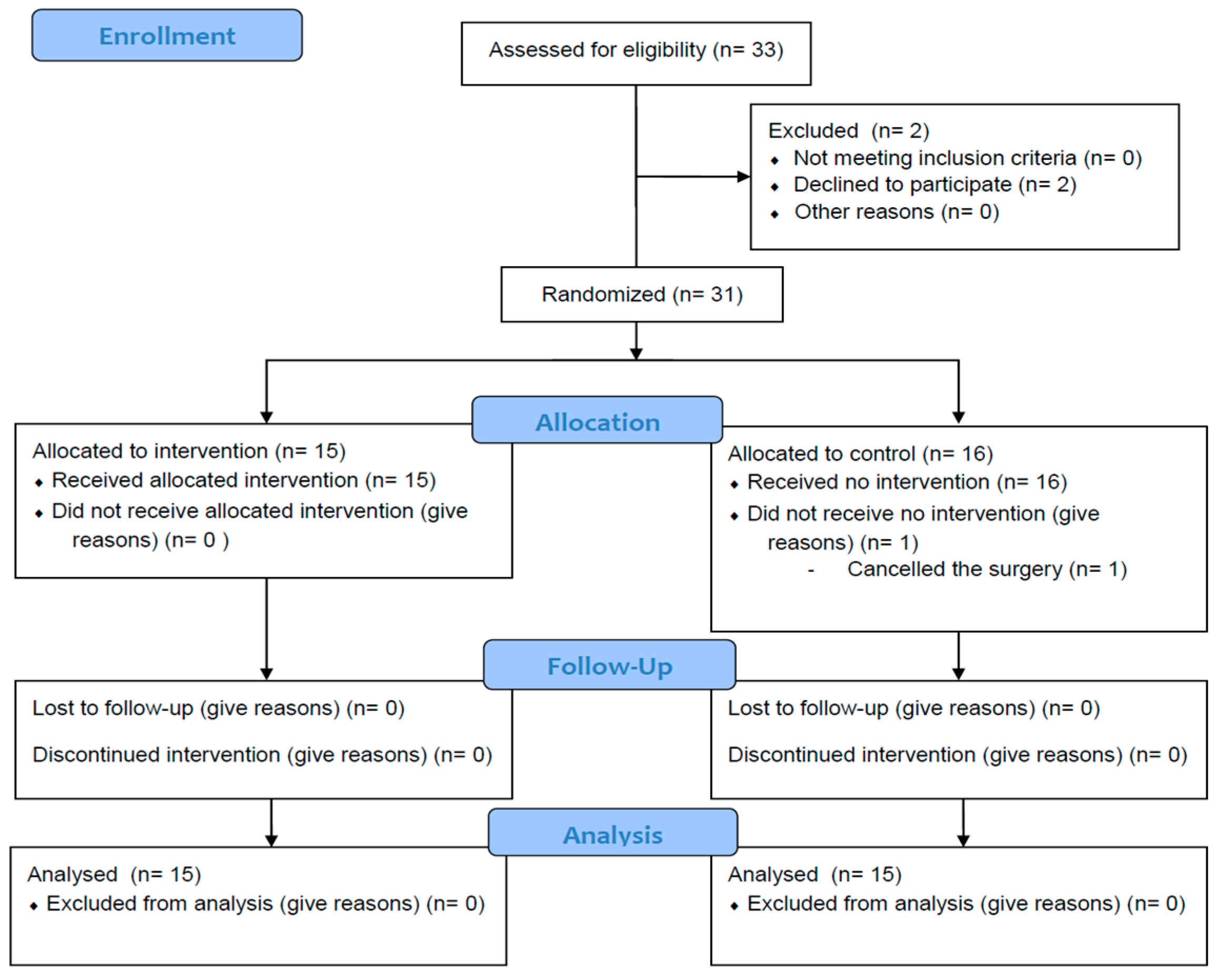
2. Materials and Methods
3. Results
3.1. Primary Outcomes
Hypotension or Hypertension Events during Intervention
3.2. Secondary Outcomes
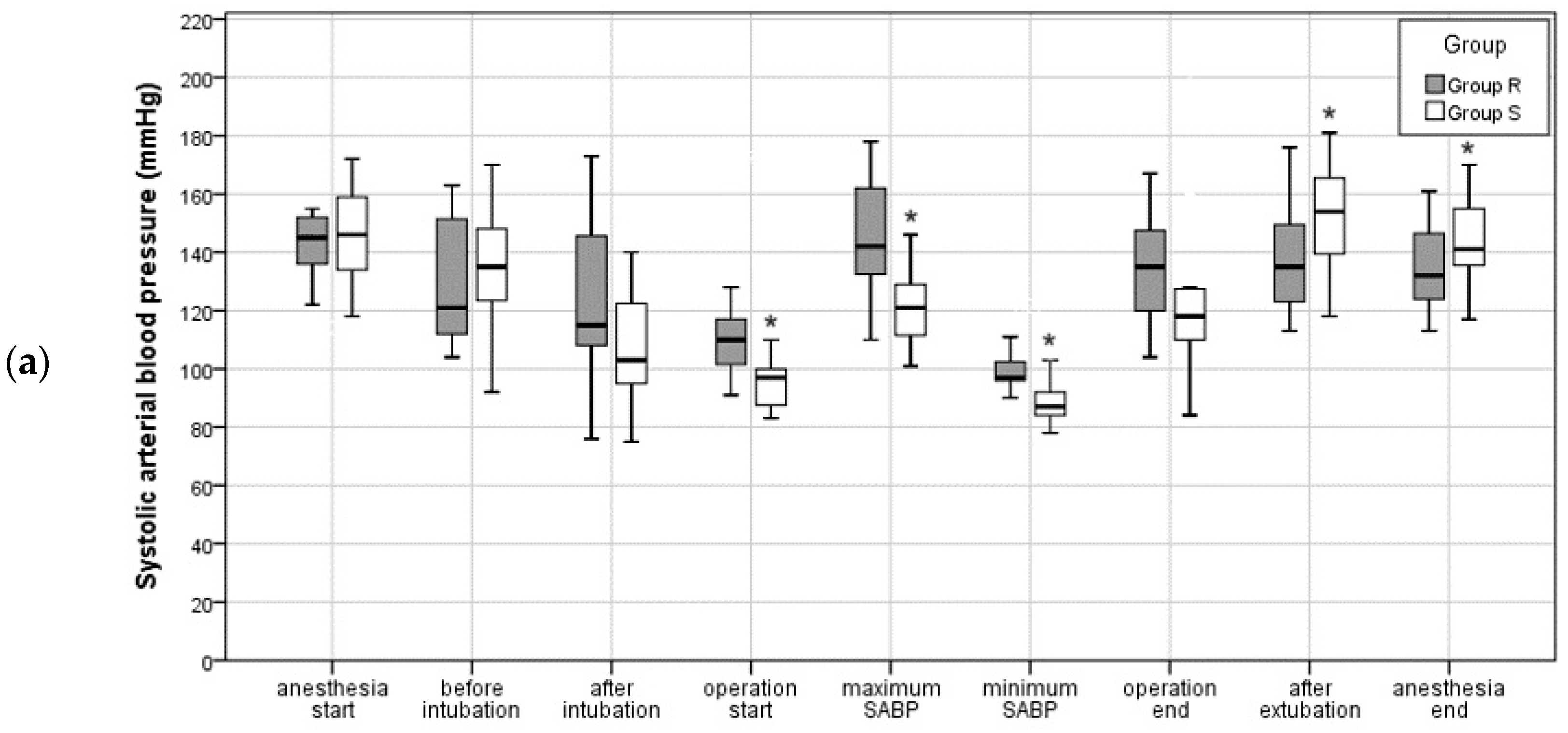
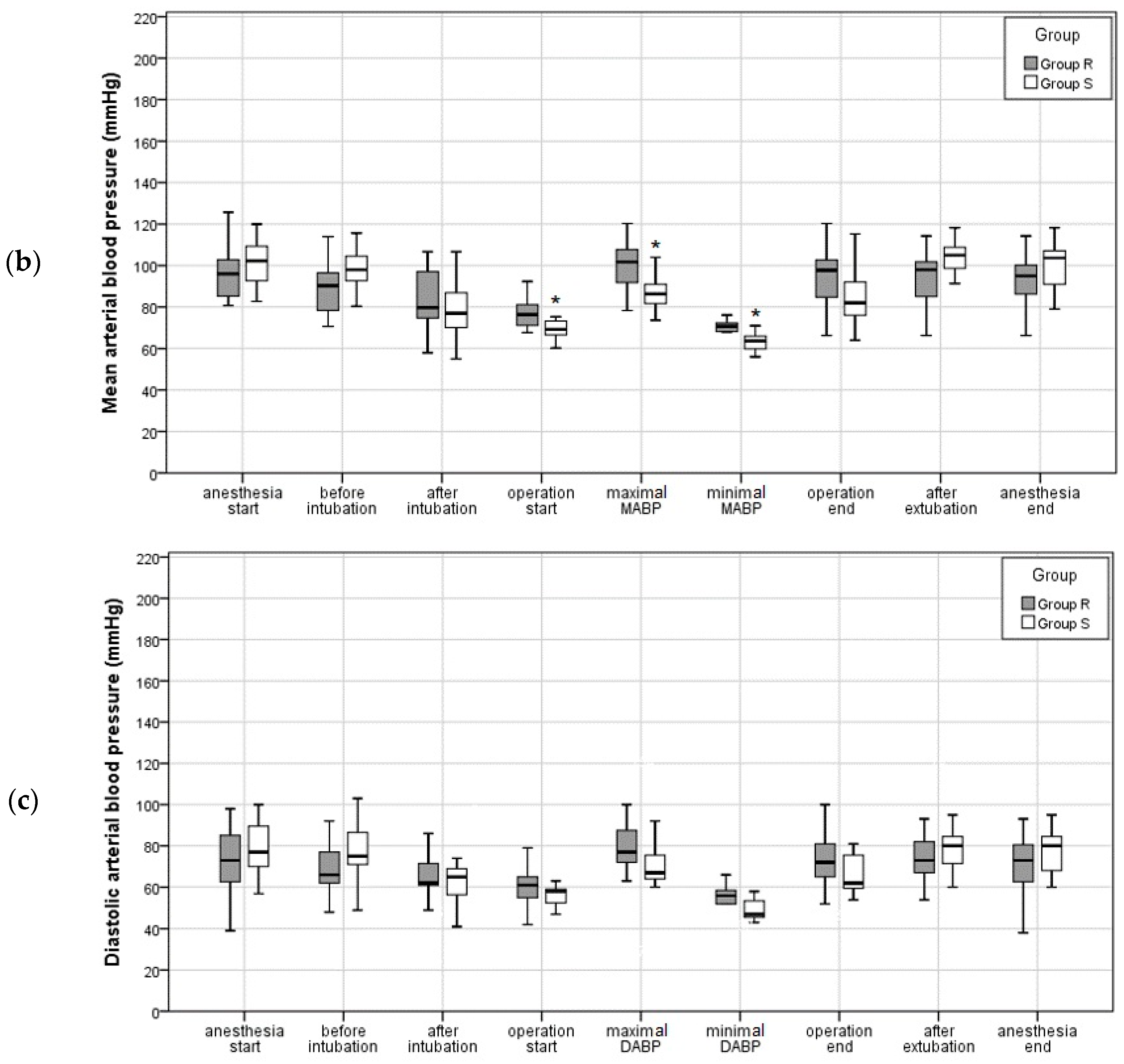
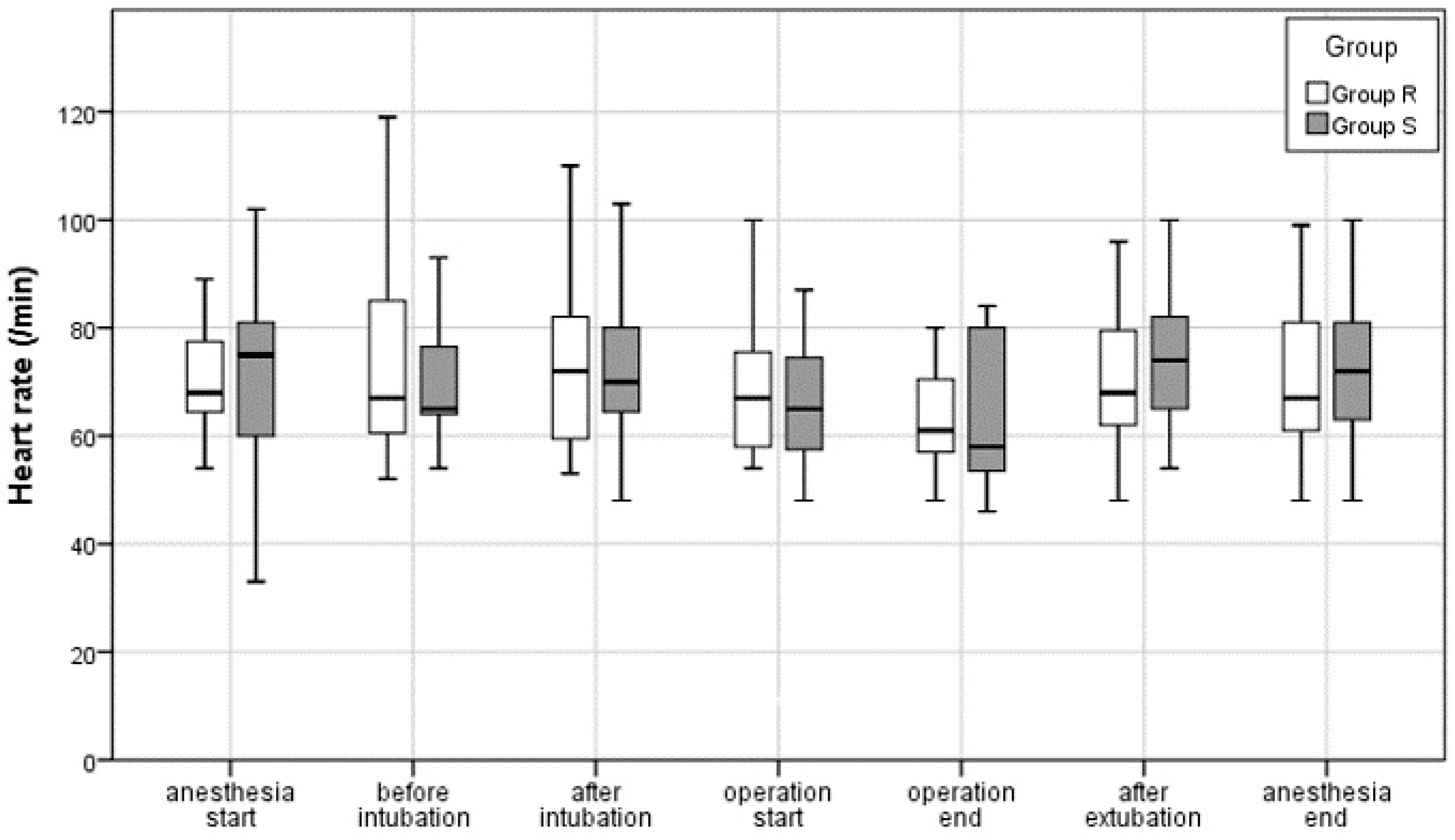
3.2.1. Intraoperative Blood Pressure and Pulse
3.2.2. Postoperative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.D., Jr.; Broderick, J.P. Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014, 13, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Abecassis, I.J.; Zeeshan, Q.; Ghodke, B.V.; Levitt, M.R.; Ellenbogen, R.G.; Sekhar, L.N. Surgical Versus Endovascular Management of Ruptured and Unruptured Intracranial Aneurysms: Emergent Issues and Future Directions. World Neurosurg. 2020, 136, 17–27. [Google Scholar] [CrossRef]
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Leslie, K.; Mitchell, P. Anaesthesia for endovascular treatment of cerebral aneurysms. J. Clin. Neurosci. 2004, 11, 468–470. [Google Scholar] [CrossRef]
- Varma, M.K.; Price, K.; Jayakrishnan, V.; Manickam, B.; Kessell, G. Anaesthetic considerations for interventional neuroradiology. Br. J. Anaesth. 2007, 99, 75–85. [Google Scholar] [CrossRef]
- Hoff, R.G.; GW, V.A.N.D.; Mettes, S.; Verweij, B.H.; Algra, A.; Rinkel, G.J.; Kalkman, C.J. Hypotension in anaesthetized patients during aneurysm clipping: Not as bad as expected? Acta Anaesthesiol. Scand. 2008, 52, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Sneyd, J.R.; Gambus, P.L.; Rigby-Jones, A.E. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: A narrative review. Br. J. Anaesth. 2021, 127, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, M.H.; Kong, H.J.; Shin, H.J.; Yang, S.; Kim, N.Y.; Chae, D. Effects of Remimazolam vs. Sevoflurane Anesthesia on Intraoperative Hemodynamics in Patients with Gastric Cancer Undergoing Robotic Gastrectomy: A Propensity Score-Matched Analysis. J. Clin. Med. 2022, 11, 2643. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lai, T.; Chen, J.; Lu, Y.; He, F.; Chen, Y.; Xie, Y. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: A randomized, double-blind, controlled trial. Pharmacol. Res. Perspect. 2021, 9, e00851. [Google Scholar] [CrossRef]
- Rex, D.K.; Bhandari, R.; Lorch, D.G.; Meyers, M.; Schippers, F.; Bernstein, D. Safety and efficacy of remimazolam in high risk colonoscopy: A randomized trial. Dig. Liver Dis. 2021, 53, 94–101. [Google Scholar] [CrossRef]
- Teixeira, M.T.; Brinkman, N.J.; Pasternak, J.J.; Abcejo, A.S. The Role of Remimazolam in Neurosurgery and in Patients with Neurological Diseases: A Narrative Review. J. Neurosurg. Anesthesiol. 2023, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; von Oettingen, G.; Larsen, K.M.; Landsfeldt, U.; Jensen, K.A.; Nielsen, E.; Cold, G.E. Effects of sevoflurane on intracranial pressure, cerebral blood flow and cerebral metabolism. A dose-response study in patients subjected to craniotomy for cerebral tumours. Acta Anaesthesiol. Scand. 1998, 42, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, L.; Stoian, A.; Rimmele, T.; Allaouchiche, B.; Chassard, D.; Boselli, E. Optimal remifentanil dosage for providing excellent intubating conditions when co-administered with a single standard dose of propofol. Anaesthesia 2009, 64, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, G.; Hirata, N.; Yoshikawa, Y.; Yamakage, M. Differential effects of remimazolam and propofol on heart rate variability during anesthesia induction. J. Anesth. 2022, 36, 239–245. [Google Scholar] [CrossRef]
- Aoki, Y.; Kurita, T.; Nakajima, M.; Imai, R.; Suzuki, Y.; Makino, H.; Kinoshita, H.; Doi, M.; Nakajima, Y. Association between remimazolam and postoperative delirium in older adults undergoing elective cardiovascular surgery: A prospective cohort study. J. Anesth. 2023, 37, 13–22. [Google Scholar] [CrossRef]
- Kim, K.M. Remimazolam: Pharmacological characteristics and clinical applications in anesthesiology. Anesth. Pain Med. 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Xu, X.; Huang, Y.; Xue, S.; Wu, A.; Jin, X.; Wang, Q.; Lyu, J.; Wang, S.; et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: A multicentered, randomized, positive-controlled, phase III clinical trial. Am. J. Transl. Res. 2020, 12, 4594–4603. [Google Scholar]
- Rex, D.K.; Bhandari, R.; Desta, T.; DeMicco, M.P.; Schaeffer, C.; Etzkorn, K.; Barish, C.F.; Pruitt, R.; Cash, B.D.; Quirk, D.; et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest. Endosc. 2018, 88, 427–437.e6. [Google Scholar] [CrossRef]
- Kim, T.K.; Kwak, H.J.; Jung, W.S.; Choi, G.B.; Park, S.Y.; Kim, J.Y. Effects of Remimazolam Anesthesia with Two Induction Doses on Hemodynamics and Recovery Profile in Older Patients: Comparison with Propofol Anesthesia. J. Clin. Med. 2023, 12, 5285. [Google Scholar] [CrossRef]
- Choi, E.K.; Jang, Y.; Park, S.J. Comparison of remimazolam and propofol induction on hemodynamic response in hypertensive patients. Medicine 2023, 102, e34358. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, J.; Shan, W.; Duan, G.; Lan, H. Effects of remimazolam combined with sufentanil on hemodynamics during anesthetic induction in elderly patients with mild hypertension undergoing orthopedic surgery of the lower limbs: A randomized controlled trial. BMC Anesthesiol. 2023, 23, 311. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Yim, S.; Choi, C.I.; Park, I.; Joung, K.W.; Song, I.A. Effects of remimazolam on hemodynamic changes during cardiac ablation for atrial fibrillation under general anesthesia: A propensity-score-matched retrospective cohort study. Can. J. Anaesth. 2023, 70, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Hirata, N.; Suzuki, T.; Morisaki, H.; Morimatsu, H.; Sakamoto, A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): Results of a multicenter, randomized, double-blind, parallel-group comparative trial. J. Anesth. 2020, 34, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Watanabe, T.; Kido, K.; Kamiya, S.; Otsuki, S.; Narasaki, S.; Toyota, Y.; Kondo, T.; Horikawa, Y.T.; Saeki, N.; et al. Remimazolam Requires Less Vasopressor Support during Induction and Maintenance of General Anesthesia in Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement: A Retrospective Analysis from a Single Center. Biomed. Res. Int. 2022, 2022, 6386606. [Google Scholar] [CrossRef] [PubMed]
- Yokose, M.; Takaki, R.; Mihara, T.; Saigusa, Y.; Yamamoto, N.; Masui, K.; Goto, T. Hypotension after general anesthesia induction using remimazolam in geriatric patients: Protocol for a double-blind randomized controlled trial. PLoS ONE 2022, 17, e0275451. [Google Scholar] [CrossRef]
- Soejima, T.; Ueda, K.; Hasegawa, S.; Motoe, H.; Okada, K.; Ito, Y.M.; Hoshino, K.; Morimoto, Y. Change in cerebral circulation during the induction of anesthesia with remimazolam. J. Anesth. 2023, 37, 92–96. [Google Scholar] [CrossRef]
| Group R (n = 15) | Group S (n = 15) | |
|---|---|---|
| Demographics | ||
| Age | 61.4 ± 15.1 | 60.8 ± 11.7 |
| Sex (male, %) | 11 (73.3) | 8 (53.3) |
| ASA-PS class | 3 [3, 3] | 3 [3, 3] |
| BMI | 23.8 ± 2.6 | 24.1 ± 2.6 |
| Cardiac comorbidities | ||
| Hypertension (%) | 11 (73.3) | 8 (53.3) |
| Coronary artery disease (%) | 2 (13.3) | 2 (13.3) |
| Arrhythmia (%) | 3 (20.0) | 1 (6.7) |
| Cardiomyopathy (%) | 1 (6.7) | 0 (0.0) |
| Pulmonary comorbidities | ||
| Asthma (%) | 1 (6.7) | 0 (0.0) |
| Pneumonia (%) | 2 (13.3) | 0 (0.0) |
| Effusion in lung (%) | 1 (6.7) | 2 (13.3) |
| Current smoking (%) | 3 (20.0) | 5 (33.3) |
| Other comorbidities | ||
| Diabetes mellitus (%) | 3 (20.0) | 3 (20.0) |
| Hypothyroidism (%) | 1 (6.7) | 0 (0.0) |
| Chronic kidney disease (%) | 2 (13.3) | 1 (6.7) |
| Cerebral hemorrhage (%) | 2 (13.3) | 3 (20.0) |
| Cerebral infarction (%) | 2 (13.3) | 0 (0.0) |
| Psychologic disorder (%) | 2 (13.3) | 4 (26.7) |
| Parkinson’s disease (%) | 0 (0.0) | 1 (6.7) |
| Chronic alcoholic (%) | 1 (6.7) | 0 (0.0) |
| Location of aneurysms | ||
| Right side (%) | 8 (53.3) | 6 (40.0) |
| Left side (%) | 6 (40.0) | 10 (66.7) |
| Anterior communicating artery (%) | 3 (20.0) | 3 (20.0) |
| Posterior communication artery (%) | 1 (6.7) | 0 (0.0) |
| Anterior cerebral artery (%) | 2 (13.3) | 4 (26.7) |
| Basilar artery (%) | 4 (26.7) | 1 (6.7) |
| Vertebral artery (%) | 2 (13.3) | 0 (0.0) |
| Paraclinoid internal carotid artery (%) | 3 (20.0) | 6 (40.0) |
| Posterior inferior cerebellar artery (%) | 0 (0.0) | 1 (6.7) |
| Internal carotid artery (%) | 1 (6.7) | 2 (13.3) |
| Intraoperative data | ||
| Duration of intervention (min) | 127.5 ± 65.2 | 103.7 ± 44.2 |
| Time from the end of intervention to extubation (min) | 11.1 ± 5.6 | 12.1 ± 7.4 |
| Duration of anesthesia (min) | 185.0 ± 67.4 | 155.0 ± 38.5 |
| Total infused fluid (mL) | 520.0 ± 273.7 | 560.0 ± 452.8 |
| Total amount of remifentanil (mg) | 0.88 ± 0.72 | 0.64 ± 0.63 |
| Intraoperative events | ||
| Intraoperative awakening (%) | 0 (0.0) | 0 (0.0) |
| Thromboembolic event (%) | 0 (0.0) | 0 (0.0) |
| Hemorrhagic event (%) | 0 (0.0) | 0 (0.0) |
| Other intraoperative events (%) | 1 (6.7) | 1 (6.7) |
| Group R (n = 15) | Group S (n = 15) | p-Value | |
|---|---|---|---|
| Hypotension Events | |||
| Number of patients who experienced hypotension (%) Number of patients to whom vasoconstrictors infused (%) | 5 (33.3) | 12 (80.0) | * 0.010 |
| 2 (13.3) | 11 (73.3) | * 0.001 | |
| Amount of phenylephrine injected (mg) | 0.35 ± 1.30 | 4.35 ± 1.74 | * <0.001 |
| Amount of ephedrine injected (mg) | 1.07 ± 2.37 | 2.67 ± 4.94 | 0.268 |
| Amount of norepinephrine injected (mg) | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.000 |
| Amount of epinephrine injected (mg) | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.000 |
| Hypertension Events | |||
| Number of patients who experienced hypotension (%) Number of patients to whom vasoconstrictors infused (%) | 10 (66.7) | 11 (73.3) | 0.690 |
| 0 (0.0) | 0 (0.0) | 1.000 | |
| Amount of nicardipine injected (mg) | 1.11 ± 1.43 | 0.73 ± 0.68 | 0.499 |
| Amount of labetalol injected (mg) | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.000 |
| Amount of esmolol injected (mg) | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, E.; Je, L.G.; Kim, J.H.; Song, Y.J.; Lim, C.H. Effects of Remimazolam versus Sevoflurane on Hemodynamics in Patients Undergoing Coil Embolization of Cerebral Aneurysm: A Prospective Randomized Controlled Trial. J. Clin. Med. 2024, 13, 3958. https://doi.org/10.3390/jcm13133958
Ko E, Je LG, Kim JH, Song YJ, Lim CH. Effects of Remimazolam versus Sevoflurane on Hemodynamics in Patients Undergoing Coil Embolization of Cerebral Aneurysm: A Prospective Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(13):3958. https://doi.org/10.3390/jcm13133958
Chicago/Turabian StyleKo, Eunji, Lee Gyeong Je, Jang Hun Kim, Yeon Jae Song, and Choon Hak Lim. 2024. "Effects of Remimazolam versus Sevoflurane on Hemodynamics in Patients Undergoing Coil Embolization of Cerebral Aneurysm: A Prospective Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 13: 3958. https://doi.org/10.3390/jcm13133958
APA StyleKo, E., Je, L. G., Kim, J. H., Song, Y. J., & Lim, C. H. (2024). Effects of Remimazolam versus Sevoflurane on Hemodynamics in Patients Undergoing Coil Embolization of Cerebral Aneurysm: A Prospective Randomized Controlled Trial. Journal of Clinical Medicine, 13(13), 3958. https://doi.org/10.3390/jcm13133958






