Is Type and Grade of Emphysema Important for Bone Mineral Density and Aortic Calcifications?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Analysis of Imaging Data
2.2.1. Lung Parenchyma Analysis
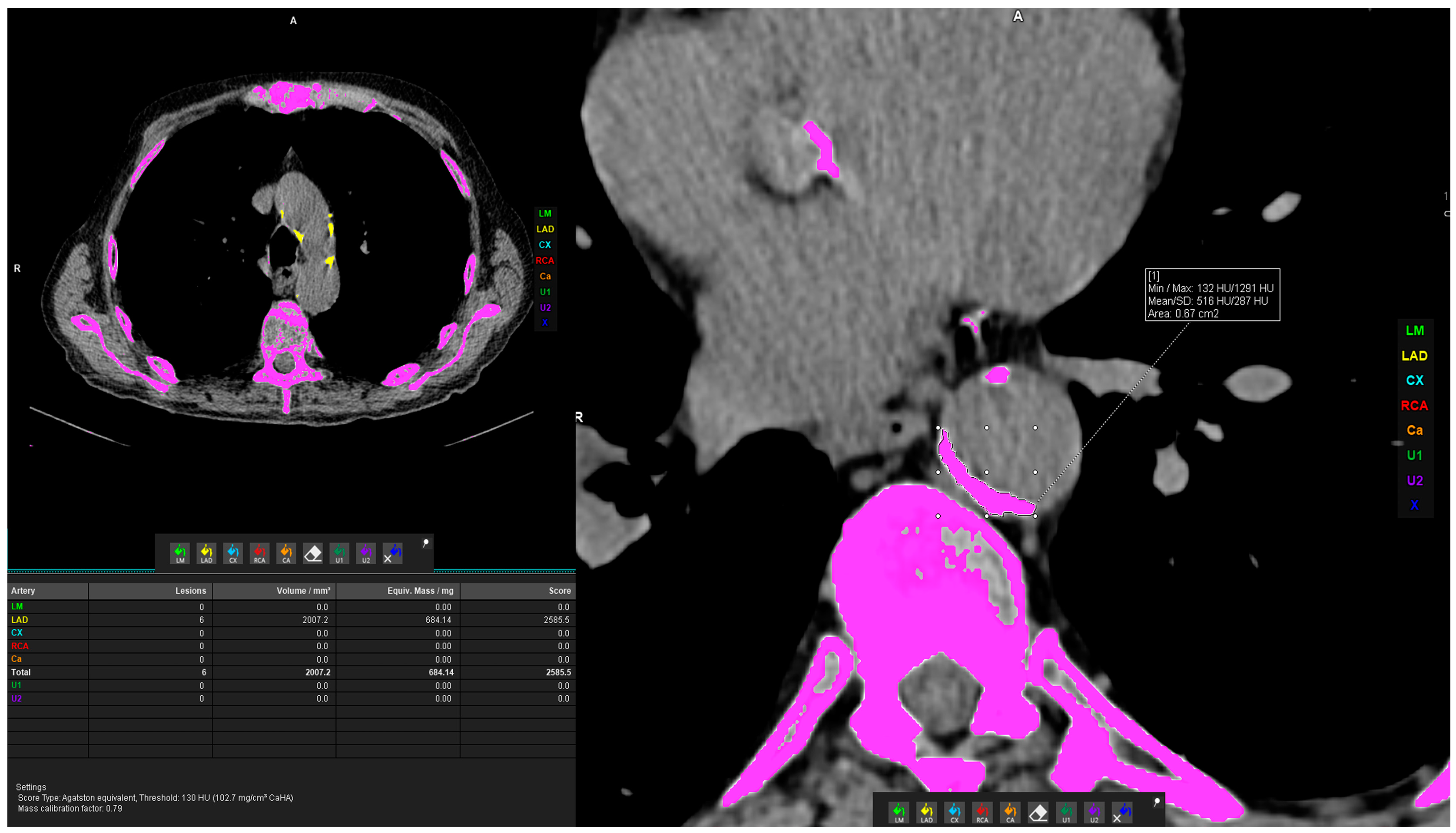
2.2.2. Vascular Calcification Measurement
2.2.3. Bone Density Measurement
2.2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kemp, S.V.; Polkey, M.I.; Shah, P.L. The Epidemiology, Etiology, Clinical Features, and Natural History of Emphysema. Thorac. Surg. Clin. 2009, 19, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Gredic, M.; Karnati, S.; Ruppert, C.; Guenther, A.; Avdeev, S.N.; Kosanovic, D. Combined Pulmonary Fibrosis and Emphysema: When Scylla and Charybdis Ally. Cells 2023, 12, 1278. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.P.; Walters, J.A.E.; Walters, E.H. Diagnosis and early detection of COPD using spirometry. J. Thorac. Dis. 2014, 6, 1557–1569. [Google Scholar] [PubMed]
- Kahnert, K.; Jörres, R.A.; Behr, J.; Welte, T. The Diagnosis and Treatment of COPD and Its Comorbidities. Dtsch. Arzteblatt Int. 2023, 120, 434–444. [Google Scholar] [CrossRef]
- Manian, P. Chronic obstructive pulmonary disease classification, phenotypes and risk assessment. J. Thorac. Dis. 2019, 11, S1761–S1766. [Google Scholar] [CrossRef] [PubMed]
- Brassington, K.; Selemidis, S.; Bozinovski, S.; Vlahos, R. Chronic obstructive pulmonary disease and atherosclerosis: Common mechanisms and novel therapeutics. Clin. Sci. 2022, 136, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, A.; Macura, K.J. Rola tomografii komputerowej w ilościowej ocenie rozedmy płuc. Pol. J. Radiol. 2012, 77, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Ricci, M.; Rosso, A.; Flacco, M.E.; Manzoli, L. Chronic Obstructive Pulmonary Disease Overdiagnosis and Overtreatment: A Meta-Analysis. J. Clin. Med. 2023, 12, 6978. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, A.B.; Sanchez-Salcedo, P.; Bastarrika, G.; Campo, A.; Berto, J.; Ocon, M.D.M.; Fernandez-Montero, A.; Celli, B.R.; Zulueta, J.J.; de-Torres, J.P. Clinical Features of Smokers with Radiological Emphysema But without Airway Limitation. Chest 2017, 151, 358–365. [Google Scholar] [CrossRef]
- Mariniello, D.F.; D’Agnano, V.; Cennamo, D.; Conte, S.; Quarcio, G.; Notizia, L.; Pagliaro, R.; Schiattarella, A.; Salvi, R.; Bianco, A.; et al. Comorbidities in COPD: Current and Future Treatment Challenges. J. Clin. Med. 2024, 13, 743. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Fukucji, Y.; Jenkins, C.; Rodriguez-Roisin, R.; van Weel, C.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef] [PubMed]
- De Miguel-Díez, J.; Fernández-Villar, A.; Doña Díaz, E.; Padilla Bernáldez, M.; Trillo-Calvo, E.; Molina París, J.; Barrecheguren, M.; Perez, J.M.V.; Ramirez Prieto, M.T. Chronic Obstructive Lung Disease: Treatment Guidelines and Recommendations for Referral and Multidisciplinary Continuity of Care. J. Clin. Med. 2024, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Sabit, R.; Bolton, C.E.; Edwards, P.H.; Pettit, R.J.; Evans, W.D.; McEniery, C.M.; Wilkinson, I.B.; Cockcroft, J.R.; Shale, D.J. Arterial Stiffness and Osteoporosis in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 175, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Bone three-dimensional microstructural features of the common osteoporotic fracture sites. World J. Orthop. 2014, 5, 486. [Google Scholar] [CrossRef]
- Bitar, A.N.; Syed Sulaiman, S.A.; Ali, I.A.H.; Khan, I.; Khan, A.H. Osteoporosis among Patients with Chronic Obstructive Pulmonary Disease: Systematic Review and Meta-analysis of Prevalence, Severity, and Therapeutic Outcomes. J. Pharm. Bioallied Sci. 2019, 11, 310–320. [Google Scholar] [PubMed]
- Corbi, G.; Bianco, A.; Turchiarelli, V.; Cellurale, M.; Fatica, F.; Daniele, A.; Mazzarella, G.; Ferrara, N. Potential Mechanisms Linking Atherosclerosis and Increased Cardiovascular Risk in COPD: Focus on Sirtuins. Int. J. Mol. Sci. 2013, 14, 12696–12713. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Romei, C.; Marzi, C.; Diciotti, S.; Picozzi, G.; Pistelli, F.; Zappa, M.; Paci, E.; Carozzi, F.; Gorini, G.; et al. Pulmonary emphysema and coronary artery calcifications at baseline LDCT and long-term mortality in smokers and former smokers of the ITALUNG screening trial. Eur. Radiol. 2023, 33, 3115–3123. [Google Scholar] [CrossRef] [PubMed]
- Papaporfyriou, A.; Bartziokas, K.; Gompelmann, D.; Idzko, M.; Fouka, E.; Zaneli, S.; Bakakos, P.; Loukides, S.; Papaioannou, A.I. Cardiovascular Diseases in COPD: From Diagnosis and Prevalence to Therapy. Life 2023, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Romme, E.A.P.M.; McAllister, D.A.; Murchison, J.T.; Van Beek, E.J.R.; Petrides, G.S.; Price, C.O.S.; Rutten, E.P.A.; Smeenk, F.W.J.M.; Wouters, E.F.M.; MacNee, W. Associations between COPD related manifestations: A cross-sectional study. Respir. Res. 2013, 14, 129. [Google Scholar] [CrossRef]
- de Jong, W.U.; de Jong, P.A.; Vliegenthart, R.; Isgum, I.; Lammers, J.W.J.; Oudkerk, M.; van der Aalst, C.; de Koning, H.J.; Hoesein, F.A.M. Association of chronic obstructive pulmonary disease and smoking status with bone density and vertebral fractures in male lung cancer screening participants. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 2224–2229. [Google Scholar] [CrossRef]
- Rentzeperi, E.; Pegiou, S.; Tsakiridis, I.; Kalogiannidis, I.; Kourtis, A.; Mamopoulos, A.; Athanasiadis, A.; Dagklis, T. Diagnosis and Management of Osteoporosis: A Comprehensive Review of Guidelines. Obstet. Gynecol. Surv. 2023, 78, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Watanabe, R.; Okazaki, R. COPD and osteoporosis: Links, risks, and treatment challenges. Int. J. Chron. Obstruct Pulmon Dis. 2016, 11, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, H.; Zhao, L.; Wang, J. Osteoporosis in COPD patients: Risk factors and pulmonary rehabilitation. Clin. Respir. J. 2022, 16, 487–496. [Google Scholar] [CrossRef]
- Lynch, D.A.; Austin, J.H.M.; Hogg, J.C.; Grenier, P.A.; Kauczor, H.U.; Bankier, A.A.; Barr, R.G.; Colby, T.V.; Galvin, J.R.; Gevenois, P.A.; et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society. Radiology 2015, 277, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Hirai, T.; Muro, S.; Haruna, A.; Terada, K.; Kinose, D.; Marumo, S.; Ogawa, E.; Hoshino, Y.; Niimi, A.; et al. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest 2008, 134, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, J.D.; Wilson, C.; Stinson, D.S.; Lynch, D.A.; Bowler, R.P.; Lutz, S.; Bonn, J.M.; Arnold, B.; McDonald, M.N.; Washko, G.R.; et al. Reduced Bone Density and Vertebral Fractures in Smokers, men and COPD Patients at Increased Risk. Ann. Am. Thorac. Soc. 2015, 12, 648–656. [Google Scholar] [CrossRef]
- Kutleša, Z.; Ordulj, I.; Perić, I.; Jerković, K.; Poljak, D.; Gavrilović, V.; Čapkun, V.; Devčić, Š.; Budimir Mršić, D. Opportunistic measures of bone mineral density at multiple skeletal sites during whole-body CT in polytrauma patients. Osteoporos. Int. 2023, 34, 775–782. [Google Scholar] [CrossRef]
- Hwang, H.J.; Lee, S.M.; Seo, J.B.; Kim, J.E.; Choi, H.Y.; Kim, N.; Lee, J.S.; Lee, S.W.; Oh, Y.M. Quantitative Vertebral Bone Density Seen on Chest CT in Chronic Obstructive Pulmonary Disease Patients: Association with Mortality in the Korean Obstructive Lung Disease Cohort. Korean J. Radiol. 2020, 21, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Li, H.; Zhu, S. An Overlooked Bone Metabolic Disorder: Cigarette Smoking-Induced Osteoporosis. Genes. 2022, 13, 806. [Google Scholar] [CrossRef]
- Dransfield, M.T.; Huang, F.; Nath, H.; Singh, S.P.; Bailey, W.C.; Washko, G.R. CT emphysema predicts thoracic aortic calcification in smokers with and without COPD. COPD J. Chronic Obstr. Pulm. Dis. 2010, 7, 404–410. [Google Scholar] [CrossRef]
- Morgan, A.D.; Zakeri, R.; Quint, J.K. Defining the relationship between COPD and CVD: What are the implications for clinical practice? Ther. Adv. Respir. Dis. 2018, 12, 1753465817750524. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Luo, F.; Ruan, G.; Peng, R.; Li, X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Perone, F.; Guglielmo, M.; Coceani, M.; La Mura, L.; Dentamaro, I.; Sabatino, J.; Gimelli, A. The Role of Multimodality Imaging Approach in Acute Aortic Syndromes: Diagnosis, Complications, and Clinical Management. Diagnostics 2023, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Vasilescu, D.M.; Hague, C.J.; Ikezoe, K.; Murphy, D.T.; Kirby, M.; Stevenson, C.S.; Verleden, S.E.; Vanaudenaerde, B.M.; Gayan-Ramirez, G.; et al. Pathological Comparisons of Paraseptal and Centrilobular Emphysema in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 202, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Pompe, E.; de Jong, P.A.; van Rikxoort, E.M.; Gallardo Estrella, L.; de Jong, W.U.; Vliegenthart, R.; Oudkerk, M.; van der Aalst, C.M.; van Ginneken, B.; Lammers, J.J.; et al. Smokers with emphysema and small airway disease on computed tomography have lower bone density. Int. J. Chron. Obstruct Pulmon Dis. 2016, 11, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- van Dort, M.J.; Driessen, J.H.M.; Geusens, P.; Romme, E.P.M.; Smeenk, F.W.J.M.; Wouters, E.F.M.; van den Bergh, J.P.W. Vertebral bone attenuation in Hounsfield Units and prevalent vertebral fractures are associated with the short-term risk of vertebral fractures in current and ex-smokers with and without COPD: A 3-year chest CT follow-up study. Osteoporos. Int. 2019, 30, 1561–1571. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Alish, C.; Pereira, S.L.; Deutz, N.E.; Heyland, D.K.; Goodpaster, B.H.; Tappenden, K.A.; Heymsfield, S.B. Implications of low muscle mass across the continuum of care: A narrative review. Ann. Med. 2018, 50, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.C.; Bon, J.M.; Mason, S.; Diaz, A.A.; Lutz, S.M.; Estepar, R.S.J.; Kinney, G.L.; Hokanson, J.E.; Rennard, S.I.; Casaburi, R.; et al. Increased chest CT derived bone and muscle measures capture markers of improved morbidity and mortality in COPD. Respir. Res. 2022, 23, 311. [Google Scholar] [CrossRef]
- Henrot, P.; Dupin, I.; Schilfarth, P.; Esteves, P.; Blervaque, L.; Zysman, M.; Gouzi, F.; Hayot, M.; Pomiès, P.; Berger, P. Main Pathogenic Mechanisms and Recent Advances in COPD Peripheral Skeletal Muscle Wasting. Int. J. Mol. Sci. 2023, 24, 6454. [Google Scholar] [CrossRef]
- Fischer, A.M.; Varga-Szemes, A.; van Assen, M.; Griffith, L.P.; Sahbaee, P.; Sperl, J.I.; Nance, J.W.; Schoepf, U.J. Comparison of Artificial Intelligence-Based Fully Automatic Chest CT Emphysema Quantification to Pulmonary Function Testing. AJR Am. J. Roentgenol. 2020, 214, 1065–1071. [Google Scholar] [CrossRef]
- Saad, M.M.; Bayoumy, A.A.; EL-Nisr, M.M.; Zaki, N.M.; Khalil, T.H.; ELSerafi, A.F. Assessment of artificial intelligence-aided chest computed tomography in diagnosis of chronic obstructive airway disease: An observational study. Egypt. J. Radiol. Nucl. Med. 2023, 54, 97. [Google Scholar] [CrossRef]
- Zhong, Z.; Yang, W.; Zhu, C.; Wang, Z. Role and progress of artificial intelligence in radiodiagnosing vascular calcification: A narrative review. Ann. Transl. Med. 2023, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Guilenea, F.N.; Casciaro, M.E.; Pascaner, A.F.; Soulat, G.; Mousseaux, E.; Craiem, D. Thoracic Aorta Calcium Detection and Quantification Using Convolutional Neural Networks in a Large Cohort of Intermediate-Risk Patients. Tomogr. Ann. Arbor. Mich. 2021, 7, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.; Liu, R.W.; Makmur, A.; Low, X.Z.; Sng, W.J.; Tan, J.H.; Kumar, N.; Hallinan, J.T.P.D. Artificial Intelligence Applications for Osteoporosis Classification Using Computed Tomography. Bioengineering 2023, 10, 1364. [Google Scholar] [CrossRef]




| Total Sample | TI ≥ 0.7 (n = 96) | TI < 0.7 (n = 104) | p Value | ||
|---|---|---|---|---|---|
| Demographic Parameters and Habits | Gender | 0.229 * | |||
| Male | 134 (67%) | 60 (62.5%) | 74 (71.2%) | ||
| Female | 66 (33%) | 36 (37.5%) | 30 (28.8%) | ||
| Age; years, median (IQR) | 64 (12) | 62 (12) | 67 (11.5) | 0.001 # | |
| Smoking | 0.277 * | ||||
| Active | 117 (58.5%) | 61 (65.6%) | 56 (54.4%) | ||
| Former | 67 (33.5%) | 27 (29%) | 40 (38.8%) | ||
| Non-smoker | 12 (6%) | 5 (5.4%) | 7 (6.8%) | ||
| Missing data | 4 (2%) | ||||
| Cigarette number/day | 20 (10) | 20 (10) | 20 (20) | 0.256 # | |
| Clinical Parameters | Arterial hypertension | 87 (43.5%) | 39 (40.6%) | 48 (46.2%) | 0.446 * |
| Diabetes | 23 (11.5%) | 13 (13.5%) | 10 (9.6%) | 0.388 * | |
| Hypercholesterolemia | 47 (23.5%) | 26 (27.1%) | 21 (20.2%) | 0.5 * | |
| Exacerbations | 40 (20%) | 12 (12.4%) | 28 (27.2%) | 0.013 * | |
| Oral corticosteroids | 42 (21%) | 17 (17.5%) | 25 (24.3%) | 0.298 * | |
| Anti-osteoporotic therapy | 14 (7%) | 8 (8.2%) | 6 (5.8%) | 0.585 * | |
| Radiological Parameters | Emphysema subtype | ||||
| Trace centrilobular | 32 (16%) | 22 (22.9%) | 10 (9.6%) | 0.012 * | |
| Mild centrilobular | 18 (9%) | 10 (10.4%) | 8 (7.7%) | 0.623 * | |
| Moderate centrilobular | 22 (11%) | 9 (9.4%) | 13 (12.5%) | 0.507 * | |
| Confluent centrilobular | 63 (31.5%) | 29 (30.2%) | 34 (32.7%) | 0.762 * | |
| Advanced destructive centrilobular | 45 (22.5%) | 11 (11.5%) | 34 (32.7%) | <0.001 * | |
| Mild paraseptal | 49 (24.5%) | 27 (28.1%) | 22 (21.2%) | 0.324 * | |
| Substantial paraseptal | 71 (35.5%) | 30 (31.3%) | 41 (39.4%) | 0.240 * | |
| Panlobular | 0 | 0 | 0 | - | |
| Total LAV%, median (IQR) | 4.3 (12.4) | 2.1 (3.7) | 12.4 (19.4) | <0.001 # | |
| Left lung LAV%, median (IQR) | 4.2 (12.25) | 1.8 (3.4) | 12.5 (17.7) | <0.001 # | |
| Right lung LAV%, median (IQR) | 3.9 (13) | 2.1 (3.1) | 12.05 (19.6) | <0.001 # | |
| TI ≥ 0.7 | TI < 0.7 | p Value | |
|---|---|---|---|
| Bone density of Th4 (HU), mean ± SD | 189.9 ± 48.8 | 165.7 ± 46.38 | 0.001 * |
| Bone density of Th8 (HU), mean ± SD | 157.0 ± 45.8 | 130.6 ± 36.2 | <0.001 * |
| Bone density of L1 (HU), mean ± SD | 125.4 ± 37.9 | 99.8 ± 31.8 | <0.001 * |
| Thoracic aorta calcifications (mm3), median (IQR) | 410 (1648.3) | 1531 (3219.2) | 0.001 # |
| Total LAV% | Left Lung LAV% | Right Lung LAV% | |
|---|---|---|---|
| Bone density of Th4 (HU) | Rho = −0.18, p = 0.012 | Rho = −0.162, p = 0.024 | Rho = −0.182, p = 0.011 |
| Bone density of Th8 (HU) | Rho = −0.204, p = 0.004 | Rho = −0.207, p = 0.004 | Rho = −0.189, p = 0.008 |
| Bone density of L1 (HU) | Rho = −0.219, p = 0.002 | Rho = −0.226, p = 0.002 | Rho = −0.193, p = 0.007 |
| Thoracic aorta calcifications (vol./mm3) | Rho = 0.325, p < 0.001 | Rho = 0.333, p < 0.001 | Rho = 0.302, p < 0.001 |
| Bone Density of Th4 (HU) | Standard β | T | p Value |
|---|---|---|---|
| Age | −0.207 | −2.907 | 0.004 |
| Total LAV% | −1.725 | −1.047 | 0.297 |
| Left lung LAV% | 1.022 | 1.182 | 0.239 |
| Right lung LAV% | 0.733 | 0.856 | 0.393 |
| TI < 0.7 | −0.212 | −2.669 | 0.008 |
| R2 = 0.119 | |||
| Bone density of Th8 (HU) | Standard β | T | p Value |
| Age | −0.359 | −5.273 | 0.000 |
| Total LAV% | −0.448 | −0.284 | 0.776 |
| Left lung LAV% | 0.245 | 0.297 | 0.767 |
| Right lung LAV% | 0.219 | 0.268 | 0.789 |
| TI < 0.7 | −0.221 | −2.936 | 0.004 |
| R2 = 0.195 | |||
| Bone density of L1 (HU) | Standard β | T | p Value |
| Age | −0.406 | −6.231 | 0.000 |
| Total LAV% | −1.871 | −1.241 | 0.216 |
| Left lung LAV% | 0.933 | 1.180 | 0.239 |
| Right lung LAV% | 1.013 | 1.294 | 0.197 |
| TI < 0.7 | −0.257 | −3.565 | 0.000 |
| R2 = 0.263 | |||
| Volume of Thoracic Aorta Calcifications | Standard β | T | p Value |
|---|---|---|---|
| Age | 0.612 | 10.715 | <0.001 |
| Total LAV% | 0.900 | 0.729 | 0.467 |
| Left lung LAV% | −0.341 | −0.524 | 0.601 |
| Right lung LAV% | −0.500 | −0.781 | 0.436 |
| TI < 0.7 | 0.089 | 1.467 | 0.144 |
| Hypercholesterolemia | 0.193 | 3.502 | 0.001 |
| Arterial hypertension | 0.060 | 1.051 | 0.295 |
| R2 = 0.539 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuković, D.; Budimir Mršić, D.; Ordulj, I.; Šarić, F.; Tandara, M.; Jerković, K.; Matana, A.; Tadić, T. Is Type and Grade of Emphysema Important for Bone Mineral Density and Aortic Calcifications? J. Clin. Med. 2024, 13, 3947. https://doi.org/10.3390/jcm13133947
Vuković D, Budimir Mršić D, Ordulj I, Šarić F, Tandara M, Jerković K, Matana A, Tadić T. Is Type and Grade of Emphysema Important for Bone Mineral Density and Aortic Calcifications? Journal of Clinical Medicine. 2024; 13(13):3947. https://doi.org/10.3390/jcm13133947
Chicago/Turabian StyleVuković, Danica, Danijela Budimir Mršić, Ivan Ordulj, Frano Šarić, Mirko Tandara, Kristian Jerković, Antonela Matana, and Tade Tadić. 2024. "Is Type and Grade of Emphysema Important for Bone Mineral Density and Aortic Calcifications?" Journal of Clinical Medicine 13, no. 13: 3947. https://doi.org/10.3390/jcm13133947
APA StyleVuković, D., Budimir Mršić, D., Ordulj, I., Šarić, F., Tandara, M., Jerković, K., Matana, A., & Tadić, T. (2024). Is Type and Grade of Emphysema Important for Bone Mineral Density and Aortic Calcifications? Journal of Clinical Medicine, 13(13), 3947. https://doi.org/10.3390/jcm13133947








