Amiodarone Administration during Cardiopulmonary Resuscitation Is Not Associated with Changes in Short-Term Mortality or Neurological Outcomes in Cardiac Arrest Patients with Shockable Rhythms
Abstract
1. Introduction
2. Methods
2.1. Study Setting and Population
2.2. Recording of Data
2.3. Propensity Score Matching
2.4. Statistics
3. Results
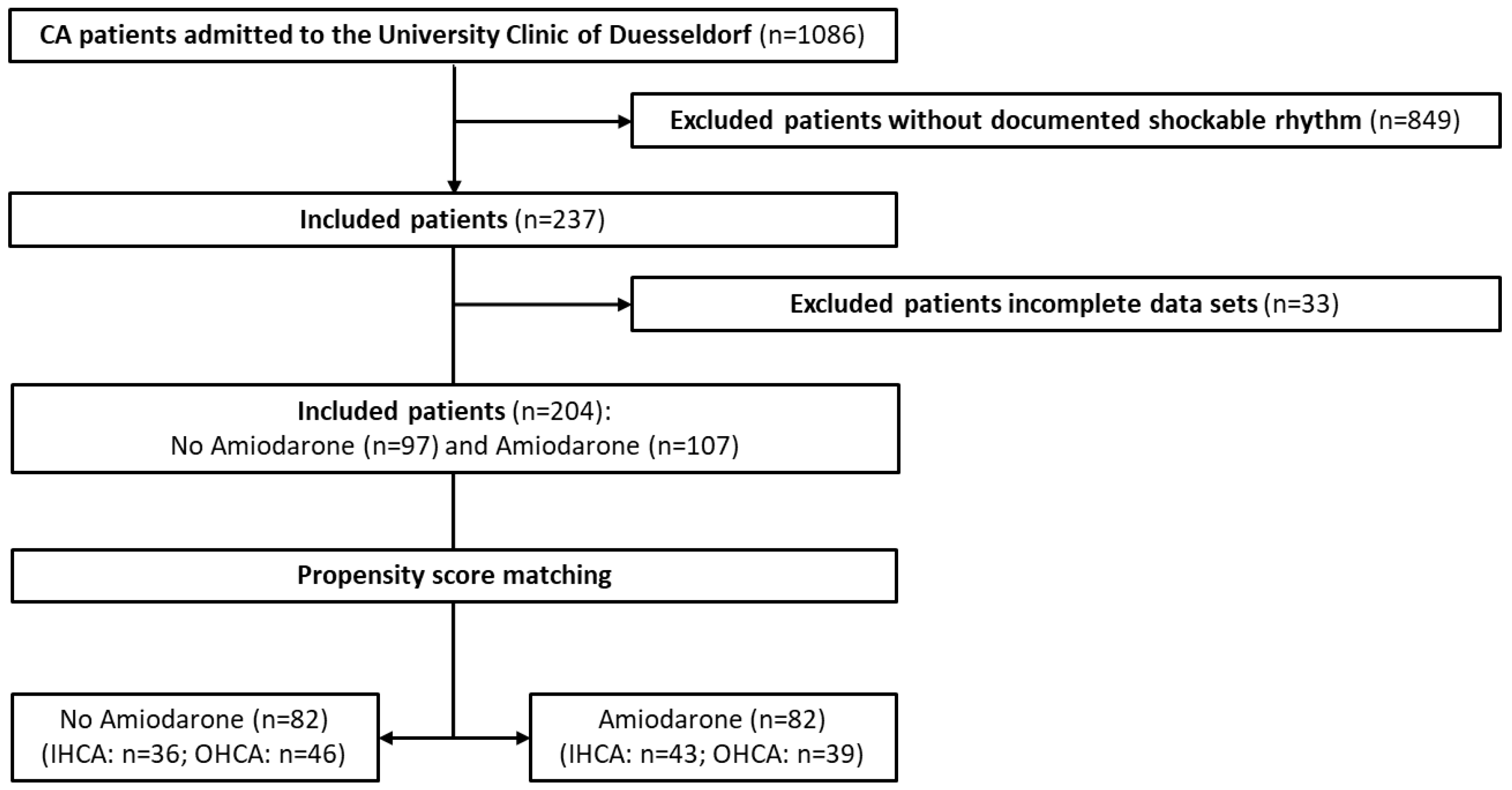
3.1. Study Patients
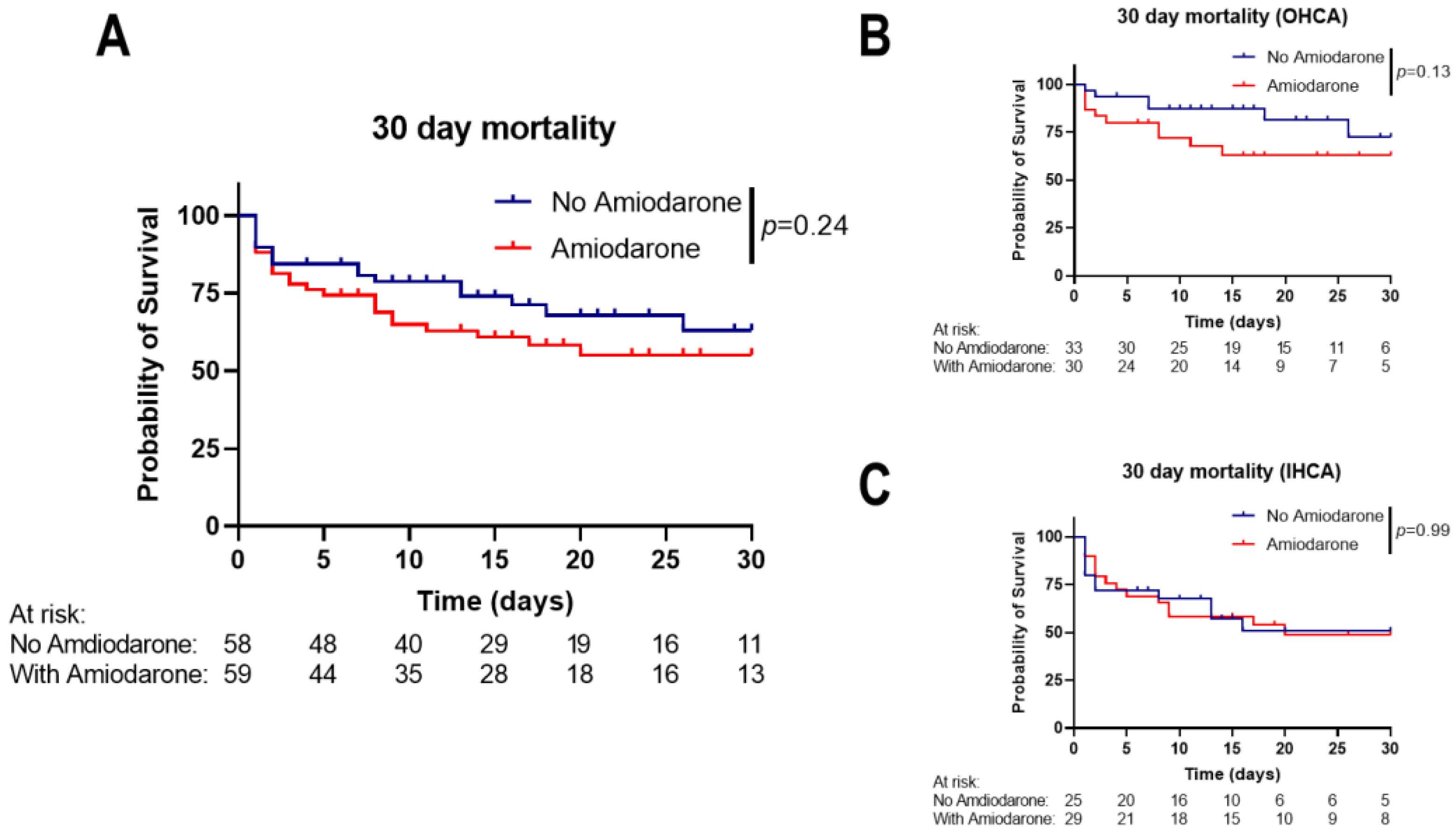
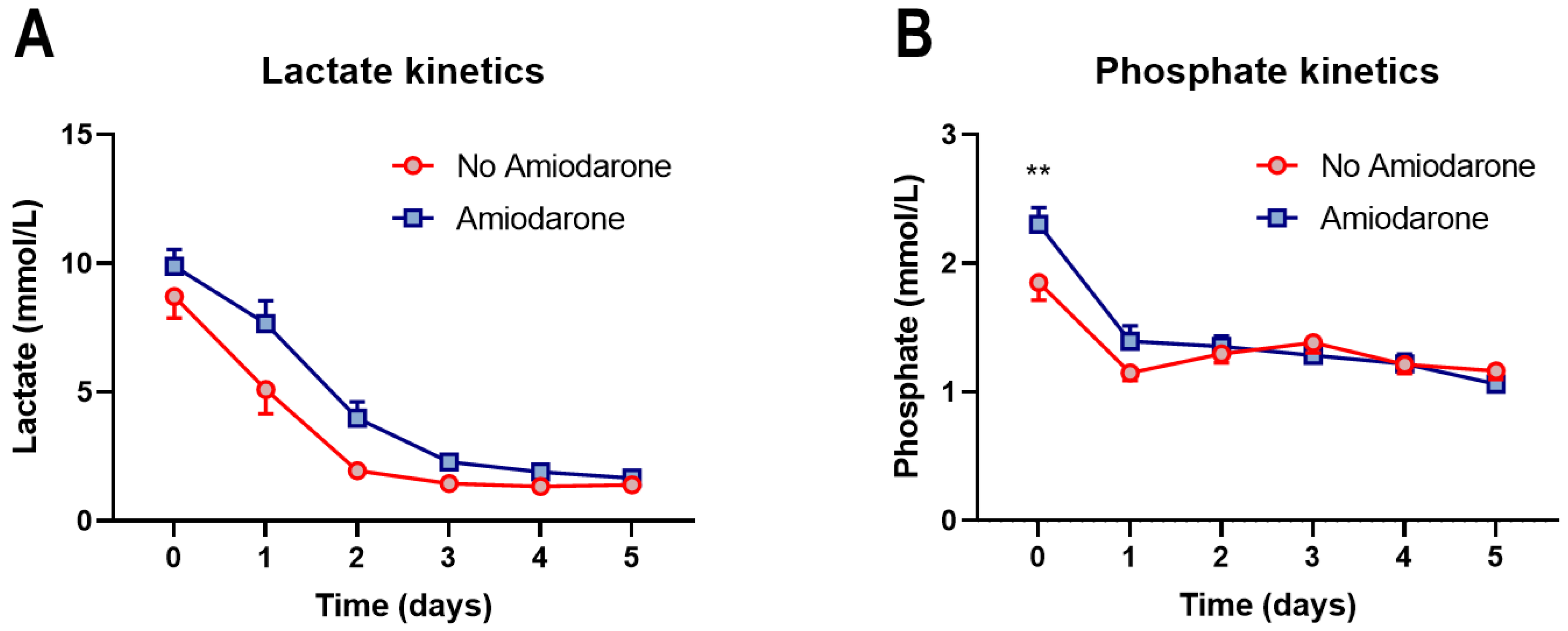
3.2. Amiodarone Administration during CPR Is Not Associated with Short-Term Mortality
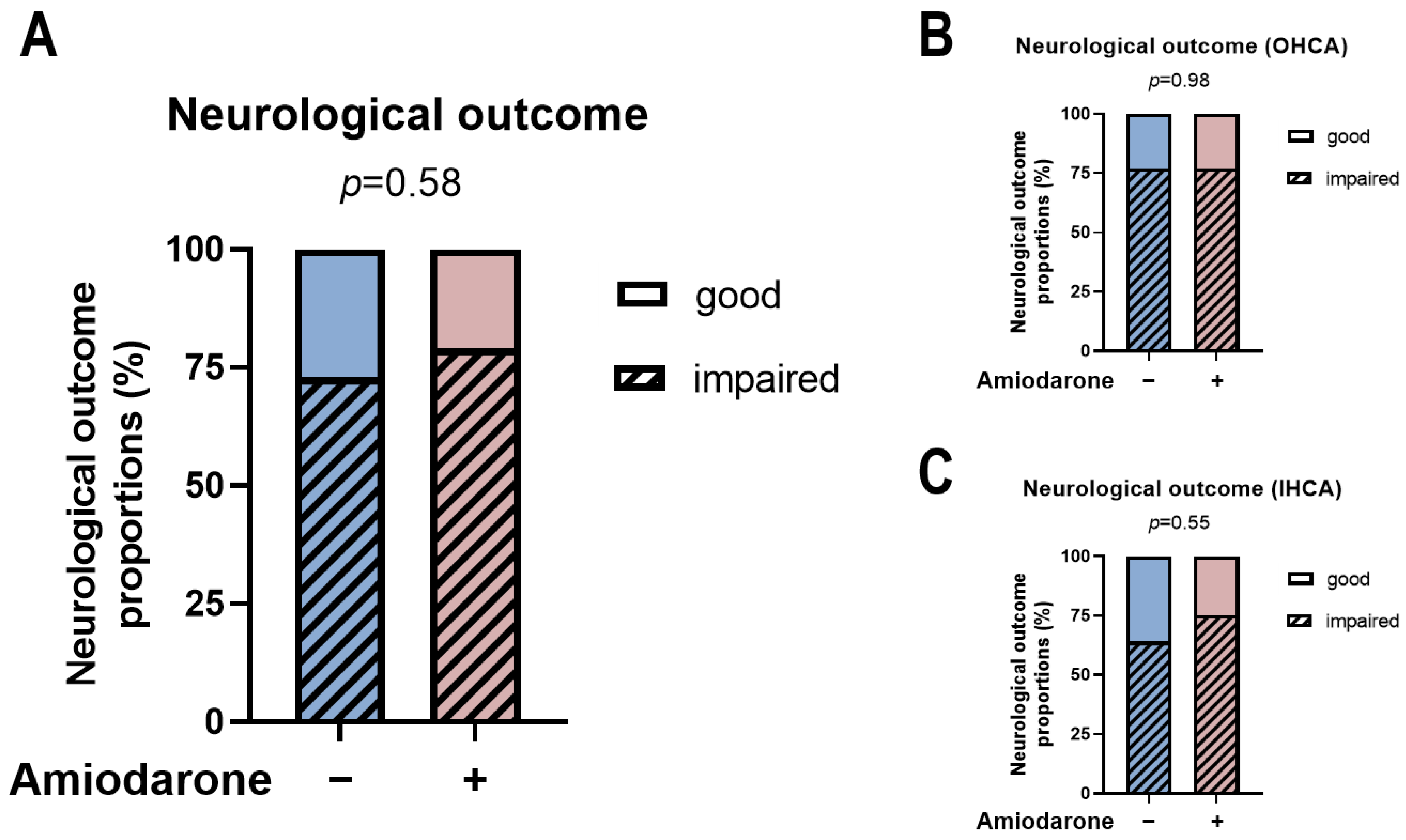
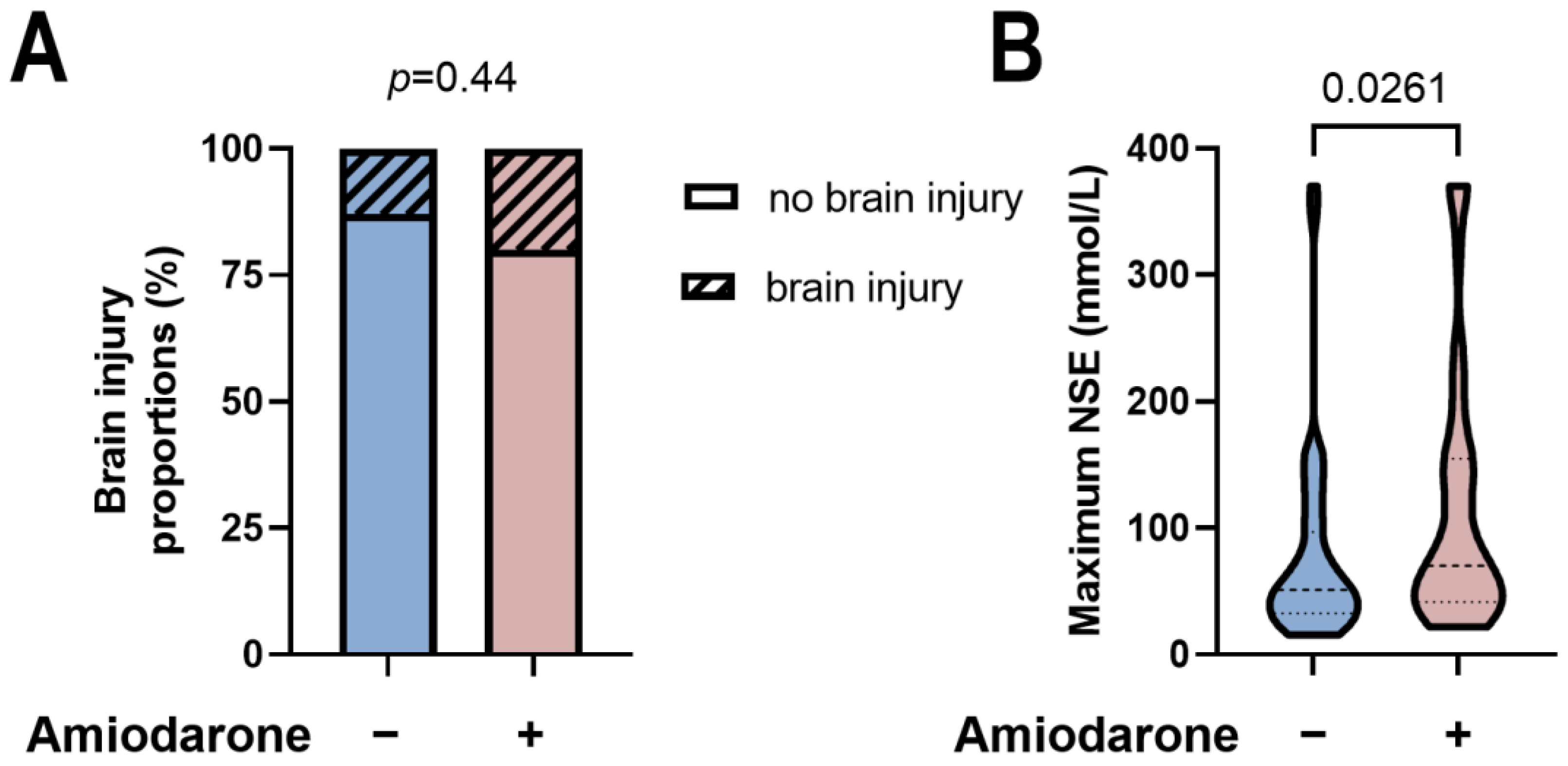
3.3. Amiodarone Administration during CPR Is Not Associated with Neurological Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adabag, A.S.; Luepker, R.V.; Roger, V.L.; Gersh, B.J. Sudden cardiac death: Epidemiology and risk factors. Nat. Rev. Cardiol. 2010, 7, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Brown, A.; Lau, D.H.; Chugh, S.S.; Albert, C.M.; Kalman, J.M.; Sanders, P. Epidemiology of Sudden Cardiac Death: Global and Regional Perspectives. Heart Lung Circ. 2019, 28, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Gan, Y.; Jiang, N.; Wang, R.; Chen, Y.; Luo, Z.; Zong, Q.; Chen, S.; Lv, C. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit. Care 2020, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Martinell, L.; Nielsen, N.; Herlitz, J.; Karlsson, T.; Horn, J.; Wise, M.P.; Undén, J.; Rylander, C. Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit. Care 2017, 21, 96. [Google Scholar] [CrossRef]
- Soloperto, R.; Magni, F.; Farinella, A.; Bogossian, E.G.; Peluso, L.; De Luca, N.; Taccone, F.S.; Annoni, F. A Comparison of Prognostic Factors in a Large Cohort of In-Hospital and Out-of-Hospital Cardiac Arrest Patients. Life 2024, 14, 403. [Google Scholar] [CrossRef]
- Bednarz, K.; Goniewicz, K.; Al-Wathinani, A.M.; Goniewicz, M. Emergency Medicine Perspectives: The Importance of Bystanders and Their Impact on On-Site Resuscitation Measures and Immediate Outcomes of Out-of-Hospital Cardiac Arrest. J. Clin. Med. 2023, 12, 6815. [Google Scholar] [CrossRef]
- Marsch, S.; Sellmann, T. Cardiopulmonary Resuscitation: Clinical Updates and Perspectives. J. Clin. Med. 2024, 13, 2717. [Google Scholar] [CrossRef]
- Nishioka, N.; Kobayashi, D.; Izawa, J.; Irisawa, T.; Yamada, T.; Yoshiya, K.; Park, C.; Nishimura, T.; Ishibe, T.; Yagi, Y.; et al. Association between serum lactate level during cardiopulmonary resuscitation and survival in adult out-of-hospital cardiac arrest: A multicenter cohort study. Sci. Rep. 2021, 11, 1639. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lee, B.K.; Jeung, K.W.; Youn, C.S.; Lee, D.H.; Lee, S.M.; Heo, T.; Min, Y.I. Prognostic value of serum phosphate level in adult patients resuscitated from cardiac arrest. Resuscitation 2018, 128, 56–62. [Google Scholar] [CrossRef]
- Duse, D.A.; Gröne, M.; Kramser, N.; Ortkemper, M.; Quast, C.; Voß, F.; Heramvand, N.; Kostev, K.; Kelm, M.; Horn, P.; et al. Elevated Initial Serum Phosphate Levels Predict Higher Mortality and Impaired Neurological Outcome in Cardiac Arrest Patients with Return of Spontaneous Circulation. Diagnostics 2023, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.R.; Wernly, B.; Binneboessel, S.; Baldia, P.; Duse, D.A.; Erkens, R.; Kelm, M.; Mamandipoor, B.; Osmani, V.; Jung, C. Failure of Lactate Clearance Predicts the Outcome of Critically Ill Septic Patients. Diagnostics 2020, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Hessulf, F.; Bhatt, D.L.; Engdahl, J.; Lundgren, P.; Omerovic, E.; Rawshani, A.; Helleryd, E.; Dworeck, C.; Friberg, H.; Redfors, B.; et al. Predicting survival and neurological outcome in out-of-hospital cardiac arrest using machine learning: The SCARS model. EBioMedicine 2023, 89, 104464. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Kondo, Y.; Sueyoshi, K.; Okamoto, K.; Tanaka, H. Early outcome prediction for out-of-hospital cardiac arrest with initial shockable rhythm using machine learning models. Resuscitation 2021, 158, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Saito, Y.; Yasufuku, Y.; Nakagomi, A.; Kitahara, H.; Kobayashi, Y.; Tahara, Y.; Yonemoto, N.; Ikeda, T.; Sato, N.; et al. Prehospital predicting factors using a decision tree model for patients with witnessed out-of-hospital cardiac arrest and an initial shockable rhythm. Sci. Rep. 2023, 13, 16180. [Google Scholar] [CrossRef] [PubMed]
- Viderman, D.; Abdildin, Y.G.; Batkuldinova, K.; Badenes, R.; Bilotta, F. Artificial Intelligence in Resuscitation: A Scoping Review. J. Clin. Med. 2023, 12, 2254. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Graesner, J.T.; Semeraro, F.; Olasveengen, T.; Soar, J.; Lott, C.; Van de Voorde, P.; Madar, J.; Zideman, D.; Mentzelopoulos, S.; et al. European Resuscitation Council Guidelines 2021: Executive summary. Resuscitation 2021, 161, 1–60. [Google Scholar] [CrossRef]
- Perkins, G.D.; Ji, C.; Deakin, C.D.; Quinn, T.; Nolan, J.P.; Scomparin, C.; Regan, S.; Long, J.; Slowther, A.; Pocock, H.; et al. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2018, 379, 711–721. [Google Scholar] [CrossRef]
- Singh, B.N.; Vaughan Williams, E.M. The effect of amiodarone, a new anti-anginal drug, on cardiac muscle. Br. J. Pharmacol. 1970, 39, 657–667. [Google Scholar] [CrossRef]
- Skrifvars, M.B.; Kuisma, M.; Boyd, J.; Määttä, T.; Repo, J.; Rosenberg, P.H.; Castren, M. The use of undiluted amiodarone in the management of out-of-hospital cardiac arrest. Acta Anaesthesiol. Scand. 2004, 48, 582–587. [Google Scholar] [CrossRef]
- Levine, J.H.; Massumi, A.; Scheinman, M.M.; Winkle, R.A.; Platia, E.V.; Chilson, D.A.; Gomes, A.; Woosley, R.L.; Intravenous Amiodarone Multicenter Trial Group. Intravenous amiodarone for recurrent sustained hypotensive ventricular tachyarrhythmias. J. Am. Coll. Cardiol. 1996, 27, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, T.; Adnet, F.; Lapandry, C. Successful resuscitation of ventricular fibrillation after low-dose amiodarone. Ann. Emerg. Med. 1998, 32, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Kudenchuk, P.J.; Cobb, L.A.; Copass, M.K.; Cummins, R.O.; Doherty, A.M.; Fahrenbruch, C.E.; Hallstrom, A.P.; Murray, W.A.; Olsufka, M.; Walsh, T. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N. Engl. J. Med. 1999, 341, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Dorian, P.; Cass, D.; Schwartz, B.; Cooper, R.; Gelaznikas, R.; Barr, A. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N. Engl. J. Med. 2002, 346, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Kudenchuk, P.J.; Leroux, B.G.; Daya, M.; Rea, T.; Vaillancourt, C.; Morrison, L.J.; Callaway, C.W.; Christenson, J.; Ornato, J.P.; Dunford, J.V.; et al. Antiarrhythmic Drugs for Nonshockable-Turned-Shockable Out-of-Hospital Cardiac Arrest: The ALPS Study (Amiodarone, Lidocaine, or Placebo). Circulation 2017, 136, 2119–2131. [Google Scholar] [CrossRef]
- Kudenchuk, P.J.; Brown, S.P.; Daya, M.; Rea, T.; Nichol, G.; Morrison, L.J.; Leroux, B.; Vaillancourt, C.; Wittwer, L.; Callaway, C.W.; et al. Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2016, 374, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Daya, M.R.; Leroux, B.G.; Dorian, P.; Rea, T.D.; Newgard, C.D.; Morrison, L.J.; Lupton, J.R.; Menegazzi, J.J.; Ornato, J.P.; Sopko, G.; et al. Survival After Intravenous Versus Intraosseous Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Shock-Refractory Cardiac Arrest. Circulation 2020, 141, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.R.; Berg, K.M.; Kudenchuk, P.J.; Del Rios, M.; Hirsch, K.G.; Link, M.S.; Kurz, M.C.; Chan, P.S.; Cabañas, J.G.; Morley, P.T.; et al. 2018 American Heart Association Focused Update on Advanced Cardiovascular Life Support Use of Antiarrhythmic Drugs During and Immediately After Cardiac Arrest: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2018, 138, e740–e749. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.; Nehme, E.; Stub, D.; Anderson, D.; Nehme, Z. The impact of time to amiodarone administration on survival from out-of-hospital cardiac arrest. Resusc. Plus 2023, 14, 100405. [Google Scholar] [CrossRef]
- Wissa, J.; Schultz, B.V.; Wilson, D.; Rashford, S.; Bosley, E.; Doan, T.N. Time to amiodarone administration and survival outcomes in refractory ventricular fibrillation. Emerg. Med. Australas. 2021, 33, 1088–1094. [Google Scholar] [CrossRef]
- Nolan, J.P.; Soar, J.; Zideman, D.A.; Biarent, D.; Bossaert, L.L.; Deakin, C.; Koster, R.W.; Wyllie, J.; Böttiger, B. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation 2010, 81, 1219–1276. [Google Scholar] [CrossRef] [PubMed]
- Monsieurs, K.G.; Nolan, J.P.; Bossaert, L.L.; Greif, R.; Maconochie, I.K.; Nikolaou, N.I.; Perkins, G.D.; Soar, J.; Truhlář, A.; Wyllie, J.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 1. Executive summary. Resuscitation 2015, 95, 1–80. [Google Scholar] [CrossRef]
- Perkins, G.D.; Jacobs, I.G.; Nadkarni, V.M.; Berg, R.A.; Bhanji, F.; Biarent, D.; Bossaert, L.L.; Brett, S.J.; Chamberlain, D.; de Caen, A.R.; et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation 2015, 132, 1286–1300. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar] [CrossRef]
- Duse, D.A.; Kelm, M.; Erkens, R. Lactate load in acute myocardial infarction: Old but gold? Int. J. Cardiol. 2024, 399, 131771. [Google Scholar] [CrossRef]
- Duse, D.A.; Voß, F.; Heyng, L.; Wolff, G.; Quast, C.; Scheiber, D.; Horn, P.; Kelm, M.; Westenfeld, R.; Jung, C.; et al. Lactate versus Phosphate as Biomarkers to Aid Mechanical Circulatory Support Decisions in Patients with Out-of-Hospital Cardiac Arrest and Return of Spontaneous Circulation. Diagnostics 2023, 13, 1523. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Grunau, B.E.; Rittenberger, J.C.; Sawyer, K.N.; Kurz, M.C.; Callaway, C.W. Association Between Duration of Resuscitation and Favorable Outcome After Out-of-Hospital Cardiac Arrest: Implications for Prolonging or Terminating Resuscitation. Circulation 2016, 134, 2084–2094. [Google Scholar] [CrossRef] [PubMed]
- Blewer, A.L.; Bigham, B.L.; Kaplan, S.; Del Rios, M.; Leary, M. Gender, Socioeconomic Status, Race, and Ethnic Disparities in Bystander Cardiopulmonary Resuscitation and Education—A Scoping Review. Healthcare 2024, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Mody, P.; Pandey, A.; Slutsky, A.S.; Segar, M.W.; Kiss, A.; Dorian, P.; Parsons, J.; Scales, D.C.; Rac, V.E.; Cheskes, S.; et al. Gender-Based Differences in Outcomes Among Resuscitated Patients With Out-of-Hospital Cardiac Arrest. Circulation 2021, 143, 641–649. [Google Scholar] [CrossRef]
- Andersen, L.W.; Bivens, M.J.; Giberson, T.; Giberson, B.; Mottley, J.L.; Gautam, S.; Salciccioli, J.D.; Cocchi, M.N.; McNally, B.; Donnino, M.W. The relationship between age and outcome in out-of-hospital cardiac arrest patients. Resuscitation 2015, 94, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Jeong, J.H.; Roh, S.-Y.; Han, K.-D.; Choi, Y.Y.; Min, K.; Shim, J.; Choi, J.-I.; Kim, Y.-H. Obesity Is Indirectly Associated with Sudden Cardiac Arrest through Various Risk Factors. J. Clin. Med. 2023, 12, 2068. [Google Scholar] [CrossRef]
- Lee, H.; Oh, J.; Kang, H.; Lim, T.H.; Ko, B.S.; Choi, H.J.; Park, S.M.; Jo, Y.H.; Lee, J.S.; Park, Y.S.; et al. Association between the body mass index and outcomes of patients resuscitated from out-of-hospital cardiac arrest: A prospective multicentre registry study. Scand. J. Trauma. Resusc. Emerg. Med. 2021, 29, 24. [Google Scholar] [CrossRef]
- Okubo, M.; Komukai, S.; Andersen, L.W.; Berg, R.A.; Kurz, M.C.; Morrison, L.J.; Callaway, C.W. Duration of cardiopulmonary resuscitation and outcomes for adults with in-hospital cardiac arrest: Retrospective cohort study. BMJ 2024, 384, e076019. [Google Scholar] [CrossRef]
- Smith, H.; Yeung, C.; Gowing, S.; Sadek, M.; Maziak, D.; Gilbert, S.; Shamji, F.; Villeneuve, P.; Sundaresan, S.; Seely, A. A review and analysis of strategies for prediction, prevention and management of post-operative atrial fibrillation after non-cardiac thoracic surgery. J. Thorac. Dis. 2018, 10 (Suppl. S32), S3799–S3808. [Google Scholar] [CrossRef]
- Paradis, N.A.; Martin, G.B.; Rivers, E.P.; Goetting, M.G.; Appleton, T.J.; Feingold, M.; Nowak, R.M. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 1990, 263, 1106–1113. [Google Scholar] [CrossRef]
- Rojas-Salvador, C.; Moore, J.C.; Salverda, B.; Lick, M.; Debaty, G.; Lurie, K.G. Effect of controlled sequential elevation timing of the head and thorax during cardiopulmonary resuscitation on cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation 2020, 149, 162–169. [Google Scholar] [CrossRef]
- Segal, N.; Metzger, A.K.; Moore, J.C.; India, L.; Lick, M.C.; Berger, P.S.; Tang, W.; Benditt, D.G.; Lurie, K.G. Correlation of end tidal carbon dioxide, amplitude spectrum area, and coronary perfusion pressure in a porcine model of cardiac arrest. Physiol. Rep. 2017, 5, e13401. [Google Scholar] [CrossRef]
- Gough, C.J.R.; Nolan, J.P. The role of adrenaline in cardiopulmonary resuscitation. Crit. Care 2018, 22, 139. [Google Scholar] [CrossRef]
- Krejci, V.; Hiltebrand, L.B.; Sigurdsson, G.H. Effects of epinephrine, norepinephrine, and phenylephrine on microcirculatory blood flow in the gastrointestinal tract in sepsis. Crit. Care Med. 2006, 34, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Trąbka-Zawicki, A.; Tomala, M.; Zeliaś, A.; Paszek, E.; Zajdel, W.; Stępień, E.; Żmudka, K. Adaptation of global hemostasis to therapeutic hypothermia in patients with out-of-hospital cardiac arrest: Thromboelastography study. Cardiol. J. 2019, 26, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.T.; Teoh, W.Y. The Effect of Prehospital Epinephrine in Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis. Prehospital Disaster Med. 2019, 34, 532–539. [Google Scholar] [CrossRef]
- Ludwin, K.; Safiejko, K.; Smereka, J.; Nadolny, K.; Cyran, M.; Yakubtsevich, R.; Jaguszewski, M.J.; Filipiak, K.J.; Szarpak, L.; Rodríguez-Núñez, A. Systematic review and meta-analysis appraising efficacy and safety of adrenaline for adult cardiopulmonary resuscitation. Cardiol. J. 2021, 28, 279–292. [Google Scholar] [CrossRef]
- Goto, Y.; Maeda, T.; Goto, Y. Effects of prehospital epinephrine during out-of-hospital cardiac arrest with initial non-shockable rhythm: An observational cohort study. Crit. Care 2013, 17, R188. [Google Scholar] [CrossRef]
- Lupton, J.R.; Neth, M.R.; Sahni, R.; Jui, J.; Wittwer, L.; Newgard, C.D.; Daya, M.R. Survival by time-to-administration of amiodarone, lidocaine, or placebo in shock-refractory out-of-hospital cardiac arrest. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2023, 30, 906–917. [Google Scholar] [CrossRef]
- Smuszkiewicz, P.; Jawień, N.; Szrama, J.; Lubarska, M.; Kusza, K.; Guzik, P. Admission Lactate Concentration, Base Excess, and Alactic Base Excess Predict the 28-Day Inward Mortality in Shock Patients. J. Clin. Med. 2022, 11, 6125. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Lactic acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef]
- Haas, S.A.; Lange, T.; Saugel, B.; Petzoldt, M.; Fuhrmann, V.; Metschke, M.; Kluge, S. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med. 2016, 42, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Cavus, E.; Bein, B.; Dörges, V.; Stadlbauer, K.H.; Wenzel, V.; Steinfath, M.; Hanss, R.; Scholz, J. Brain tissue oxygen pressure and cerebral metabolism in an animal model of cardiac arrest and cardiopulmonary resuscitation. Resuscitation 2006, 71, 97–106. [Google Scholar] [CrossRef]
- Magliocca, A.; Olivari, D.; De Giorgio, D.; Zani, D.; Manfredi, M.; Boccardo, A.; Cucino, A.; Sala, G.; Babini, G.; Ruggeri, L.; et al. LUCAS Versus Manual Chest Compression During Ambulance Transport: A Hemodynamic Study in a Porcine Model of Cardiac Arrest. J. Am. Heart Assoc. 2019, 8, e011189. [Google Scholar] [CrossRef] [PubMed]
- Broman, M.; Wilsson, A.M.J.; Hansson, F.; Klarin, B. Analysis of Hypo- and Hyperphosphatemia in an Intensive Care Unit Cohort. Anesth. Analg. 2017, 124, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Dundar, Z.D.; Cander, B.; Gul, M.; Karabulut, K.U.; Kocak, S.; Girisgin, S.; Mehmetoglu, I.; Toy, H. Serum intestinal fatty acid binding protein and phosphate levels in the diagnosis of acute intestinal ischemia: An experimental study in rabbits. J. Emerg. Med. 2012, 42, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Cerullo, P.; Brancaccio, P.; Anzilotti, S.; Vinciguerra, A.; Cuomo, O.; Fiorino, F.; Severino, B.; Di Vaio, P.; Di Renzo, G.; Annunziato, L.; et al. Acute and long-term NCX activation reduces brain injury and restores behavioral functions in mice subjected to neonatal brain ischemia. Neuropharmacology 2018, 135, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, G.; Sirabella, R.; Anzilotti, S.; Di Renzo, G.; Annunziato, L. Does Na+/Ca2+ exchanger, NCX, represent a new druggable target in stroke intervention? Transl. Stroke Res. 2014, 5, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Tamura, M.; Itoh, Y.; Ukai, Y. Cerebroprotective action of a Na+/Ca2+ channel blocker NS-7. I. Effect on the cerebral infarction and edema at the acute stage of permanent middle cerebral artery occlusion in rats. Brain Res. 2001, 890, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kotoda, M.; Hishiyama, S.; Ishiyama, T.; Mitsui, K.; Matsukawa, T. Amiodarone exacerbates brain injuries after hypoxic-ischemic insult in mice. BMC Neurosci. 2019, 20, 62. [Google Scholar] [CrossRef]
- Gemba, T.; Ninomiya, M.; Matsunaga, K.; Ueda, M. Effects of a novel calcium antagonist, S-(+)-methyl-4,7-dihydro-3-isobutyl-6- methyl-4-(3-nitrophenyl)thieno [2,3-b]pyridine-5-carboxylate (S-312-d), on ischemic amino acid release and neuronal injury in stroke-prone spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 1993, 265, 463–467. [Google Scholar]
- Matsumori, A.; Ono, K.; Nishio, R.; Nose, Y.; Sasayama, S. Amiodarone inhibits production of tumor necrosis factor-alpha by human mononuclear cells: A possible mechanism for its effect in heart failure. Circulation 1997, 96, 1386–1389. [Google Scholar] [CrossRef]
- Sandroni, C.; D’Arrigo, S.; Cacciola, S.; Hoedemaekers, C.W.E.; Kamps, M.J.A.; Oddo, M.; Taccone, F.S.; Di Rocco, A.; Meijer, F.J.A.; Westhall, E.; et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: A systematic review. Intensive Care Med. 2020, 46, 1803–1851. [Google Scholar] [CrossRef] [PubMed]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M.; et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132, S465–S482. [Google Scholar] [CrossRef]
- Wang, C.H.; Chang, W.T.; Su, K.I.; Huang, C.H.; Tsai, M.S.; Chou, E.; Lu, T.C.; Chen, W.J.; Lee, C.C.; Chen, S.C. Neuroprognostic accuracy of blood biomarkers for post-cardiac arrest patients: A systematic review and meta-analysis. Resuscitation 2020, 148, 108–117. [Google Scholar] [CrossRef]
- Schmechel, D.; Marangos, P.J.; Brightman, M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature 1978, 276, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.S.; Huesgen, K.W.; Wang, K.K.; Mark, K.; Tyndall, J.A. Prognostic utility of neuroinjury biomarkers in post out-of-hospital cardiac arrest (OHCA) patient management. Med. Hypotheses 2017, 105, 34–47. [Google Scholar] [CrossRef] [PubMed]
| Standardized Mean Differences | ||
|---|---|---|
| Parameter | Before Matching | After Matching |
| Type of cardiac arrest | −0.505 | −0.176 |
| Age | −0.058 | −0.026 |
| Gender | −0.031 | −0.031 |
| Duration of cardiopulmonary resuscitation | 0.628 | 0.238 |
| Patients Characteristics | |||
|---|---|---|---|
| Characteristics | No Amiodarone | Amiodarone | p-Value |
| n | 82 | 82 | |
| Age (y) | 66.7 ± 12.9 | 66.3 ± 13.5 | 0.87 |
| Gender [male] (%) | 64 (78%) | 63 (77%) | 0.85 |
| Pre-existing illness (%) | |||
| Cardiac disease | 64 (78%) | 63 (77%) | 0.85 |
| Pulmonic disease | 7 (9%) | 16 (20%) | 0.04 |
| Renal disease | 17 (21%) | 15 (18%) | 0.69 |
| Neurological disease | 6 (7%) | 7 (9%) | 0.77 |
| Malignancy | 8 (10%) | 4 (5%) | 0.23 |
| No known illness | 10 (12%) | 12 (15%) | 0.64 |
| Witnessed arrest (%) | 58 (71%) | 75 (91%) | 0.0007 |
| Bystander CPR (%) | 13 (16%) | 15 (18%) | 0.67 |
| CPR cause (%) | 0.86 | ||
| Cardiac cause | 62 (76%) | 61 (74%) | |
| Other cause | 20 (24%) | 21 (6%) | |
| CPR duration (mins) | 28 ± 29 | 36.6 ± 35 | 0.09 |
| Location of cardiac arrest (%) | 0.27 | ||
| IHCA | 46 (56%) | 39 (48%) | |
| OHCA | 36 (44%) | 43 (52%) | |
| Return of spontaneous consciousness (%) | 69 (84%) | 68 (83%) | 0.83 |
| Coronary intervention after CPR (%) | 36 (44%) | 42 (51%) | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramser, N.; Duse, D.A.; Gröne, M.; Stücker, B.; Voß, F.; Tokhi, U.; Jung, C.; Horn, P.; Kelm, M.; Erkens, R. Amiodarone Administration during Cardiopulmonary Resuscitation Is Not Associated with Changes in Short-Term Mortality or Neurological Outcomes in Cardiac Arrest Patients with Shockable Rhythms. J. Clin. Med. 2024, 13, 3931. https://doi.org/10.3390/jcm13133931
Kramser N, Duse DA, Gröne M, Stücker B, Voß F, Tokhi U, Jung C, Horn P, Kelm M, Erkens R. Amiodarone Administration during Cardiopulmonary Resuscitation Is Not Associated with Changes in Short-Term Mortality or Neurological Outcomes in Cardiac Arrest Patients with Shockable Rhythms. Journal of Clinical Medicine. 2024; 13(13):3931. https://doi.org/10.3390/jcm13133931
Chicago/Turabian StyleKramser, Nicolas, Dragos Andrei Duse, Michael Gröne, Bernd Stücker, Fabian Voß, Ursala Tokhi, Christian Jung, Patrick Horn, Malte Kelm, and Ralf Erkens. 2024. "Amiodarone Administration during Cardiopulmonary Resuscitation Is Not Associated with Changes in Short-Term Mortality or Neurological Outcomes in Cardiac Arrest Patients with Shockable Rhythms" Journal of Clinical Medicine 13, no. 13: 3931. https://doi.org/10.3390/jcm13133931
APA StyleKramser, N., Duse, D. A., Gröne, M., Stücker, B., Voß, F., Tokhi, U., Jung, C., Horn, P., Kelm, M., & Erkens, R. (2024). Amiodarone Administration during Cardiopulmonary Resuscitation Is Not Associated with Changes in Short-Term Mortality or Neurological Outcomes in Cardiac Arrest Patients with Shockable Rhythms. Journal of Clinical Medicine, 13(13), 3931. https://doi.org/10.3390/jcm13133931






