Case Studies of a Simulation Workflow to Improve Bone Healing Assessment in Impending Non-Unions
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Data
2.2. Digital Twin of the Fracture Situation
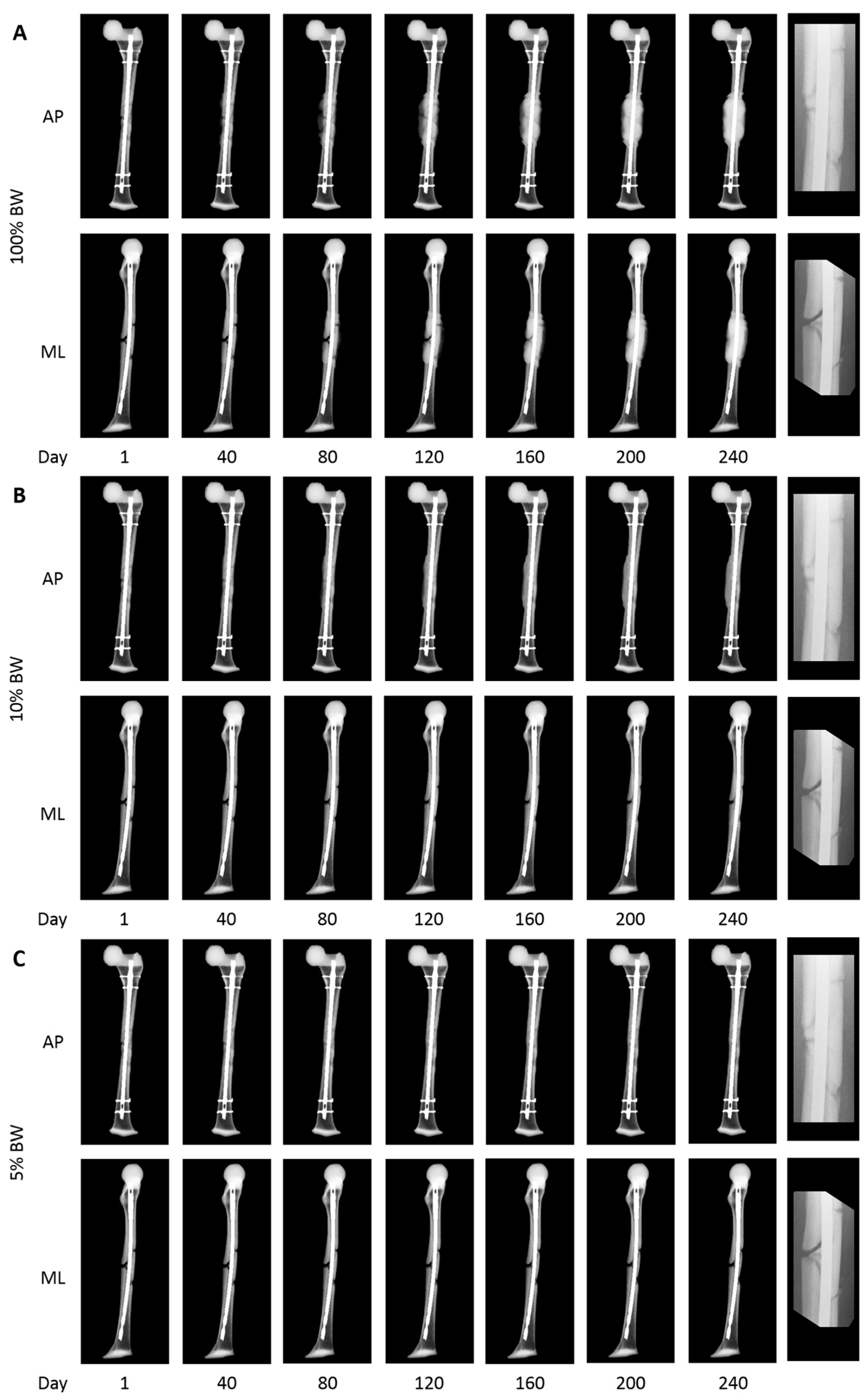
2.3. Application to the Clinical Cases
3. Results
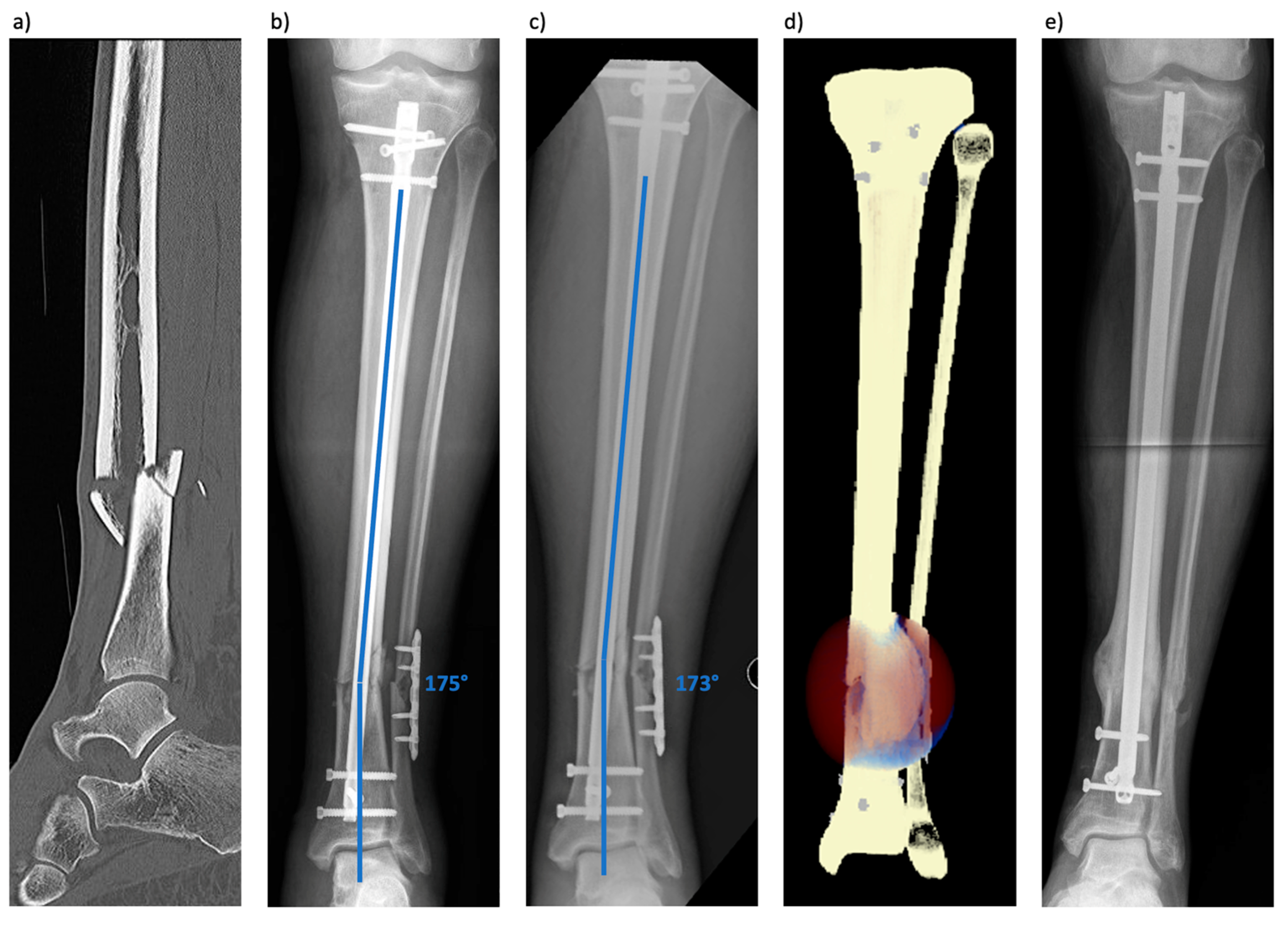
3.1. Patient 1 (Tibial Fracture)
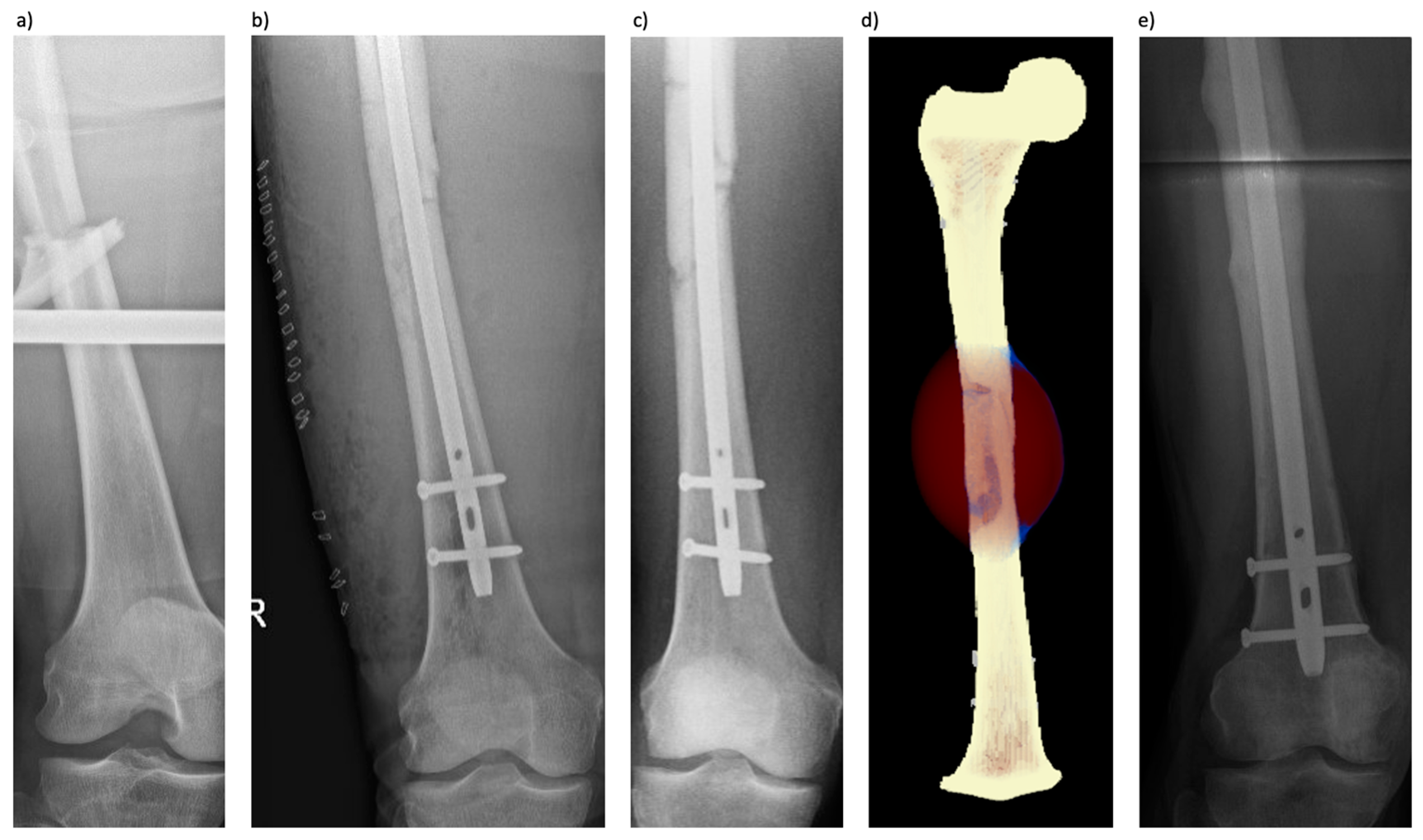
3.2. Patient 2 (Femoral Fracture)
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zura, R.; Xiong, Z.; Einhorn, T.; Watson, J.T.; Ostrum, R.F.; Prayson, M.J.; Della Rocca, G.J.; Mehta, S.; McKinley, T.; Wang, Z.; et al. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg. 2016, 151, e162775. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.J.; Menger, M.M.; Reumann, M.K.; Histing, T. Pseudarthrosen beim Erwachsenen—Ein Update. Orthopädie Und Unfallchirurgie Up2date 2022, 17, 537–558. [Google Scholar] [CrossRef]
- Nicholson, J.A.; Makaram, N.; Simpson, A.; Keating, J.F. Fracture nonunion in long bones: A literature review of risk factors and surgical management. Injury 2021, 52, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.R.; Taitsman, L.A.; Barei, D.P.; Nork, S.E. Femoral nonunion: Risk factors and treatment options. J. Am. Acad. Orthop. Surg. 2008, 16, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Copuroglu, C.; Calori, G.M.; Giannoudis, P.V. Fracture non-union: Who is at risk? Injury 2013, 44, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Santolini, E.; West, R.; Giannoudis, P.V. Risk factors for long bone fracture non-union: A stratification approach based on the level of the existing scientific evidence. Injury 2015, 46, S8–S19. [Google Scholar] [CrossRef]
- Brinker, M.R.; Hanus, B.D.; Sen, M.; O’Connor, D.P. The devastating effects of tibial nonunion on health-related quality of life. J. Bone Joint Surg. Am. 2013, 95, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Biehl, C.; Budak, M.; Thormann, U.; Heiss, C.; Alt, V. Diaphyseal long bone nonunions—Types, aetiology, economics, and treatment recommendations. Int. Orthop. 2018, 42, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef]
- Bhandari, M.; Guyatt, G.H.; Swiontkowski, M.F.; Tornetta, P., 3rd; Sprague, S.; Schemitsch, E.H. A lack of consensus in the assessment of fracture healing among orthopaedic surgeons. J. Orthop. Trauma 2002, 16, 562–566. [Google Scholar] [CrossRef]
- Ferreira, N.; Marais, L.; Aldous, C. Challenges and controversies in defining and classifying tibial non-unions. SA Orthop. J. 2014, 13, 52–56. [Google Scholar]
- Wittauer, M.; Burch, M.A.; McNally, M.; Vandendriessche, T.; Clauss, M.; Della Rocca, G.J.; Giannoudis, P.V.; Metsemakers, W.J.; Morgenstern, M. Definition of long-bone nonunion: A scoping review of prospective clinical trials to evaluate current practice. Injury 2021, 52, 3200–3205. [Google Scholar] [CrossRef]
- Neumaier, M.; Biberthaler, P. Hypertrophe Pseudarthrose. In Knochendefekte und Pseudarthrosen; Biberthaler, P., van Griensven, M., Eds.; Springer: Berlin, Heidelberg, 2017; pp. 77–100. [Google Scholar] [CrossRef]
- Schmidmaier, G. Non Unions. Unfallchirurg 2020, 123, 669–670. [Google Scholar] [CrossRef]
- Simpson, A. The forgotten phase of fracture healing: The need to predict nonunion. Bone Joint Res. 2017, 6, 610–611. [Google Scholar] [CrossRef]
- Claes, L. Biomechanical Principles and Mechanobiologic Aspects of Flexible and Locked Plating. J. Orthop. Trauma 2011, 25, S4–S7. [Google Scholar] [CrossRef]
- Claes, L. Mechanobiology of fracture healing part 1: Principles. Unfallchirurg 2017, 120, 14–22. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Marmor, M.T.; Dailey, H.; Marcucio, R.; Hunt, A.C. Biomedical research models in the science of fracture healing—Pitfalls & promises. Injury 2020, 51, 2118–2128. [Google Scholar] [CrossRef]
- Braun, B.J.; Orth, M.; Diebels, S.; Wickert, K.; Andres, A.; Gawlitza, J.; Bücker, A.; Pohlemann, T.; Roland, M. Individualized Determination of the Mechanical Fracture Environment After Tibial Exchange Nailing-A Simulation-Based Feasibility Study. Front. Surg. 2021, 8, 749209. [Google Scholar] [CrossRef]
- Orth, M.; Ganse, B.; Andres, A.; Wickert, K.; Warmerdam, E.; Müller, M.; Diebels, S.; Roland, M.; Pohlemann, T. Simulation-based prediction of bone healing and treatment recommendations for lower leg fractures: Effects of motion, weight-bearing and fibular mechanics. Front. Bioeng. Biotechnol. 2023, 11, 1067845. [Google Scholar] [CrossRef]
- Niemeyer, F.; Claes, L.; Ignatius, A.; Meyers, N.; Simon, U. Simulating lateral distraction osteogenesis. PLoS ONE 2018, 13, e0194500. [Google Scholar] [CrossRef]
- Engelhardt, L.; Niemeyer, F.; Christen, P.; Müller, R.; Stock, K.; Blauth, M.; Urban, K.; Ignatius, A.; Simon, U. Simulating Metaphyseal Fracture Healing in the Distal Radius. Biomechanics 2021, 1, 29–42. [Google Scholar] [CrossRef]
- Degenhart, C.; Engelhardt, L.; Niemeyer, F.; Erne, F.; Braun, B.; Gebhard, F.; Schütze, K. Computer-Based Mechanobiological Fracture Healing Model Predicts Non-Union of Surgically Treated Diaphyseal Femur Fractures. J. Clin. Med. 2023, 12, 3461. [Google Scholar] [CrossRef]
- Simon, U.; Augat, P.; Utz, M.; Claes, L. A numerical model of the fracture healing process that describes tissue development and revascularisation. Comput. Methods Biomech. Biomed. Eng. 2011, 14, 79–93. [Google Scholar] [CrossRef]
- Pauwels, F. Eine neue Theorie über den Einfluß mechanischer Reize auf die Differenzierung der Stützgewebe. Z. Anat. Entwicklungsgeschichte 1960, 121, 478–515. [Google Scholar] [CrossRef]
- Claes, L.E.; Heigele, C.A. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J. Biomech. 1999, 32, 255–266. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Heller, M.O.; Bergmann, G.; Kassi, J.P.; Claes, L.; Haas, N.P.; Duda, G.N. Determination of muscle loading at the hip joint for use in pre-clinical testing. J. Biomech. 2005, 38, 1155–1163. [Google Scholar] [CrossRef]
- Zhao, D.; Banks, S.A.; D’Lima, D.D.; Colwell, C.W., Jr.; Fregly, B.J. In vivo medial and lateral tibial loads during dynamic and high flexion activities. J. Orthop. Res. 2007, 25, 593–602. [Google Scholar] [CrossRef]
- O’Halloran, K.; Coale, M.; Costales, T.; Zerhusen, T., Jr.; Castillo, R.C.; Nascone, J.W.; O’Toole, R.V. Will My Tibial Fracture Heal? Predicting Nonunion at the Time of Definitive Fixation Based on Commonly Available Variables. Clin. Orthop. Relat. Res. 2016, 474, 1385–1395. [Google Scholar] [CrossRef]
- Chloros, G.D.; Kanakaris, N.K.; Vun, J.S.H.; Howard, A.; Giannoudis, P.V. Scoring systems for early prediction of tibial fracture non-union: An update. Int. Orthop. 2021, 45, 2081–2091. [Google Scholar] [CrossRef]
- Chloros, G.D.; Howard, A.; Giordano, V.; Giannoudis, P.V. Radiographic Long Bone Fracture Healing Scores: Can they predict non-union? Injury 2020, 51, 1693–1695. [Google Scholar] [CrossRef]
- Dailey, H.L.; Schwarzenberg, P.; Daly, C.J.; Boran, S.A.M.; Maher, M.M.; Harty, J.A. Virtual Mechanical Testing Based on Low-Dose Computed Tomography Scans for Tibial Fracture: A Pilot Study of Prediction of Time to Union and Comparison with Subjective Outcomes Scoring. J. Bone Joint. Surg. Am. 2019, 101, 1193–1202. [Google Scholar] [CrossRef]
- Millar, M.J.; Wilkinson, A.; Navarre, P.; Steiner, J.; Vohora, A.; Hardidge, A.; Edwards, E. Nail Fit: Does Nail Diameter to Canal Ratio Predict the Need for Exchange Nailing in the Setting of Aseptic, Hypertrophic Femoral Nonunions? J. Orthop. Trauma 2018, 32, 245–250. [Google Scholar] [CrossRef]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Ghiasi, M.S.; Chen, J.E.; Rodriguez, E.K.; Vaziri, A.; Nazarian, A. Computational modeling of human bone fracture healing affected by different conditions of initial healing stage. BMC Musculoskelet. Disord. 2019, 20, 562. [Google Scholar] [CrossRef]
- Wang, M.; Yang, N.; Wang, X. A review of computational models of bone fracture healing. Med. Biol. Eng. Comput. 2017, 55, 1895–1914. [Google Scholar] [CrossRef]
- Claes, L. Improvement of clinical fracture healing—What can be learned from mechano-biological research? J. Biomech. 2021, 115, 110148. [Google Scholar] [CrossRef]
- Glatt, V.; Evans, C.H.; Tetsworth, K. A Concert between Biology and Biomechanics: The Influence of the Mechanical Environment on Bone Healing. Front Physiol. 2016, 7, 678. [Google Scholar] [CrossRef]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114–139. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maisenbacher, T.C.; Libicher, S.; Erne, F.; Menger, M.M.; Reumann, M.K.; Schindler, Y.; Niemeyer, F.; Engelhardt, L.; Histing, T.; Braun, B.J. Case Studies of a Simulation Workflow to Improve Bone Healing Assessment in Impending Non-Unions. J. Clin. Med. 2024, 13, 3922. https://doi.org/10.3390/jcm13133922
Maisenbacher TC, Libicher S, Erne F, Menger MM, Reumann MK, Schindler Y, Niemeyer F, Engelhardt L, Histing T, Braun BJ. Case Studies of a Simulation Workflow to Improve Bone Healing Assessment in Impending Non-Unions. Journal of Clinical Medicine. 2024; 13(13):3922. https://doi.org/10.3390/jcm13133922
Chicago/Turabian StyleMaisenbacher, Tanja C., Saskia Libicher, Felix Erne, Maximilian M. Menger, Marie K. Reumann, Yannick Schindler, Frank Niemeyer, Lucas Engelhardt, Tina Histing, and Benedikt J. Braun. 2024. "Case Studies of a Simulation Workflow to Improve Bone Healing Assessment in Impending Non-Unions" Journal of Clinical Medicine 13, no. 13: 3922. https://doi.org/10.3390/jcm13133922
APA StyleMaisenbacher, T. C., Libicher, S., Erne, F., Menger, M. M., Reumann, M. K., Schindler, Y., Niemeyer, F., Engelhardt, L., Histing, T., & Braun, B. J. (2024). Case Studies of a Simulation Workflow to Improve Bone Healing Assessment in Impending Non-Unions. Journal of Clinical Medicine, 13(13), 3922. https://doi.org/10.3390/jcm13133922






