Bone: A Neglected Endocrine Organ?
Abstract
1. Bone as an Endocrine Organ
2. Materials and Methods
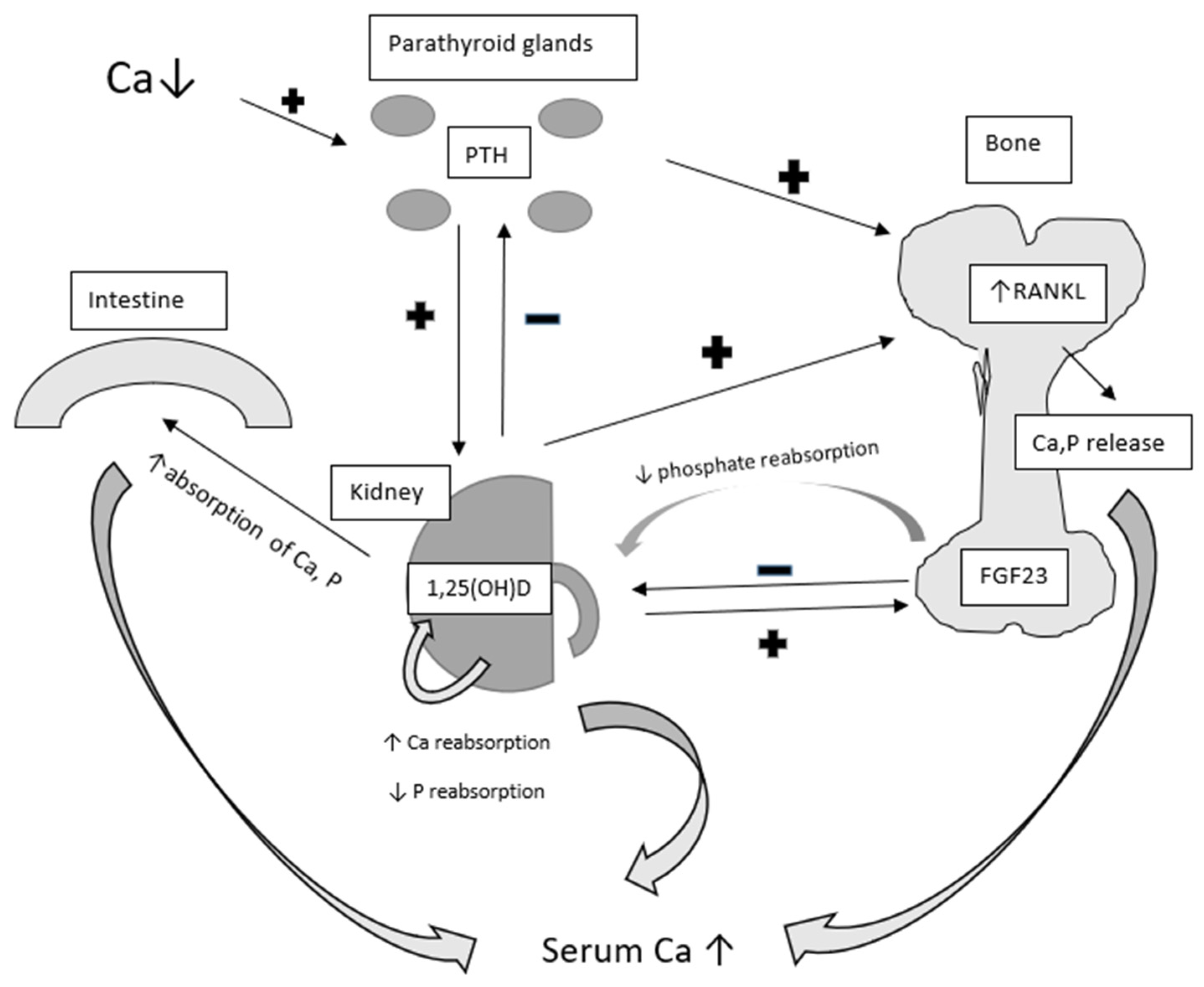
3. Parathormone, Vitamin D, and Calcium—Mineral Metabolism
4. Osteocalcin and Its Profound Role
4.1. Osteocalcin and the Gonads
4.2. Osteocalcin and Cognition
5. Neuropeptide Y and Its Role in the Regulation of Bone Formation and Bone Resorption
5.1. Neuropeptide Y and Bone Formation
5.2. NPY and Bone Resorption
5.3. Other Mechanisms of NPY Action on Bones
6. Bone Turnover Markers
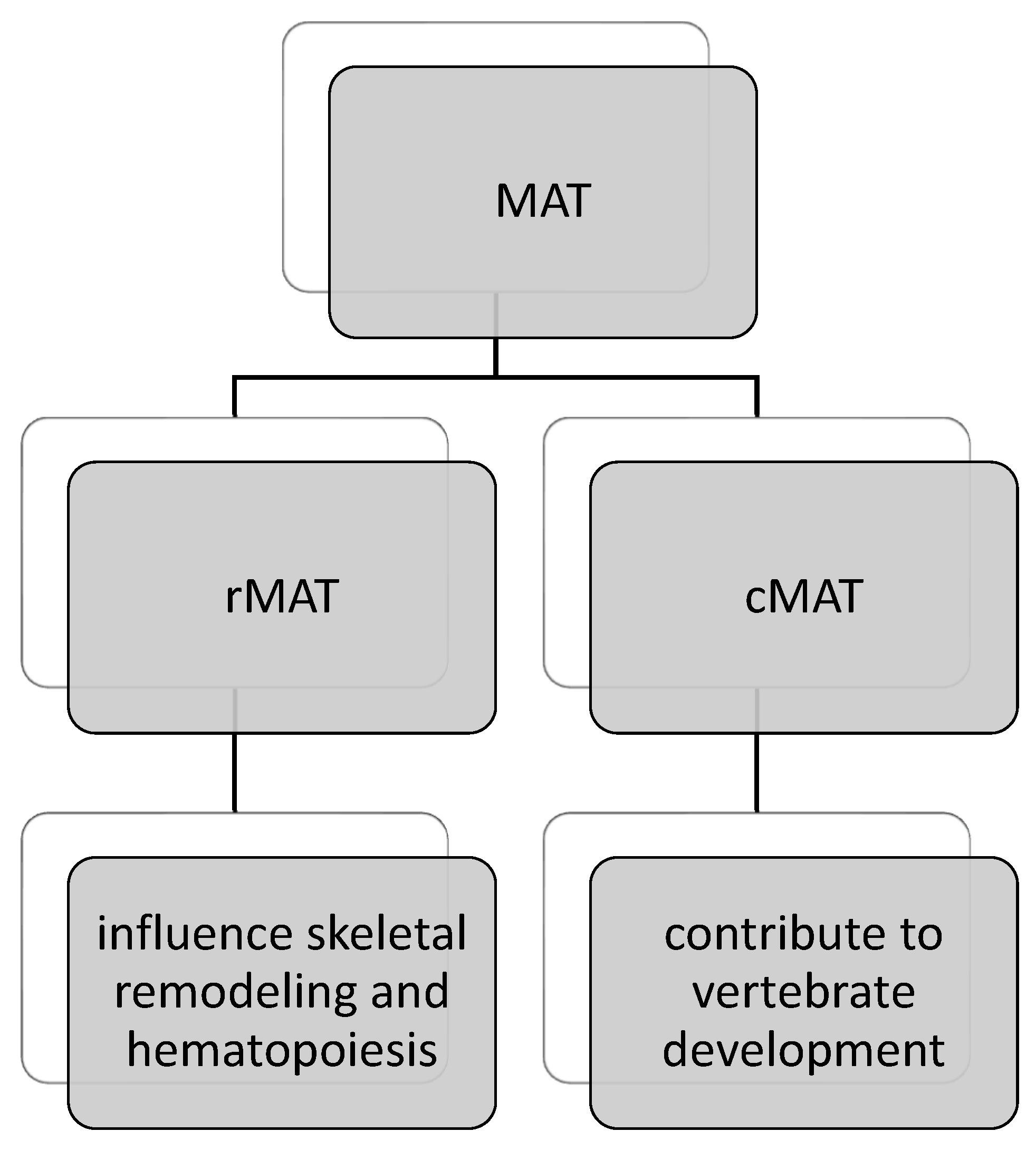
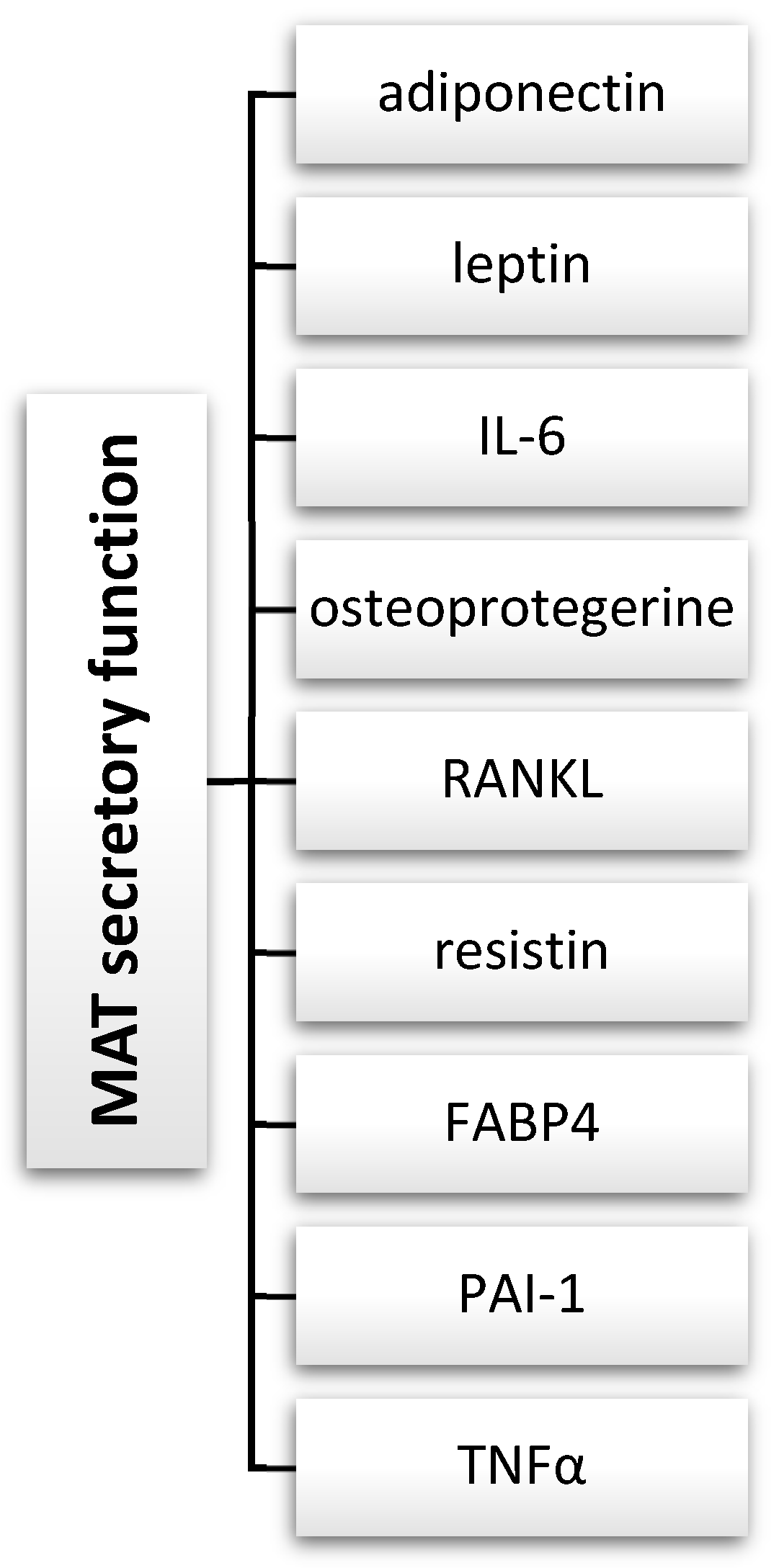
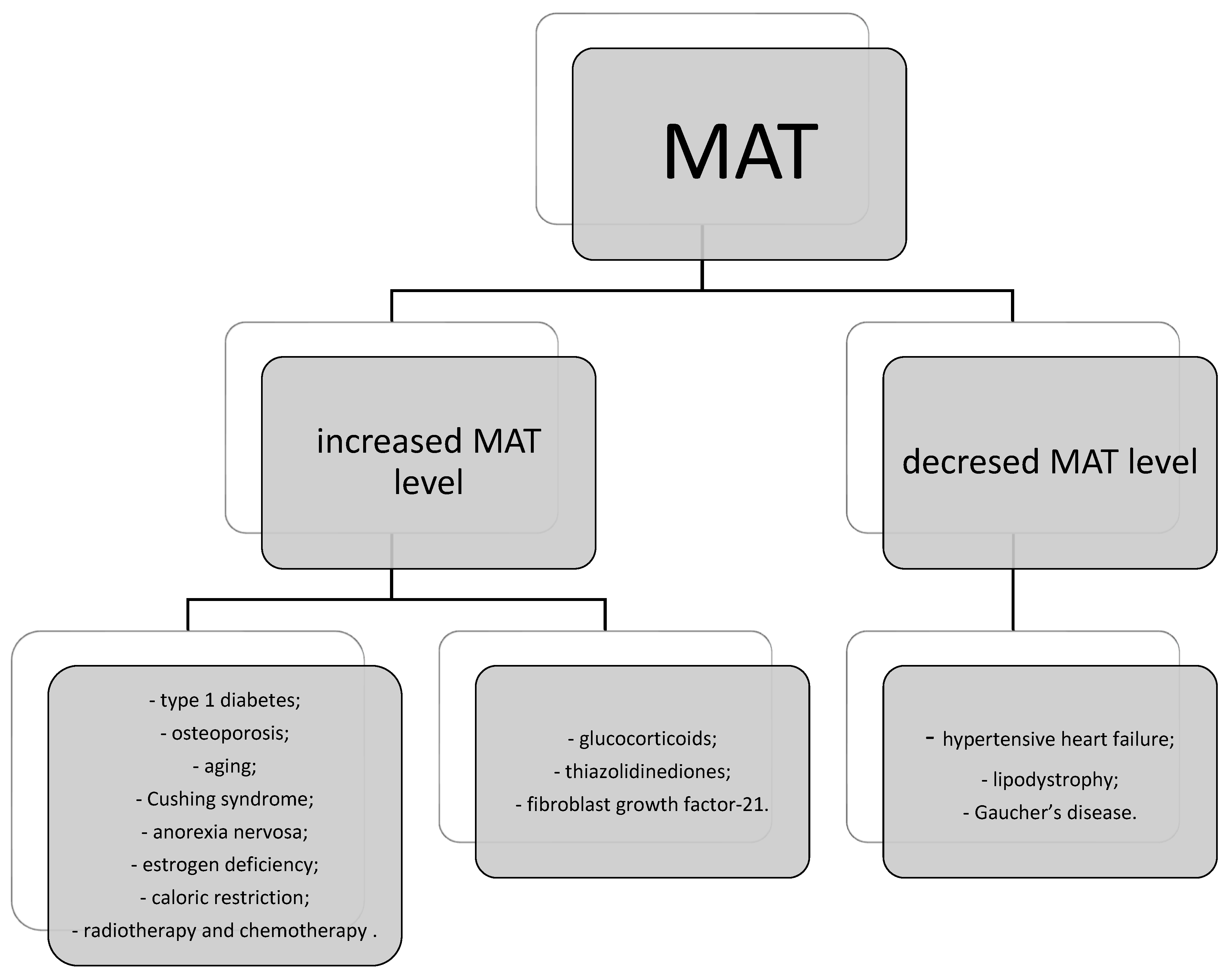
7. Bone Marrow Adipose Tissue (MAT) and Mesenchymal Stem Cells (MSCs) as Endocrine and Immunomodulatory Systems
8. Novel Bone-Derived Substances
8.1. Osteopontin
8.2. Lipocalin 2
8.3. Other Novel Bone-Derived Factors
9. Impact of Bone Hormones on Other Tissues and Organs
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| 1,25(OH)D | calcitriol (1,25-dihydroxycholecalcyferol) |
| 25(OH)D | 25-hydroxycholecalciferol |
| bone ALP | bone-specific alkaline phosphatase |
| BSP | bone sialoprotein |
| Ca | calcium |
| Ca2+ | ionic calcium |
| CTX | C-telopeptides of type I collagen |
| Dkk | Dickkopfs proteins |
| DMP 1 | dentin matrix phospoprotein 1 |
| DPD | deoxypyridinoline |
| DPP-4 | dipeptidyl peptidase 4 |
| DSPP | dentin sialophosphoprotein |
| ERK | extracellular-signal-regulated kinase |
| FABP4 | fatty-acid-binding protein 4 |
| FGF-23 | fibroblast growth factor 23 |
| GABA | gamma-aminobutyric acid |
| HSPCs | hematopoietic stem/progenitor cells |
| IGF-1 | insulin-like growth factor 1 |
| IL-6 | interleukin 6 |
| LCN 2 | lipocalin 2 |
| LPR | low-density lipoprotein receptor |
| MAT | marrow adipose tissue (rMAT regulated MAT; cMAT constitutive MAT) |
| MEPE | matrix extracellular phosphoglycoprotein |
| MSCs | mesenchymal stromal/stem cells |
| NaPi2a | sodium-dependent phosphatase transport protein 2a; sodium-phosphate cotransporer 2a |
| NPY | neuropeptide Y |
| NTX | N-telopeptides of type I collagen |
| OCN | osteocalcin |
| OPG | osteoprotegrin |
| OPN | osteopontin |
| P | phosphorus |
| P1CP | C-terminal propeptide of type 1 collagen |
| P1NP | N-terminal propeptide of type 1 collagen |
| PAI-1 | human plasminogen activator inhibitor-1 |
| POSTN | periostin |
| PTH | parathyroid hormone |
| PYD | pyridinoline |
| RANK | receptor activator for nuclear factor kB |
| RANKL | receptor activator for nuclear factor kB ligand |
| S1P | sphingolisine-1-phosphatase |
| TNFα | tumor necrosis factor α |
| TRACP5b | tartrate-resistant acid phosphatase type 5b |
| VEGF | vascular endothelial growth factor |
| Y1R, Y2R, Y5R | NPY receptors |
References
- Nishimura, R. Bone and Cartilage Biology. Int. J. Mol. Sci. 2023, 24, 5264. [Google Scholar] [CrossRef] [PubMed]
- Niwczyk, O.; Grymowicz, M.; Szczęsnowicz, A.; Hajbos, M.; Kostrzak, A.; Budzik, M.; Maciejewska-Jeske, M.; Bala, G.; Smolarczyk, R.; Męczekalski, B. Bones and Hormones: Interaction between Hormones of the Hypothalamus, Pituitary, Adipose Tissue and Bone. Int. J. Mol. Sci. 2023, 24, 6840. [Google Scholar] [CrossRef]
- Rizzoli, R.; Chevalley, T. Bone health: Biology and nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; An, Y.-Z.; Guo, Q.; Zhou, H.-Y.; Luo, X.-H. Energy homeostasis in the bone. Trends Endocrinol. Metab. 2024, 35, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, A.; Vidyasagar, S.; Amrutha Sukumar, C. Osteocalcin and Metabolic Syndrome. Clin. Med. Insights. Endocrinol. Diabetes 2023, 16, 11795514231206728. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, Z.; Yang, T.; Ying, H.; Zhang, Y.; Liu, S. Bone Regulates Glucose Metabolism as an Endocrine Organ through Osteocalcin. Int. J. Endocrinol. 2015, 2015, 967673. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. Osteocalcin: A Multifaceted Bone-Derived Hormone. Annu. Rev. Nutr. 2023, 43, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Dastghaib, S.; Koohpeyma, F.; Shams, M.; Saki, F.; Alizadeh, A. New concepts in regulation and function of the FGF23. Clin. Exp. Med. 2023, 23, 1055–1066. [Google Scholar] [CrossRef]
- Xie, D.; Wang, Q.; Huang, W.; Zhao, L. Dipeptidyl-peptidase-4 inhibitors have anti-inflammatory effects in patients with type 2 diabetes. Eur. J. Clin. Pharmacol. 2023, 79, 1291–1301. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Kousteni, S. Lipocalin 2-A bone-derived anorexigenic and β-cell promoting signal: From mice to humans. J. Diabetes 2024, 16, e13504. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.; Monier-Faugere, M.-C.; Mawad, H.; David, V.; Malluche, H.H. FGF-23 and sclerostin in serum and bone of CKD patients. Clin. Nephrol. 2023, 99, 209–218. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.-R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Mazur, C.M.; Wein, M.N. Sclerostin and Osteocalcin: Candidate Bone-Produced Hormones. Front. Endocrinol. 2021, 12, 584147. [Google Scholar] [CrossRef]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.; Dennison, E.; Kovacs, C.S.; Mannstadt, M.; Rizzoli, R.; Brandi, M.L.; Clarke, B.; Thakker, R.V. Hormonal regulation of biomineralization. Nat. Rev. Endocrinol. 2021, 17, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, P. The skeleton in primary hyperparathyroidism: A review focusing on bone remodeling, structure, mass, and fracture. APMIS. Suppl. 2001, 102, 1–52. [Google Scholar]
- Saini, V.; Marengi, D.A.; Barry, K.J.; Fulzele, K.S.; Heiden, E.; Liu, X.; Dedic, C.; Maeda, A.; Lotinun, S.; Baron, R.; et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J. Biol. Chem. 2013, 288, 20122–20134. [Google Scholar] [CrossRef]
- Kaleem, I.; Alexander, J.; Hisbulla, M.; Kannichamy, V.; Mishra, V.; Banerjee, A.; Gandhi, A.B.; Khan, S. A Review of the Relationship of the Cerebrospinal Fluid Changes During the Dysregulation of Parathyroid Hormone With Psychiatric or Neurological Manifestations. Cureus 2021, 13, e12679. [Google Scholar] [CrossRef]
- Murray, R.D.; Holthouser, K.; Clark, B.J.; Salyer, S.A.; Barati, M.T.; Khundmiri, S.J.; Lederer, E.D. Parathyroid hormone (PTH) decreases sodium-phosphate cotransporter type IIa (NpT2a) mRNA stability. Am. J. Physiol. Renal Physiol. 2013, 304, F1076–F1085. [Google Scholar] [CrossRef] [PubMed]
- Latic, N.; Erben, R.G. Interaction of Vitamin D with Peptide Hormones with Emphasis on Parathyroid Hormone, FGF23, and the Renin-Angiotensin-Aldosterone System. Nutrients 2022, 14, 5186. [Google Scholar] [CrossRef] [PubMed]
- Allgrove, J. Physiology of Calcium, Phosphate, Magnesium and Vitamin D. Endocr. Dev. 2015, 28, 7–32. [Google Scholar] [CrossRef]
- Wesseling-Perry, K. FGF-23 in bone biology. Pediatr. Nephrol. 2010, 25, 603–608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin D. Compr. Physiol. 2016, 6, 561–601. [Google Scholar] [CrossRef] [PubMed]
- Latic, N.; Erben, R.G. FGF23 and Vitamin D Metabolism. JBMR Plus 2021, 5, e10558. [Google Scholar] [CrossRef] [PubMed]
- Hamedanian, L.; Badehnoosh, B.; Razavi-Khorasani, N.; Mohammadpour, Z.; Mozaffari-Khosravi, H. Evaluation of vitamin D status, parathyroid hormone, and calcium among Iranian pregnant women with preeclampsia: A case-control study. Int. J. Reprod. Biomed. 2019, 17, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Popescu, M.; Ghenea, A.; Ţuculină, M.; Valea, A. Editorial: Calcium and parathormone: An update on the clinical presentation and new therapies. Front. Endocrinol. 2023, 14, 1199056. [Google Scholar] [CrossRef] [PubMed]
- Rendina-Ruedy, E.; Rosen, C.J. Parathyroid hormone (PTH) regulation of metabolic homeostasis: An old dog teaches us new tricks. Mol. Metab. 2022, 60, 101480. [Google Scholar] [CrossRef]
- Lourida, I.; Thompson-Coon, J.; Dickens, C.M.; Soni, M.; Kuźma, E.; Kos, K.; Llewellyn, D.J. Parathyroid hormone, cognitive function and dementia: A systematic review. PLoS ONE 2015, 10, e0127574. [Google Scholar] [CrossRef]
- van Ballegooijen, A.J.; Reinders, I.; Visser, M.; Brouwer, I.A. Parathyroid hormone and cardiovascular disease events: A systematic review and meta-analysis of prospective studies. Am. Heart J. 2013, 165, 655–664.e5. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.C.; van der Eerden, B.C.J. Osteocalcin-A Versatile Bone-Derived Hormone. Front. Endocrinol. 2018, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Saravanan, S.; d’Adesky, N.D.; Bramlett, H.; Perez-Pinzon, M.A.; Raval, A.P. Osteocalcin, ovarian senescence, and brain health. Front. Neuroendocrinol. 2020, 59, 100861. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Quarles, L.D. Novel bone endocrine networks integrating mineral and energy metabolism. Curr. Osteoporos. Rep. 2013, 11, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Tsao, Y.-T.; Huang, Y.-J.; Wu, H.-H.; Liu, Y.-A.; Liu, Y.-S.; Lee, O.K. Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Komori, T. What is the function of osteocalcin? J. Oral Biosci. 2020, 62, 223–227. [Google Scholar] [CrossRef]
- Mundy, G.R.; Poser, J.W. Chemotactic activity of the gamma-carboxyglutamic acid containing protein in bone. Calcif. Tissue Int. 1983, 35, 164–168. [Google Scholar] [CrossRef]
- Patti, A.; Gennari, L.; Merlotti, D.; Dotta, F.; Nuti, R. Endocrine actions of osteocalcin. Int. J. Endocrinol. 2013, 2013, 846480. [Google Scholar] [CrossRef]
- Villafán-Bernal, J.R.; Sánchez-Enríquez, S.; Muñoz-Valle, J.F. Molecular modulation of osteocalcin and its relevance in diabetes (Review). Int. J. Mol. Med. 2011, 28, 283–293. [Google Scholar] [CrossRef]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef]
- Schwetz, V.; Pieber, T.; Obermayer-Pietsch, B. The endocrine role of the skeleton: Background and clinical evidence. Eur. J. Endocrinol. 2012, 166, 959–967. [Google Scholar] [CrossRef]
- Ferron, M.; Hinoi, E.; Karsenty, G.; Ducy, P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. USA 2008, 105, 5266–5270. [Google Scholar] [CrossRef]
- Kanazawa, I.; Yamaguchi, T.; Yano, S.; Yamauchi, M.; Yamamoto, M.; Sugimoto, T. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 2007, 8, 51. [Google Scholar] [CrossRef]
- Karsenty, G. Update on the Biology of Osteocalcin. Endocr. Pract. 2017, 23, 1270–1274. [Google Scholar] [CrossRef]
- Oury, F.; Sumara, G.; Sumara, O.; Ferron, M.; Chang, H.; Smith, C.E.; Hermo, L.; Suarez, S.; Roth, B.L.; Ducy, P.; et al. Endocrine regulation of male fertility by the skeleton. Cell 2011, 144, 796–809. [Google Scholar] [CrossRef]
- Adrian, T.E.; Allen, J.M.; Bloom, S.R.; Ghatei, M.A.; Rossor, M.N.; Roberts, G.W.; Crow, T.J.; Tatemoto, K.; Polak, J.M. Neuropeptide Y distribution in human brain. Nature 1983, 306, 584–586. [Google Scholar] [CrossRef]
- Vähätalo, L.H.; Ruohonen, S.T.; Ailanen, L.; Savontaus, E. Neuropeptide Y in noradrenergic neurons induces obesity in transgenic mouse models. Neuropeptides 2016, 55, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Igwe, J.C.; Jiang, X.; Paic, F.; Ma, L.; Adams, D.J.; Baldock, P.A.; Pilbeam, C.C.; Kalajzic, I. Neuropeptide Y is expressed by osteocytes and can inhibit osteoblastic activity. J. Cell. Biochem. 2009, 108, 621–630. [Google Scholar] [CrossRef]
- Matic, I.; Matthews, B.G.; Kizivat, T.; Igwe, J.C.; Marijanovic, I.; Ruohonen, S.T.; Savontaus, E.; Adams, D.J.; Kalajzic, I. Bone-specific overexpression of NPY modulates osteogenesis. J. Musculoskelet. Neuronal Interact. 2012, 12, 209–218. [Google Scholar] [PubMed]
- Chen, Q.-C.; Zhang, Y. The Role of NPY in the Regulation of Bone Metabolism. Front. Endocrinol. 2022, 13, 833485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.-Y.; Liu, Y.-W.; Rao, S.-S.; Tan, Y.-J.; Qian, Y.-X.; Xia, K.; Huang, J.; Liu, X.-X.; Hong, C.-G.; et al. Neuronal Induction of Bone-Fat Imbalance through Osteocyte Neuropeptide Y. Adv. Sci. 2021, 8, e2100808. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.J.; Doyle, K.L.; Sainsbury, A.; Enriquez, R.F.; Hort, Y.J.; Riepler, S.J.; Baldock, P.A.; Herzog, H. Critical role for Y1 receptors in mesenchymal progenitor cell differentiation and osteoblast activity. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 1736–1747. [Google Scholar] [CrossRef]
- Baldock, P.A.; Lin, S.; Zhang, L.; Karl, T.; Shi, Y.; Driessler, F.; Zengin, A.; Hörmer, B.; Lee, N.J.; Wong, I.P.L.; et al. Neuropeptide y attenuates stress-induced bone loss through suppression of noradrenaline circuits. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 2238–2249. [Google Scholar] [CrossRef]
- Ma, W.H.; Liu, Y.J.; Wang, W.; Zhang, Y.Z. Neuropeptide Y, substance P, and human bone morphogenetic protein 2 stimulate human osteoblast osteogenic activity by enhancing gap junction intercellular communication. Braz. J. Med. Biol. Res. 2015, 48, 299–307. [Google Scholar] [CrossRef]
- Wu, W.; Peng, S.; Shi, Y.; Li, L.; Song, Z.; Lin, S. NPY promotes macrophage migration by upregulating matrix metalloproteinase-8 expression. J. Cell. Physiol. 2021, 236, 1903–1912. [Google Scholar] [CrossRef]
- Amano, S.; Arai, M.; Goto, S.; Togari, A. Inhibitory effect of NPY on isoprenaline-induced osteoclastogenesis in mouse bone marrow cells. Biochim. Biophys. Acta 2007, 1770, 966–973. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, N.; Jin, H.K.; Bae, J.-S. Neuropeptide Y-based recombinant peptides ameliorate bone loss in mice by regulating hematopoietic stem/progenitor cell mobilization. BMB Rep. 2017, 50, 138–143. [Google Scholar] [CrossRef]
- Wu, J.-Q.; Jiang, N.; Yu, B. Mechanisms of action of neuropeptide Y on stem cells and its potential applications in orthopaedic disorders. World J. Stem Cells 2020, 12, 986–1000. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, M.; Liang, J.; Yang, K.; Li, F.; Zhou, Z.; Qiu, M.; Chen, H.; Cai, Z.; Cui, W.; et al. Neuropeptide Y-Mediated Gut Microbiota Alterations Aggravate Postmenopausal Osteoporosis. Adv. Sci. 2023, 10, e2303015. [Google Scholar] [CrossRef]
- Long, H.; Ahmed, M.; Ackermann, P.; Stark, A.; Li, J. Neuropeptide Y innervation during fracture healing and remodeling. A study of angulated tibial fractures in the rat. Acta Orthop. 2010, 81, 639–646. [Google Scholar] [CrossRef]
- Szulc, P. Bone turnover: Biology and assessment tools. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 725–738. [Google Scholar] [CrossRef]
- Brown, J.P.; Don-Wauchope, A.; Douville, P.; Albert, C.; Vasikaran, S.D. Current use of bone turnover markers in the management of osteoporosis. Clin. Biochem. 2022, 109–110, 1–10. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Niedźwiedzki, T.; Filipowska, J. Bone remodeling in the context of cellular and systemic regulation: The role of osteocytes and the nervous system. J. Mol. Endocrinol. 2015, 55, R23–R36. [Google Scholar] [CrossRef]
- Garnero, P. New developments in biological markers of bone metabolism in osteoporosis. Bone 2014, 66, 46–55. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef]
- Boyce, B.F. Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 2013, 92, 860–867. [Google Scholar] [CrossRef]
- Tian, J.; Ma, S.; Xie, W.-Q.; Zhang, Y.-M.; Tao, L.; Li, Y.-S.; Xiao, W.-F. Sphingosine 1-phosphate and osteoporosis: Pathophysiology and therapeutic aspects-a narrative review. Ann. Palliat. Med. 2021, 10, 4799–4805. [Google Scholar] [CrossRef]
- Xu, H.; Duan, J.; Ning, D.; Li, J.; Liu, R.; Yang, R.; Jiang, J.X.; Shang, P. Role of Wnt signaling in fracture healing. BMB Rep. 2014, 47, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration—What Do We Know So Far. Front. Cell Dev. Biol. 2018, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Semënov, M.; Tamai, K.; He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005, 280, 26770–26775. [Google Scholar] [CrossRef] [PubMed]
- Baetta, R.; Banfi, C. Dkk (Dickkopf) Proteins. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Sapir-Koren, R.; Livshits, G. Osteocyte control of bone remodeling: Is sclerostin a key molecular coordinator of the balanced bone resorption-formation cycles? Osteoporos. Int. 2014, 25, 2685–2700. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Balemans, W.; Ebeling, M.; Patel, N.; Van Hul, E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; Van Den Ende, J.; Willems, P.; et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef] [PubMed]
- van Bezooijen, R.L.; Bronckers, A.L.; Gortzak, R.A.; Hogendoorn, P.C.W.; van der Wee-Pals, L.; Balemans, W.; Oostenbroek, H.J.; Van Hul, W.; Hamersma, H.; Dikkers, F.G.; et al. Sclerostin in mineralized matrices and van Buchem disease. J. Dent. Res. 2009, 88, 569–574. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther. Adv. Musculoskelet. Dis. 2014, 6, 48–57. [Google Scholar] [CrossRef]
- Koide, M.; Kobayashi, Y. Regulatory mechanisms of sclerostin expression during bone remodeling. J. Bone Miner. Metab. 2019, 37, 9–17. [Google Scholar] [CrossRef]
- Kobayakawa, T.; Miyazaki, A.; Saito, M.; Suzuki, T.; Takahashi, J.; Nakamura, Y. Denosumab versus romosozumab for postmenopausal osteoporosis treatment. Sci. Rep. 2021, 11, 11801. [Google Scholar] [CrossRef] [PubMed]
- Dorafshan, S.; Razmi, M.; Safaei, S.; Gentilin, E.; Madjd, Z.; Ghods, R. Periostin: Biology and function in cancer. Cancer Cell Int. 2022, 22, 315. [Google Scholar] [CrossRef] [PubMed]
- Chapurlat, R.D.; Confavreux, C.B. Novel biological markers of bone: From bone metabolism to bone physiologya. Rheumatology 2016, 55, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, Y.; Zhang, Q. Emerging Role of Extracellular Vesicles in Bone Remodeling. J. Dent. Res. 2018, 97, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhou, X.; Chen, L.; Huang, S.; Leung, V.; Wu, N.; Pan, H.; Zhen, W.; Lu, W.; Peng, S. The Regulatory Roles of MicroRNAs in Bone Remodeling and Perspectives as Biomarkers in Osteoporosis. BioMed Res. Int. 2016, 2016, 1652417. [Google Scholar] [CrossRef] [PubMed]
- Baier, S.R.; Wan, Y. MicroRNA Exert Macro Effects on Cancer Bone Metastasis. Curr. Osteoporos. Rep. 2016, 14, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Taipaleenmäki, H. Secreted microRNAs in bone metastasis. J. Bone Miner. Metab. 2023, 41, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Scheller, E.L.; Learman, B.S.; Parlee, S.D.; Simon, B.R.; Mori, H.; Ning, X.; Bree, A.J.; Schell, B.; Broome, D.T.; et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014, 20, 368–375. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Horowitz, M.C.; MacDougald, O.A.; Scheller, E.L.; Rodeheffer, M.S.; Rosen, C.J.; Klibanski, A. Marrow fat and bone--new perspectives. J. Clin. Endocrinol. Metab. 2013, 98, 935–945. [Google Scholar] [CrossRef]
- Kricun, M.E. Red-yellow marrow conversion: Its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985, 14, 10–19. [Google Scholar] [CrossRef]
- Scheller, E.L.; Doucette, C.R.; Learman, B.S.; Cawthorn, W.P.; Khandaker, S.; Schell, B.; Wu, B.; Ding, S.-Y.; Bredella, M.A.; Fazeli, P.K.; et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat. Commun. 2015, 6, 7808. [Google Scholar] [CrossRef] [PubMed]
- Justesen, J.; Stenderup, K.; Ebbesen, E.N.; Mosekilde, L.; Steiniche, T.; Kassem, M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001, 2, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Fumoto, T.; Naoe, Y.; Ikeda, K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J. Biol. Chem. 2014, 289, 16699–16710. [Google Scholar] [CrossRef] [PubMed]
- Devlin, M.J.; Cloutier, A.M.; Thomas, N.A.; Panus, D.A.; Lotinun, S.; Pinz, I.; Baron, R.; Rosen, C.J.; Bouxsein, M.L. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Botolin, S.; McCabe, L.R. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology 2007, 148, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Wroblewski, V.J.; Koester, A.; Chen, Y.-F.; Clutinger, C.K.; Tigno, X.T.; Hansen, B.C.; Shanafelt, A.B.; Etgen, G.J. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007, 148, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Tornvig, L.; Mosekilde, L.I.; Justesen, J.; Falk, E.; Kassem, M. Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice. Calcif. Tissue Int. 2001, 69, 46–50. [Google Scholar] [CrossRef]
- Vande Berg, B.C.; Malghem, J.; Lecouvet, F.E.; Devogelaer, J.P.; Maldague, B.; Houssiau, F.A. Fat conversion of femoral marrow in glucocorticoid-treated patients: A cross-sectional and longitudinal study with magnetic resonance imaging. Arthritis Rheum. 1999, 42, 1405–1411. [Google Scholar] [CrossRef]
- Scheller, E.L.; Rosen, C.J. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann. N. Y. Acad. Sci. 2014, 1311, 14–30. [Google Scholar] [CrossRef]
- Maas, M.; Hollak, C.E.M.; Akkerman, E.M.; Aerts, J.M.F.G.; Stoker, J.; Den Heeten, G.J. Quantification of skeletal involvement in adults with type I Gaucher’s disease: Fat fraction measured by Dixon quantitative chemical shift imaging as a valid parameter. AJR Am. J. Roentgenol. 2002, 179, 961–965. [Google Scholar] [CrossRef]
- Pastores, G.M.; Meere, P.A. Musculoskeletal complications associated with lysosomal storage disorders: Gaucher disease and Hurler-Scheie syndrome (mucopolysaccharidosis type I). Curr. Opin. Rheumatol. 2005, 17, 70–78. [Google Scholar] [CrossRef] [PubMed]
- SHILLINGFORD, J.P. The red bone marrow in heart failure. J. Clin. Pathol. 1950, 3, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Sulston, R.J.; Cawthorn, W.P. Bone marrow adipose tissue as an endocrine organ: Close to the bone? Horm. Mol. Biol. Clin. Investig. 2016, 28, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Berner, H.S.; Lyngstadaas, S.P.; Spahr, A.; Monjo, M.; Thommesen, L.; Drevon, C.A.; Syversen, U.; Reseland, J.E. Adiponectin and its receptors are expressed in bone-forming cells. Bone 2004, 35, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Park, J.-H.; Ju, S.-K.; You, K.-H.; Ko, J.S.; Kim, H.-M. Leptin receptor isoform expression in rat osteoblasts and their functional analysis. FEBS Lett. 2002, 528, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Patsch, J.M.; Li, X.; Baum, T.; Yap, S.P.; Karampinos, D.C.; Schwartz, A.V.; Link, T.M. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Worthley, D.L.; Churchill, M.; Compton, J.T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M.G.; Uygur, A.; Hayakawa, Y.; et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 2015, 160, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Maurin, A.C.; Chavassieux, P.M.; Frappart, L.; Delmas, P.D.; Serre, C.M.; Meunier, P.J. Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone 2000, 26, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Wu, X.; Rivas, D.; Gimble, J.M.; Duque, G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J. Cell. Mol. Med. 2010, 14, 982–991. [Google Scholar] [CrossRef]
- Kelly, K.A.; Tanaka, S.; Baron, R.; Gimble, J.M. Murine bone marrow stromally derived BMS2 adipocytes support differentiation and function of osteoclast-like cells in vitro. Endocrinology 1998, 139, 2092–2101. [Google Scholar] [CrossRef][Green Version]
- Fan, Y.; Hanai, J.-I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef]
- Brown, J.M.; Nemeth, K.; Kushnir-Sukhov, N.M.; Metcalfe, D.D.; Mezey, E. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2011, 41, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hematti, P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp. Hematol. 2009, 37, 1445–1453. [Google Scholar] [CrossRef]
- Kay, L.J.; Yeo, W.W.; Peachell, P.T. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br. J. Pharmacol. 2006, 147, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Bianchi, G.; Bertolotto, M.; Montecucco, F.; Busca, A.; Dallegri, F.; Ottonello, L.; Pistoia, V. Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells 2008, 26, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-X.; Zhang, Y.; Liu, B.; Zhang, S.-X.; Wu, Y.; Yu, X.-D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Manz, R.A.; Thiel, A.; Radbruch, A. Lifetime of plasma cells in the bone marrow. Nature 1997, 388, 133–134. [Google Scholar] [CrossRef]
- Di Rosa, F.; Gebhardt, T. Bone Marrow T Cells and the Integrated Functions of Recirculating and Tissue-Resident Memory T Cells. Front. Immunol. 2016, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.C.; Coley, S.M.; Wherry, E.J.; Ahmed, R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J. Immunol. 2005, 174, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, E.; Cooper, M.D. Memory B lymphocytes migrate to bone marrow in humans. Proc. Natl. Acad. Sci. USA 1997, 94, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Addo, R.K.; Heinrich, F.; Heinz, G.A.; Schulz, D.; Sercan-Alp, Ö.; Lehmann, K.; Tran, C.L.; Bardua, M.; Matz, M.; Löhning, M.; et al. Single-cell transcriptomes of murine bone marrow stromal cells reveal niche-associated heterogeneity. Eur. J. Immunol. 2019, 49, 1372–1379. [Google Scholar] [CrossRef]
- McGrath, M.; Dong, J.; Mashreghi, M.-F.; Chang, H.-D.; Radbruch, A. Rethinking ‘immunology memory’: Local and systemic immunity provided by bone marrow-resident memory cells. Scand. J. Immunol. 2022, 96, e13232. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Bailey, S.; Karsenty, G.; Gundberg, C.; Vashishth, D. Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann. N. Y. Acad. Sci. 2017, 1409, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Thurner, P.J.; Chen, C.G.; Ionova-Martin, S.; Sun, L.; Harman, A.; Porter, A.; Ager, J.W., 3rd; Ritchie, R.O.; Alliston, T. Osteopontin deficiency increases bone fragility but preserves bone mass. Bone 2010, 46, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Haylock, D.N.; Nilsson, S.K. Osteopontin: A bridge between bone and blood. Br. J. Haematol. 2006, 134, 467–474. [Google Scholar] [CrossRef]
- Kruger, T.E.; Miller, A.H.; Godwin, A.K.; Wang, J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit. Rev. Oncol. Hematol. 2014, 89, 330–341. [Google Scholar] [CrossRef]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Abella, V.; Scotece, M.; Conde, J.; Gómez, R.; Lois, A.; Pino, J.; Gómez-Reino, J.J.; Lago, F.; Mobasheri, A.; Gualillo, O. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers Biochem. Indic. Expo. Response Susceptibility Chem. 2015, 20, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Mosialou, I.; Shikhel, S.; Liu, J.-M.; Maurizi, A.; Luo, N.; He, Z.; Huang, Y.; Zong, H.; Friedman, R.A.; Barasch, J.; et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 2017, 543, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, K.S.L.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.K.; Chow, W.-S.; Wat, N.M.S.; Xu, J.Y.; Hoo, R.L.C.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Song, C. Crosstalk between bone and other organs. Med. Rev. 2022, 2, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Brennan-Speranza, T.C.; Levinger, I.; Yeap, B.B. Undercarboxylated Osteocalcin: Experimental and Human Evidence for a Role in Glucose Homeostasis and Muscle Regulation of Insulin Sensitivity. Nutrients 2018, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Mendias, C.L.; Gumucio, J.P.; Davis, M.E.; Bromley, C.W.; Davis, C.S.; Brooks, S.V. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve 2012, 45, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Braverman, E.R.; Chen, T.J.H.; Chen, A.L.C.; Arcuri, V.; Kerner, M.M.; Bajaj, A.; Carbajal, J.; Braverman, D.; Downs, B.W.; Blum, K. Age-related increases in parathyroid hormone may be antecedent to both osteoporosis and dementia. BMC Endocr. Disord. 2009, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, A.; Hsu, Y.-M.S.; Day, R.B.; Schuettpelz, L.G.; Christopher, M.J.; Borgerding, J.N.; Nagasawa, T.; Link, D.C. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013, 495, 227–230. [Google Scholar] [CrossRef]
- Pittas, A.G.; Harris, S.S.; Eliades, M.; Stark, P.; Dawson-Hughes, B. Association between serum osteocalcin and markers of metabolic phenotype. J. Clin. Endocrinol. Metab. 2009, 94, 827–832. [Google Scholar] [CrossRef]
- Guo, H.; Wang, C.; Jiang, B.; Ge, S.; Cai, J.; Zhou, Y.; Ying, R.; Zha, K.; Zhou, J.; Wang, N.; et al. Association of Insulin Resistance and β-cell Function With Bone Turnover Biomarkers in Dysglycemia Patients. Front. Endocrinol. 2021, 12, 554604. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Kountouras, J.; Makras, P.; Papatheodorou, A.; Kokkoris, P.; Sakellariou, G.T.; Terpos, E. Circulating sclerostin and Dickkopf-1 levels in patients with nonalcoholic fatty liver disease. J. Bone Miner. Metab. 2016, 34, 447–456. [Google Scholar] [CrossRef] [PubMed]

| Bone Formation Markers | Bone Resorption Markers |
|---|---|
| Serm total osteocalcin | Pyridinoline (PYD) |
| Bone-specific alkaline phosphatase (bone ALP) | Deoxypyridinoline (DPD) |
| N-terminal propeptide of type 1 collgen (P1NP) | C-telopeptide of type 1 collagen (CTX) |
| N-telopeptide of type 1 collagen (NTX) | |
| C-terminal propeptide of type 1 collagen (P1CP) | Tartrate-resistant acid phosphatase type 5b (TRACP5b) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szeliga, A.; Grymowicz, M.; Kostrzak, A.; Smolarczyk, R.; Bala, G.; Smolarczyk, K.; Meczekalski, B.; Suchta, K. Bone: A Neglected Endocrine Organ? J. Clin. Med. 2024, 13, 3889. https://doi.org/10.3390/jcm13133889
Szeliga A, Grymowicz M, Kostrzak A, Smolarczyk R, Bala G, Smolarczyk K, Meczekalski B, Suchta K. Bone: A Neglected Endocrine Organ? Journal of Clinical Medicine. 2024; 13(13):3889. https://doi.org/10.3390/jcm13133889
Chicago/Turabian StyleSzeliga, Anna, Monika Grymowicz, Anna Kostrzak, Roman Smolarczyk, Gregory Bala, Katarzyna Smolarczyk, Blazej Meczekalski, and Katarzyna Suchta. 2024. "Bone: A Neglected Endocrine Organ?" Journal of Clinical Medicine 13, no. 13: 3889. https://doi.org/10.3390/jcm13133889
APA StyleSzeliga, A., Grymowicz, M., Kostrzak, A., Smolarczyk, R., Bala, G., Smolarczyk, K., Meczekalski, B., & Suchta, K. (2024). Bone: A Neglected Endocrine Organ? Journal of Clinical Medicine, 13(13), 3889. https://doi.org/10.3390/jcm13133889






