The Optimal Lowest Instrumented Vertebra to Prevent the Distal Adding-On Phenomenon in Patients Undergoing Selective Thoracic Fusion for Adolescent Idiopathic Scoliosis with Lenke Type 1A and 1B Curves: Comparison of Nine Selection Criteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Surgical Protocol
2.3. Searching for the LIV Selection Criteria
2.4. Radiographic Measurements
2.5. Outcomes Measures
2.6. Statistical Analysis
3. Results
3.1. Baseline Data
3.2. Suggested LIV Location According to the Criteria
3.3. Comparison of Preventive Ability against DA by Odds Ratio
3.4. Agreement of LIV among the Nine LIV Selection Criteria
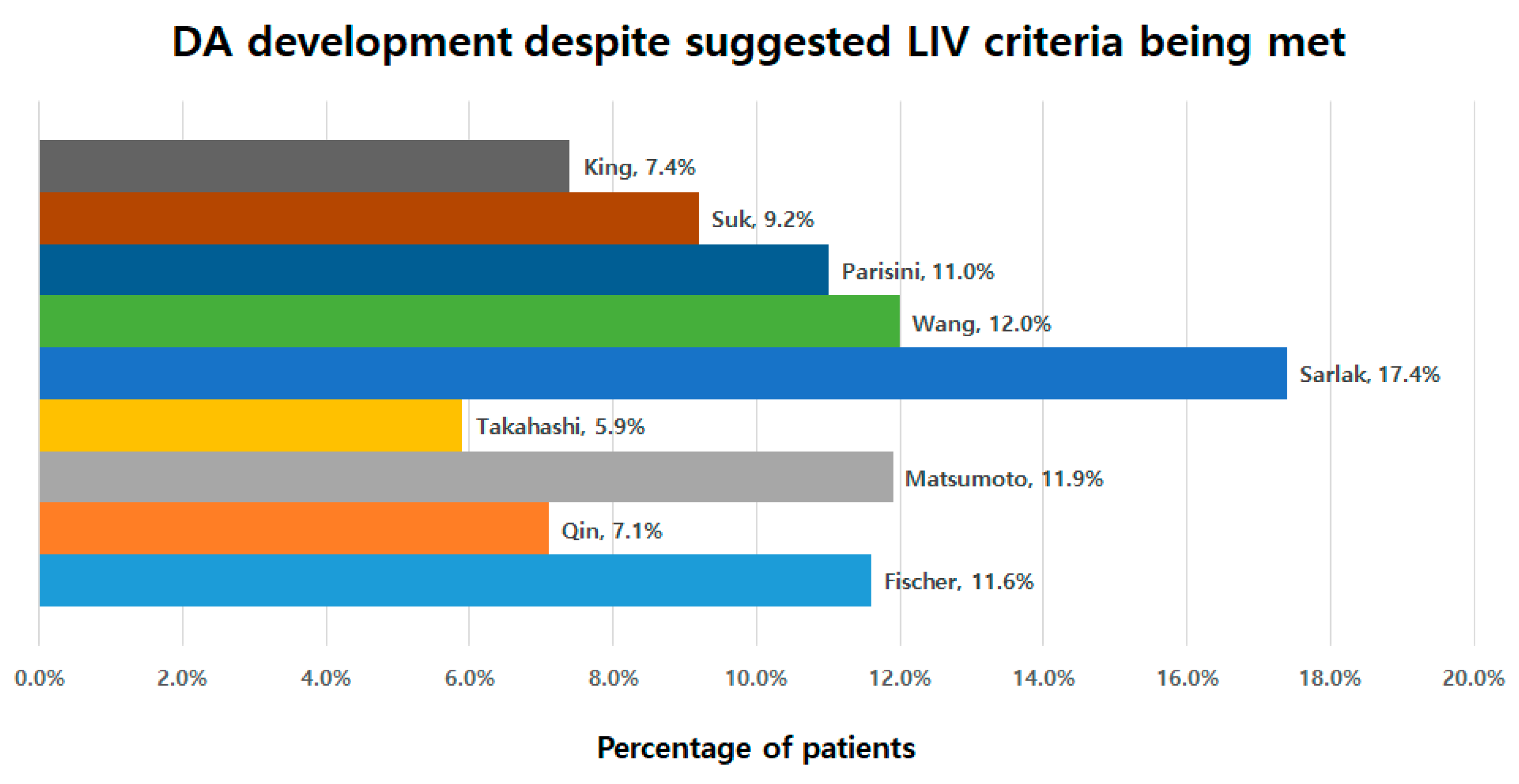
3.5. Development of DA despite Suggested LIV Criteria Being Met
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobbs, M.B.; Lenke, L.G.; Kim, Y.J.; Kamath, G.; Peelle, M.W.; Bridwell, K.H. Selective posterior thoracic fusions for adolescent idiopathic scoliosis: Comparison of hooks versus pedicle screws. Spine 2006, 31, 2400–2404. [Google Scholar] [CrossRef]
- Lehman, R.A., Jr.; Lenke, L.G.; Keeler, K.A.; Kim, Y.J.; Buchowski, J.M.; Cheh, G.; Kuhns, C.A.; Bridwell, K.H. Operative treatment of adolescent idiopathic scoliosis with posterior pedicle screw-only constructs: Minimum three-year follow-up of one hundred fourteen cases. Spine 2008, 33, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Lenke, L.G.; Betz, R.R.; Harms, J.; Bridwell, K.H.; Clements, D.H.; Lowe, T.G.; Blanke, K. Adolescent idiopathic scoliosis: A new classification to determine extent of spinal arthrodesis. J. Bone Jt. Surg. Am. 2001, 83, 1169–1181. [Google Scholar] [CrossRef]
- Kim, H.J.; Lenke, L.G.; Pizones, J.; Castelein, R.; Trobisch, P.D.; Yagi, M.; Kelly, M.P.; Chang, D.G. Adolescent Idiopathic Scoliosis: Is the Feasible Option of Minimally Invasive Surgery using Posterior Approach? Asian Spine J. 2024, 18, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Ikegami, S.; Oba, H.; Uehara, M.; Kuraishi, S.; Takizawa, T.; Munakata, R.; Hatakenaka, T.; Kamanaka, T.; Miyaoka, Y.; et al. Postoperative Residual Coronal Decompensation Inhibits Self-image Improvement in Adolescent Patients with Idiopathic Scoliosis. Asian Spine J. 2023, 17, 149–155. [Google Scholar] [CrossRef]
- Neradi, D.; Kumar, V.; Kumar, S.; Sodavarapu, P.; Goni, V.; Dhatt, S.S. Minimally Invasive Surgery versus Open Surgery for Adolescent Idiopathic Scoliosis: A Systematic Review and Meta-Analysis. Asian Spine J. 2022, 16, 279–289. [Google Scholar] [CrossRef]
- Wang, Y.; Hansen, E.S.; Hoy, K.; Wu, C.; Bunger, C.E. Distal adding-on phenomenon in Lenke 1A scoliosis: Risk factor identification and treatment strategy comparison. Spine 2011, 36, 1113–1122. [Google Scholar] [CrossRef]
- Fujii, T.; Kawabata, S.; Suzuki, S.; Tsuji, O.; Nori, S.; Okada, E.; Nagoshi, N.; Yagi, M.; Michikawa, T.; Nakamura, M.; et al. Can Postoperative Distal Adding-On be Predicted in Lenke Type 1B and 1C Curves with Intraoperative Radiographs? Spine 2022, 47, E215–E221. [Google Scholar] [CrossRef]
- Li, Y.; Bai, H.; Liu, C.; Zhao, Y.; Feng, Y.; Li, T.; Wang, X.; Zhang, Y.; Lei, W.; Zhao, X.; et al. Distal Adding-On Phenomenon in Lenke IA and Lenke IIA: Risk Analysis and Selection of the Lowest Instrumented Vertebra. World Neurosurg. 2020, 136, e171–e180. [Google Scholar] [CrossRef]
- Suk, S.I.; Lee, S.M.; Chung, E.R.; Kim, J.H.; Kim, W.J.; Sohn, H.M. Determination of distal fusion level with segmental pedicle screw fixation in single thoracic idiopathic scoliosis. Spine 2003, 28, 484–491. [Google Scholar] [CrossRef]
- Parisini, P.; Di Silvestre, M.; Lolli, F.; Bakaloudis, G. Selective thoracic surgery in the Lenke type 1A: King III and King IV type curves. Eur. Spine J. 2009, 18 (Suppl. S1), 82–88. [Google Scholar] [CrossRef] [PubMed]
- Sarlak, A.Y.; Atmaca, H.; Kim, W.J.; Musaoglu, R.; Tosun, B. Radiographic features of the Lenke 1A curves to help to determine the optimum distal fusion level selection. Spine 2011, 36, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Newton, P.O.; Ugrinow, V.L.; Bastrom, T.P. Selective thoracic fusion in adolescent idiopathic scoliosis: Factors influencing the selection of the optimal lowest instrumented vertebra. Spine 2011, 36, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Watanabe, K.; Hosogane, N.; Kawakami, N.; Tsuji, T.; Uno, K.; Suzuki, T.; Ito, M.; Yanagida, H.; Yamaguchi, T.; et al. Postoperative distal adding-on and related factors in Lenke type 1A curve. Spine 2013, 38, 737–744. [Google Scholar] [CrossRef]
- Fischer, C.R.; Lenke, L.G.; Bridwell, K.H.; Boachie-Adjei, O.; Gupta, M.; Kim, Y.J. Optimal Lowest Instrumented Vertebra for Thoracic Adolescent Idiopathic Scoliosis. Spine Deform. 2018, 6, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Sun, W.; Xu, L.; Liu, Z.; Qiu, Y.; Zhu, Z. Selecting the Last “Substantially” Touching Vertebra as Lowest Instrumented Vertebra in Lenke Type 1A Curve: Radiographic Outcomes With a Minimum of 2-year Follow-Up. Spine 2016, 41, E742–E750. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Luk, K.D.K.; Zhang, C.; Sun, J.; Wang, G. Relationship between Fusion Mass Shift and Postoperative Distal Adding-on in Lenke 1 Adolescent Idiopathic Scoliosis after Selective Thoracic Fusion. Asian Spine J. 2023, 17, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- King, H.A.; Moe, J.H.; Bradford, D.S.; Winter, R.B. The selection of fusion levels in thoracic idiopathic scoliosis. J. Bone Jt. Surg. Am. 1983, 65, 1302–1313. [Google Scholar] [CrossRef]
- Kim, D.H.; Hyun, S.J.; Kim, K.J. Selection of Fusion Level for Adolescent Idiopathic Scoliosis Surgery: Selective Fusion versus Postoperative Decompensation. J. Korean Neurosurg. Soc. 2021, 64, 473–485. [Google Scholar] [CrossRef]
- Helenius, I.; Remes, V.; Yrjonen, T.; Ylikoski, M.; Schlenzka, D.; Helenius, M.; Poussa, M. Comparison of long-term functional and radiologic outcomes after Harrington instrumentation and spondylodesis in adolescent idiopathic scoliosis: A review of 78 patients. Spine 2002, 27, 176–180. [Google Scholar] [CrossRef]
- Sanchez-Raya, J.; Bago, J.; Pellise, F.; Cuxart, A.; Villanueva, C. Does the lower instrumented vertebra have an effect on lumbar mobility, subjective perception of trunk flexibility, and quality of life in patients with idiopathic scoliosis treated by spinal fusion? Clin. Spine Surg. 2012, 25, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.K.; Rosner, M.K.; Lehman, R.A., Jr.; Polly, D.W., Jr.; Schroeder, T.M.; Kuklo, T.R. Reliability of end, neutral, and stable vertebrae identification in adolescent idiopathic scoliosis. Spine 2005, 30, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Blondel, B.; Lafage, V.; Schwab, F.; Farcy, J.P.; Bollini, G.; Jouve, J.L. Reciprocal sagittal alignment changes after posterior fusion in the setting of adolescent idiopathic scoliosis. Eur. Spine J. 2012, 21, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.L.; Pelletier, Y.; Solla, F.; Rampal, V. Surgical increase in thoracic kyphosis increases unfused lumbar lordosis in selective fusion for thoracic adolescent idiopathic scoliosis. Eur. Spine J. 2018, 28, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Colacchio, N.D.; Schwab, F.J.; Lafage, V.; Roye, D.P.; Vitale, M.G. Flatback Revisited: Reciprocal Loss of Lumbar Lordosis Following Selective Thoracic Fusion in the Setting of Adolescent Idiopathic Scoliosis. Spine Deform. 2015, 3, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hai, Y. Sagittal plane analysis of selective posterior thoracic spinal fusion in adolescent idiopathic scoliosis: A comparison study of all pedicle screw and hybrid instrumentation. J. Spinal Disord. Tech. 2014, 27, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.O.; Yaszay, B.; Upasani, V.V.; Pawelek, J.B.; Bastrom, T.P.; Lenke, L.G.; Lowe, T.; Crawford, A.; Betz, R.; Lonner, B.; et al. Preservation of thoracic kyphosis is critical to maintain lumbar lordosis in the surgical treatment of adolescent idiopathic scoliosis. Spine 2010, 35, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Ries, Z.; Harpole, B.; Graves, C.; Gnanapragasam, G.; Larson, N.; Weintstein, S.; Mendoza-Lattes, S.A. Selective Thoracic Fusion of Lenke I and II Curves Affects Sagittal Profiles But Not Sagittal or Spinopelvic Alignment: A Case-Control Study. Spine 2015, 40, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Dumpa, S.R.; Shetty, A.P.; Aiyer, S.N.; Kanna, R.M.; Rajasekaran, S. Reciprocal Changes in Sagittal Alignment in Adolescent Idiopathic Scoliosis Patients Following Strategic Pedicle Screw Fixation. Asian Spine J. 2018, 12, 300–308. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, C.S.; Lee, K.J.; Lee, J.W.; Park, J.S. Analysis of the Change Patterns of Sagittal Alignment Values After Selective Thoracic Fusion in Lenke 1 Adolescent Idiopathic Scoliosis According to Preoperative Thoracic Kyphosis Status. Clin. Spine Surg. 2020, 33, E352–E358. [Google Scholar] [CrossRef]
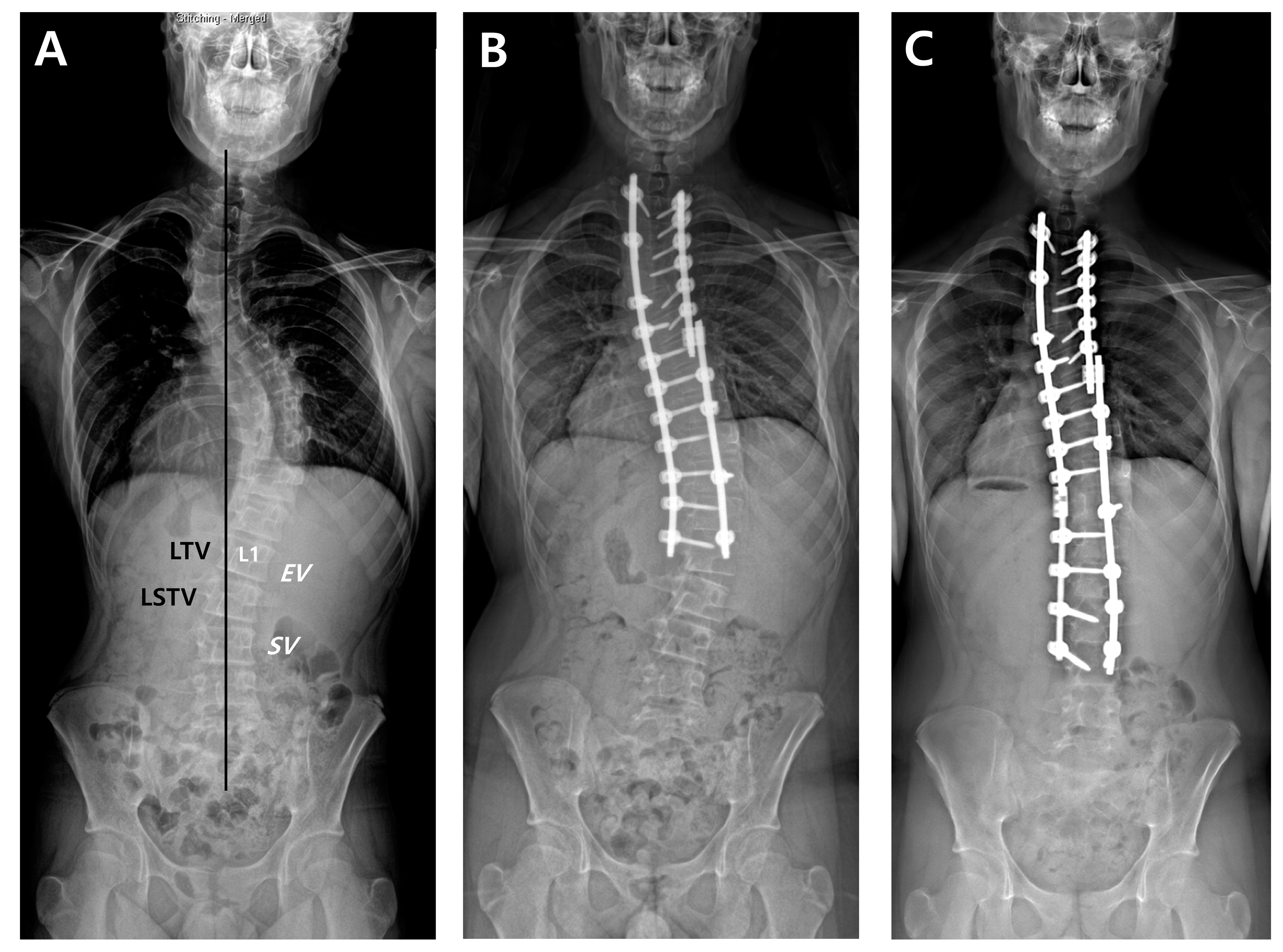

| Study | Curve Type | No. of Patients | Mean Age (Years) | Mean FU (Years) | Suggestion |
|---|---|---|---|---|---|
| King et al. (1983) [18] | King 3, 4, 5 | 405 | 14.8 | 4.0 | SV |
| Suk et al. (2003) [10] | King 3, 4 | 42 | 15.5 | 4.2 | Diff. between NV and EV: ≤1 levels → NV ≥2 level → NV-1 |
| Parisini et al. (2009) [11] | Lenke 1A | 31 | 16.3 | Min. 2 | Direction of rotation at LEV+1 is equal to that of thoracic curve and diff. between SV and EV is ≥3 level → L2 or L3. Otherwise → SV-2 or SV-3 |
| Wang et al. (2011) [7] | Lenke 1A | 45 | - | 3.6 | 1st vertebrae >10 mm from CSVL |
| Sarlak et al. (2011) [12] | Lenke 1A | 36 | 15.8 | 4.3 | L3 tilt (−) → LEV-1 L3 tilt (+) → LEV |
| Takahashi et al. (2011) [13] | Lenke 1B, 1C, 3C | 172 | 14 | 2 | SV+1 |
| Matsumoto et al. (2013) [14] | Lenke 1A | 112 | 16.1 | 3.6 | LTV |
| Qin et al. (2016) [16] | Lenke 1A | 104 | 14.5 | Min. 2 | LSTV or nSTV+1 |
| Fischer et al. (2018) [15] | Lenke 1, 2 | 544 | 14.7 | 4.1 | LTV within NV-2 |
| King | Suk | Parisini | Wang | Sarlak | Takahashi | Matsumoto | Qin | Fischer | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 54 | 109 | 118 | 117 | 136 | 17 | 109 | 84 | 112 | - |
| Female (%) | 83.3% | 80.7% | 82.2% | 82.9% | 82.4% | 88.2% | 81.7% | 82.1% | 83.9% | 0.999 |
| Age (years) | 14.5 | 14.9 | 14.7 | 14.7 | 14.9 | 13.2 | 14.7 | 14.6 | 14.6 | 0.536 |
| Lenke 1A (%) | 81.5% | 79.8% | 79.7% | 79.5% | 80.1% | 85.1% | 78.9% | 77.4% | 78.6% | 0.770 |
| Pre CA (°) | 54.8° | 56.1° | 56.4° | 55.5° | 56.0° | 52.9° | 55.3° | 54.9° | 55.1° | 0.913 |
| Flexibility (%) | 53.0% | 51.6% | 50.7% | 51.9% | 51.8% | 53.9% | 52.4% | 52.3% | 52.7% | 0.993 |
| Post CA (°) | 19.2° | 20.2° | 20.6° | 19.9° | 19.8° | 15.1° | 19.8° | 20.0° | 19.5° | 0.653 |
| Correction rate (%) | 65.3% | 64.5% | 63.9% | 64.7% | 65.2% | 71.1% | 64.7% | 64.0% | 65.1% | 0.775 |
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Nine criteria | |||
| King | 13.1 ± 1.7 | 9.0 | 16.0 |
| Suk | 12.1 ± 1.4 | 9.0 | 15.0 |
| Parisini | 11.4 ± 1.9 | 7.0 | 14.0 |
| Wang | 12.1 ± 1.4 | 8.0 | 15.0 |
| Sarlak | 11.7 ± 1.0 | 8.0 | 14.0 |
| Takahashi | 14.1 ± 1.7 | 10.0 | 17.0 |
| Matsumoto | 12.0 ± 1.6 | 8.0 | 16.0 |
| Qin | 12.6 ± 1.6 | 9.0 | 16.0 |
| Fischer | 12.1 ± 1.6 | 8.0 | 16.0 |
| Current Study population | |||
| EV | 11.7 ± 1.0 | 8.0 | 14.0 |
| NV | 12.3 ± 1.6 | 9.0 | 16.0 |
| SV | 13.1 ± 1.7 | 9.0 | 16.0 |
| Postoperative actual LIV | 12.3 ± 1.1 | 9.0 | 14.0 |
| B | S.E | Wald | p | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| King | ||||||
| Proximal to LIV vs. at LIV | −1.190 | 0.653 | 3.323 | 0.068 | 0.304 | 0.085–1.094 |
| At LIV vs. distal to LIV | −0.251 | 1.195 | 0.044 | 0.833 | 0.778 | 0.075–8.095 |
| Suk | ||||||
| Proximal to LIV vs. at LIV | −1.319 | 0.513 | 6.608 | 0.010 | 0.267 | 0.098–0.731 |
| At LIV vs. distal to LIV | −1.487 | 0.816 | 3.324 | 0.068 | 0.226 | 0.046–1.118 |
| Parisini | ||||||
| Proximal to LIV vs. at LIV | −1.471 | 0.734 | 4.012 | 0.045 | 0.230 | 0.054–0.969 |
| At LIV vs. distal to LIV | −0.306 | 0.705 | 0.188 | 0.665 | 0.736 | 0.185–2.935 |
| Wang | ||||||
| Proximal to LIV vs. at LIV | −1.240 | 0.533 | 5.407 | 0.020 | 0.289 | 0.102–0.823 |
| At LIV vs. distal to LIV | −0.413 | 0.592 | 0.486 | 0.486 | 0.662 | 0.208–2.111 |
| Sarlak | ||||||
| Proximal to LIV vs. at LIV | −1.463 | 0.754 | 3.766 | 0.052 | 0.231 | 0.053–1.015 |
| At LIV vs. distal to LIV | −0.138 | 0.484 | 0.081 | 0.775 | 0.871 | 0.337–2.251 |
| Takahashi | ||||||
| Proximal to LIV vs. at LIV | −0.784 | 1.704 | 0.533 | 0.465 | 0.457 | 0.056–3.745 |
| At LIV vs. distal to LIV | −1.131 | 0.229 | 1.487 | 0.222 | −0.323 | 0.052–1.985 |
| Matsumoto | ||||||
| Proximal to LIV vs. at LIV | −0.768 | 0.501 | 2.348 | 0.125 | 0.464 | 0.174–1.239 |
| At LIV vs. distal to LIV | −1.162 | 0.689 | 2.844 | 0.092 | 0.313 | 0.081–1.207 |
| Qin | ||||||
| Proximal to LIV vs. at LIV | −1.563 | 0.544 | 8.241 | 0.004 | 0.210 | 0.072–0.609 |
| At LIV vs. distal to LIV | −0.611 | 1.125 | 0.295 | 0.587 | 0.543 | 0.060–4.922 |
| Fischer | ||||||
| Proximal to LIV vs. at LIV | −0.135 | 0.507 | 0.071 | 0.790 | 1.145 | 0.424–3.092 |
| At LIV vs. distal to LIV | −1.511 | 0.674 | 5.020 | 0.025 | 0.221 | 0.059–0.828 |
| King | Suk | Parisini | Wang | Sarlak | Takahashi | Matsumoto | Qin | Fischer | |
|---|---|---|---|---|---|---|---|---|---|
| King | 1 | 0.614 | 0.621 | 0.727 | 0.487 | 0.850 | 0.789 | 0.916 | 0.776 |
| Suk | 1 | 0.604 | 0.710 | 0.678 | 0.406 | 0.710 | 0.690 | 0.778 | |
| Parisini | 1 | 0.698 | 0.561 | 0.422 | 0.827 | 0.673 | 0.786 | ||
| Wang | 1 | 0.720 | 0.477 | 0.872 | 0.833 | 0.851 | |||
| Sarlak | 1 | 0.295 | 0.706 | 0.611 | 0.681 | ||||
| Takahashi | 1 | 0.534 | 0.682 | 0.527 | |||||
| Matsumoto | 1 | 0.885 | 0.960 | ||||||
| Qin | 1 | 0.871 | |||||||
| Fischer | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-J.; Park, J.-S.; Kang, D.-H.; Lee, C.-S. The Optimal Lowest Instrumented Vertebra to Prevent the Distal Adding-On Phenomenon in Patients Undergoing Selective Thoracic Fusion for Adolescent Idiopathic Scoliosis with Lenke Type 1A and 1B Curves: Comparison of Nine Selection Criteria. J. Clin. Med. 2024, 13, 3859. https://doi.org/10.3390/jcm13133859
Park S-J, Park J-S, Kang D-H, Lee C-S. The Optimal Lowest Instrumented Vertebra to Prevent the Distal Adding-On Phenomenon in Patients Undergoing Selective Thoracic Fusion for Adolescent Idiopathic Scoliosis with Lenke Type 1A and 1B Curves: Comparison of Nine Selection Criteria. Journal of Clinical Medicine. 2024; 13(13):3859. https://doi.org/10.3390/jcm13133859
Chicago/Turabian StylePark, Se-Jun, Jin-Sung Park, Dong-Ho Kang, and Chong-Suh Lee. 2024. "The Optimal Lowest Instrumented Vertebra to Prevent the Distal Adding-On Phenomenon in Patients Undergoing Selective Thoracic Fusion for Adolescent Idiopathic Scoliosis with Lenke Type 1A and 1B Curves: Comparison of Nine Selection Criteria" Journal of Clinical Medicine 13, no. 13: 3859. https://doi.org/10.3390/jcm13133859
APA StylePark, S.-J., Park, J.-S., Kang, D.-H., & Lee, C.-S. (2024). The Optimal Lowest Instrumented Vertebra to Prevent the Distal Adding-On Phenomenon in Patients Undergoing Selective Thoracic Fusion for Adolescent Idiopathic Scoliosis with Lenke Type 1A and 1B Curves: Comparison of Nine Selection Criteria. Journal of Clinical Medicine, 13(13), 3859. https://doi.org/10.3390/jcm13133859








