Expression of Hormones’ Receptors in Human Corneal Endothelium from Fuchs’ Dystrophy: A Possible Gender’ Association
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations and STUDY Population
2.2. Clinical Assessment and Routine Biological Tests
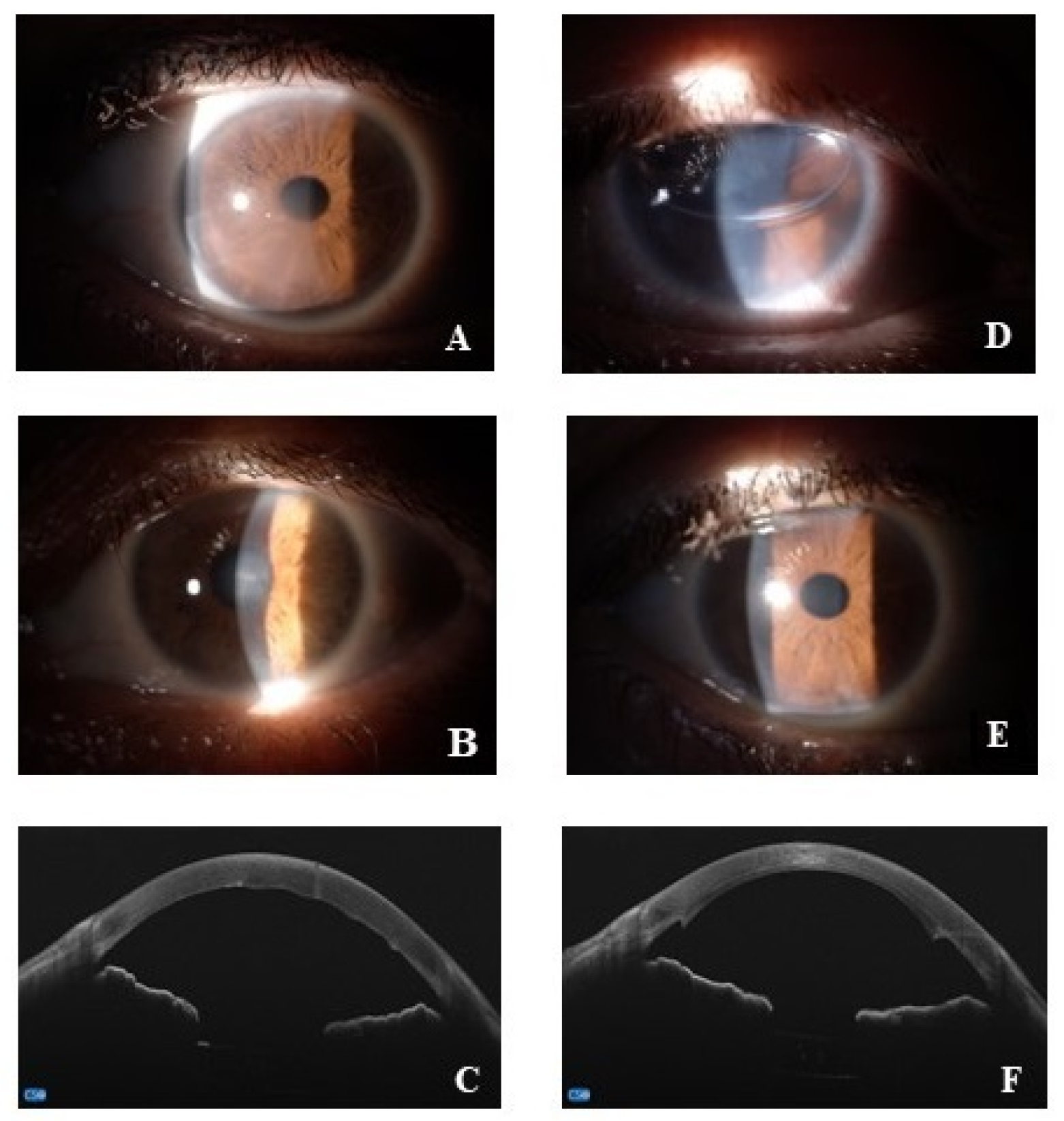
2.3. Surgical Procedures
2.4. Molecular Analysis: Tissue Processing and 2-Step Real-Time RT-PCR Analysis
2.5. Biochemical Analysis: SDS-PAGE and IntDen Analysis
2.6. Statistical Analysis
3. Results
3.1. PR, ERα, AR and SHBG Transcripts’ Expression in Fuchs Endothelia
3.2. Fuchs-Endothelia Synthesizes Growth Factors and Matrix Enzymes’ Transcripts
3.3. Fuchs-Endothelia Display a Th1 Phenotype
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jun, A.S. One hundred years of Fuchs’ dystrophy. Ophthalmology 2010, 117, 859–860.e814. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Azizi, B.; Jurkunas, U.V. Fuchs Endothelial Corneal Dystrophy. Ocul. Surf. 2010, 8, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Tone, S.O.; Kocaba, V.; Böhm, M.; Wylegala, A.; White, T.L.; Jurkunas, U.V. Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis. Prog. Retin. Eye Res. 2021, 80, 100863. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, F.; Ortiz-Morales, G.; O′Connor-Cordova, M.A.; Sancén-Herrera, J.P.; Zavala, J.; Valdez-Garcia, J.E. Fuchs endo-thelial corneal dystrophy: An updated review. Int. Ophthalmol. 2024, 44, 61. [Google Scholar] [CrossRef] [PubMed]
- Hamill, C.E.; Schmedt, T.; Jurkunas, U. Fuchs endothelial cornea dystrophy: A review of the genetics behind disease development. Semin. Ophthalmol. 2013, 28, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhang, S.; Nielsen, E.; Ivarsen, A.R.; Liang, C.; Li, Q.; Thomsen, K.; Hjortdal, J.Ø.; Dong, M. The Ultrastructures and Mechanical Properties of the Descement′s Membrane in Fuchs Endothelial Corneal Dystrophy. Sci. Rep. 2016, 6, 23096. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.A.; Figueiredo, F.C.; Connon, C.J. Use of biomaterials in corneal endothelial repair. Ther. Adv. Ophthalmol. 2021, 13, 25158414211058249. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Louttit, M.D.; Kopplin, L.J.; Igo, R.P., Jr.; Fondran, J.R.; Tagliaferri, A.; Bardenstein, D.; Aldave, A.J.; Croasdale, C.R.; Price, M.O.; Rosenwasser, G.O.; et al. A multicenter study to map genes for Fuchs endothelial corneal dystrophy: Baseline characteristics and heritability. Cornea 2012, 31, 26–35. [Google Scholar] [CrossRef]
- Aiello, F.; Gallo Afflitto, G.; Ceccarelli, F.; Cesareo, M.; Nucci, C. Global Prevalence of Fuchs Endothelial Corneal Dystrophy (FECD) in Adult Population: A Systematic Review and Meta-Analysis. J. Ophthalmol. 2022, 2022, 3091695. [Google Scholar] [CrossRef]
- Zhang, X.; Igo, R.P., Jr.; Fondran, J.; Mootha, V.V.; Oliva, M.; Hammersmith, K.; Sugar, A.; Lass, J.H.; Iyengar, S.K.; Fuchs′ Genetics Multi-Center Study Group. Association of smoking and other risk factors with Fuchs’ endothelial corneal dystrophy severity and corneal thickness. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5829–5835. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.W. Targeting the NRF2 pathway: A promising approach for corneal endothelial dysfunction. Curr. Opin. Pharmacol. 2024, 74, 102429. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Owczarek, K.; Lewandowska, U. The Role of Metalloproteinases and Their Tissue Inhibitors on Ocular Diseases: Focusing on Potential Mechanisms. Int. J. Mol. Sci. 2022, 23, 4256. [Google Scholar] [CrossRef] [PubMed]
- Kocaba, V.; Katikireddy, K.R.; Gipson, I.; Price, M.O.; Price, F.W.; Jurkunas, U.V. Association of the gutta-induced microenvironment with corneal endothelial cell behavior and demise in fuchs endothelial corneal dystrophy. JAMA Ophthalmol. 2018, 136, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.M.; Zenkel, M.; Schlötzer-Schrehardt, U.; Bachmann, B.O.; Tourtas, T.; Kruse, F.E. Extracellular matrix alterations in late-onset Fuchs’ corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3700–3708. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, M.; Lajoix, A.D.; Desmetz, C. Experimental Models to Study Endothelial to Mesenchymal Transition in Myocardial Fibrosis and Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 382. [Google Scholar] [CrossRef]
- Katikireddy, K.R.; White, T.L.; Miyajima, T.; Vasanth, S.; Raoof, D.; Chen, Y.; Price, M.O.; Price, F.W.; Jurkunas, U.V. NQO1 downregulation potentiates menadione-induced endothelial-mesenchymal transition during rosette formation in Fuchs endothelial corneal dystrophy. Free Radic. Biol. Med. 2018, 116, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Sornelli, F.; Lambiase, A.; Mantelli, F.; Aloe, L. NGF and NGF-receptor expression of cultured immortalized human corneal endothelial cells. Mol. Vis. 2010, 16, 1439–1447. [Google Scholar] [PubMed]
- Gorimanipalli, B.; Khamar, P.; Sethu, S.; Shetty, R. Hormones and dry eye disease. Indian J. Ophthalmol. 2023, 71, 1276–1284. [Google Scholar] [CrossRef]
- Yamaguchi, T. Inflammatory Response in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES192–DES199. [Google Scholar] [CrossRef]
- du Toit, R.; Vega, J.A.; Fonn, D.; Simpson, T. Diurnal variation of corneal sensitivity and thickness. Cornea 2003, 22, 205–209. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Lu, A.; Beeson, C. Maternal Corneal Thickness During Pregnancy. Am. J. Ophthalmol. 1988, 105, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Cho, K.; Srikumaran, D. Fuchs Dystrophy and Cataract: Diagnosis, Evaluation and Treatment. Ophthalmol. Ther. 2023, 12, 691–704. [Google Scholar] [CrossRef]
- Seitzman, G.D.; Gottsch, J.D.; Stark, W.J. Cataract surgery in patients with Fuchs’ corneal dystrophy: Expanding recommendations for cataract surgery without simultaneous keratoplasty. Ophthalmology 2005, 112, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Price, F.W., Jr. Endothelial cell loss after descemet stripping with endothelial keratoplasty influencing factors and 2-year trend. Ophthalmology 2008, 115, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Price, F.W., Jr. Descemet’s stripping with endothelial keratoplasty: Comparative outcomes with microkeratome-dissected and manually dissected donor tissue. Ophthalmology 2006, 113, 1936–1942. [Google Scholar] [CrossRef]
- Yeh, P.; Colby, K. Corneal Endothelial Dystrophies, 4th ed.; Foster, C., Dimitri, T., Eds.; The Cornea: Scientific Foundations and Clinical Practice; Lippincott William and Wilkins: Philadelphia, PA, USA, 2005; Volume 47, p. 849. [Google Scholar]
- Wilson, S.E.; Bourne, W.M. Fuchs’ dystrophy. Cornea 1988, 7, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Zoega, G.M.; Fujisawa, A.; Sasaki, H.; Kubota, A.; Sasaki, K.; Kitagawa, K.; Jonasson, F. Prevalence and risk factors for cornea guttata in the Reykjavik Eye Study. Ophthalmology 2006, 113, 565–569. [Google Scholar] [CrossRef]
- Stapleton, F.; Abad, J.C.; Barabino, S.; Burnett, A.; Iyer, G.; Lekhanont, K.; Li, T.; Liu, Y.; Navas, A.; Obinwanne, C.J.; et al. TFOS lifestyle: Impact of societal challenges on the ocular surface. Ocul. Surf. 2023, 28, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.I.; Poppelaars, F.; Wagner, B.D.; Palestine, A.G.; Patnaik, J.L.; Holers, V.M.; Frazer-Abel, A.A.; Mathias, M.T.; Manoharan, N.; Fonteh, C.N.; et al. Sex and age-related differences in complement factors among patients with intermediate age-related macular degeneration. Transl. Vis. Sci. Technol. 2022, 11, 22. [Google Scholar] [CrossRef]
- Cailing, L.; Miyajima, T.; Melangath, G.; Miyai, T.; Vasanth, S.; Deshpande, N.; Kumar, V.; Ong Tone, S.; Gupta, R.; Zhu, S.; et al. Ultraviolet A light induces DNA damage and estrogen-DNA adducts in Fuchs endothelial corneal dystrophy causing females to be more affected. Proc. Natl. Acad. Sci. USA 2020, 117, 573–583. [Google Scholar] [CrossRef]
- Adamis, A.P.; Filatov, V.; Tripathi, B.J. Fuchs’ endothelial dystrophy of the cornea. Surv. Ophthalmol. 1993, 38, 149–168. [Google Scholar] [CrossRef]
- Miyajima, T.; Melangath, G.; Zhu, S.; Deshpande, N.; Vasanth, S.; Mondal, B.; Kumar, V.; Chen, Y.; Price, M.O.; Price, F.W., Jr.; et al. Loss of NQO1 generates genotoxic estrogen-DNA adducts in Fuchs endothelial corneal dystrophy. Free Radic. Biol. Med. 2020, 147, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Hadeyama, T.; Nakayasu, K.; Ha, N.T.; Nakamura, S. Expression of estrogen receptors alpha and beta, androgen receptors and progesterone receptors in human cornea. Nippon. Ganka Gakkai Zasshi 2002, 106, 557–564. [Google Scholar] [PubMed]
- Vecsei, P.V.; Kircher, K.; Kaminski, S.; Nagel, G.; Breitenecker, G.; Kohlberger, P.D. Immunohistochemical detection of estrogen and progesterone receptor in human cornea. Maturitas 2000, 36, 169–172. [Google Scholar] [CrossRef]
- Kumar, V.; Deshpande, N.; Parekh, M.; Wong, R.; Ashraf, S.; Zahid, M.; Hui, H.; Miall, A.; Kimpton, S.; Price, M.O.; et al. Estrogen genotoxicity causes preferential development of Fuchs endothelial corneal dystrophy in females. Redox Biol. 2024, 69, 102986. [Google Scholar] [CrossRef]
- Okumura, N.; Hashimoto, K.; Kitahara, M.; Okuda, H.; Ueda, E.; Watanabe, K.; Nakahara, M.; Sato, T.; Kinoshita, S.; Tourtas, T.; et al. Activation of TGF-β signaling induces cell death via the unfolded protein response in Fuchs endothelial corneal dystrophy. Sci. Rep. 2017, 7, 6801. [Google Scholar] [CrossRef] [PubMed]
- Fiolk, R.; Wylęgała, E.; Toborek, M.; Szkodny, D.; Czuba, Z.; Wylęgała, A. Fuch’s Endothelial Corneal Dystrophy in Cataract Patients Is Associated with Elevated Levels of Inflammatory Chemokines, but Not Growth Factors, in the Aqueous Humor. Int. J. Mol. Sci. 2024, 25, 1894. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.L.; Balzamino, B.O.; Micera, A. Nerve Growth Factor: A Focus on Neuroscience and Therapy. Curr. Neuropharmacol. 2015, 13, 294–303. [Google Scholar] [CrossRef]
- Tuft, S.J.; Coster, D.J. The corneal endothelium. Eye 1990, 4 Pt 3, 389–424. [Google Scholar] [CrossRef]
- Gavin, K.M.; Seals, D.R.; Silver, A.E.; Moreau, K.L. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J. Clin. Endocrinol. Metab. 2009, 94, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, R.; Caselgrandi, P. Sex Hormones and Their Effects on Ocular Disorders and Pathophysiology: Current Aspects and Our Experience. Int. J. Mol. Sci. 2022, 23, 3269. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mueller, C.; Wuebbolt, C.; Kilcullen, S.; Nayyar, V.; Gonzalez, B.C.; Fard, A.M.; Floss, J.C.; Morales, M.J.; Patel, S.P. Selective effects of estradiol on human corneal endothelial cells. Sci. Rep. 2023, 13, 15279. [Google Scholar] [CrossRef] [PubMed]



| Referring Gene | Forward and Reverse Sequences | Accession Number |
|---|---|---|
| H3 | F: 5′-GTC TGC AGG CTG GCA TAG AAG-3′ R: 5′-TCG CCT TCT GGG TTG AGT G-3′ | NM005324.4 |
| 18S | F: 5′-GGA GAG GGA GCC TGA GAA C-3′ R: 5′-AGG GCC TCG AAA GAG TCC T-3′ | NR003286 |
| Target gene | ||
| PR | F: 5′-TAC GGA GCC AGC AGA AGT CC-3′ R: 5′-TGA AGC TCT CAG TCC CTC GC-3′ | X51730.1 |
| ERα | F: 5′-GTG GTG CCC CTC TAT GAC CT-3′ R: 5′-TGC CTC CCC CGT GAT GTA AT-3′ | X03635 |
| AR | F: 5′-GGG GAC ATG CGT TTG GAG AC-3′ R: 5′-CTG TTT CCC TTC AGC GGC TC-3′ | M34233 |
| SHBG | F: 5′-AAA TCA CTC CCT CTG GGT CC-3′ R: 5′-AAG TCA AGA TGG AGG GGG AC-3′ | BC112186.1 |
| VEGF A | F: 5′-TGA CAG GGA AGA GGA GGA GA-3′ R: 5′-CGG TGT TCC CAA AAC TGG-3′ | AF022375.1 |
| βNGF | F: 5′-TGA AGC TGC AGA CAC TCA GG-3′ R: 5′-CAC CTC CTT GCC CTT GAT GT-3′ | BC126150.1 |
| TGFβ1 | F: 5′-GAG ATG AGG GTT TCC ACG AG-3′ R: 5′-GCG CCG AGA TGT AGT TAT CC-3′ | BC017288 |
| MMP1 | F: 5′-TCC CAG AGA GCA GCT TCA GT-3′ R: 5′-CCT ATC CAG GGT GAC ACC AG-3′ | BC013875 |
| MMP7 | F: 5′-GAG CTC ATG GGG ACT CCT AC-3′ R: 5′-ACT GCT ACC ATC CGT CCA G-3′ | BC003635 |
| IFNγ | F: 5′-ACC TAA GCA AGA TCC CAT GGG-3′ R: 5′-TGG GTA CAG TCA CAG TTG TCA A-3′ | NM_000619.3 |
| IL-10 | F: 5′-GCC TGA CCA CGC TTT CTA GC-3′ R: 5′-GGC TCC CTG GTT TCT CTT CC-3′ | M57627 |
| Pt. | Gender | Age | CCT (µm) | Phakia/PseudoPhakia |
|---|---|---|---|---|
| 1 | F | 70 | 601 | Phakia |
| 2 | F | 84 | 945 | Phakia |
| 3 | F | 73 | 599 | Combined PHACO + IOL |
| 4 | F | 71 | 669 | Combined PHACO + IOL |
| 5 | M | 69 | 693 | Previous PHACO + IOL |
| 6 | M | 88 | 616 | Combined PHACO + IOL |
| 7 | F | 76 | 745 | Previous PHACO + IOL |
| 8 | F | 70 | 750 | Previous PHACO + IOL |
| 9 | F | 57 | 538 | Combined PHACO + IOL |
| 10 | F | 78 | 659 | Phakia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Piano, M.; Abicca, I.; Dinu, V.; Roszkowska, A.M.; Micera, A.; Schiano-Lomoriello, D. Expression of Hormones’ Receptors in Human Corneal Endothelium from Fuchs’ Dystrophy: A Possible Gender’ Association. J. Clin. Med. 2024, 13, 3787. https://doi.org/10.3390/jcm13133787
De Piano M, Abicca I, Dinu V, Roszkowska AM, Micera A, Schiano-Lomoriello D. Expression of Hormones’ Receptors in Human Corneal Endothelium from Fuchs’ Dystrophy: A Possible Gender’ Association. Journal of Clinical Medicine. 2024; 13(13):3787. https://doi.org/10.3390/jcm13133787
Chicago/Turabian StyleDe Piano, Maria, Irene Abicca, Valentin Dinu, Anna Maria Roszkowska, Alessandra Micera, and Domenico Schiano-Lomoriello. 2024. "Expression of Hormones’ Receptors in Human Corneal Endothelium from Fuchs’ Dystrophy: A Possible Gender’ Association" Journal of Clinical Medicine 13, no. 13: 3787. https://doi.org/10.3390/jcm13133787
APA StyleDe Piano, M., Abicca, I., Dinu, V., Roszkowska, A. M., Micera, A., & Schiano-Lomoriello, D. (2024). Expression of Hormones’ Receptors in Human Corneal Endothelium from Fuchs’ Dystrophy: A Possible Gender’ Association. Journal of Clinical Medicine, 13(13), 3787. https://doi.org/10.3390/jcm13133787








