Impact of Periodontitis on Endothelial Risk Dysfunction and Oxidative Stress Improvement in Patients with Cardiovascular Disease
Abstract
1. Introduction
2. Materials and Methods
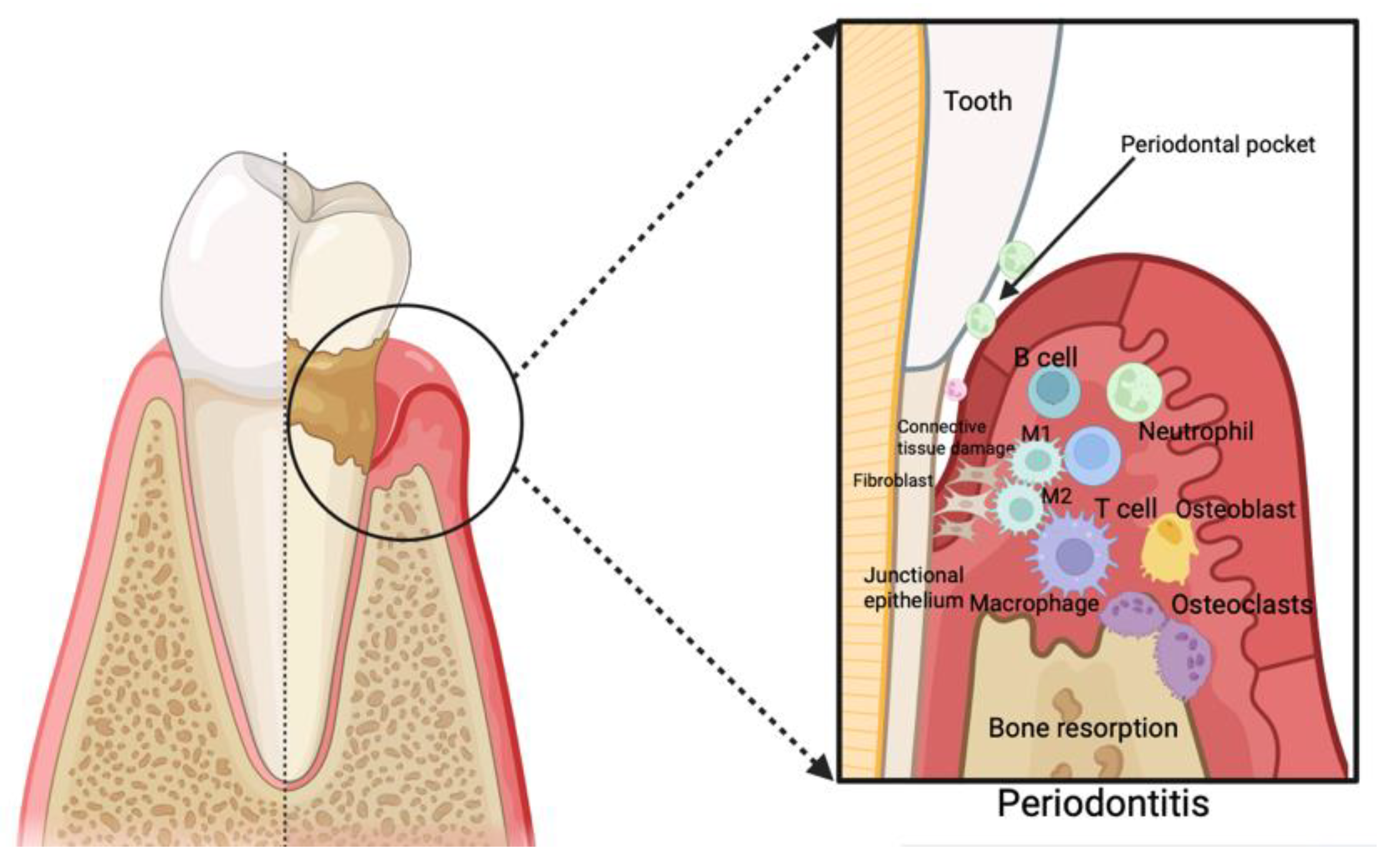
3. Periodontitis as an Inflammatory Disease and Its Correlation with Cardiovascular Disease
4. Impact of Periodontal Therapy on Endothelial Dysfunction in Individuals with Periodontitis
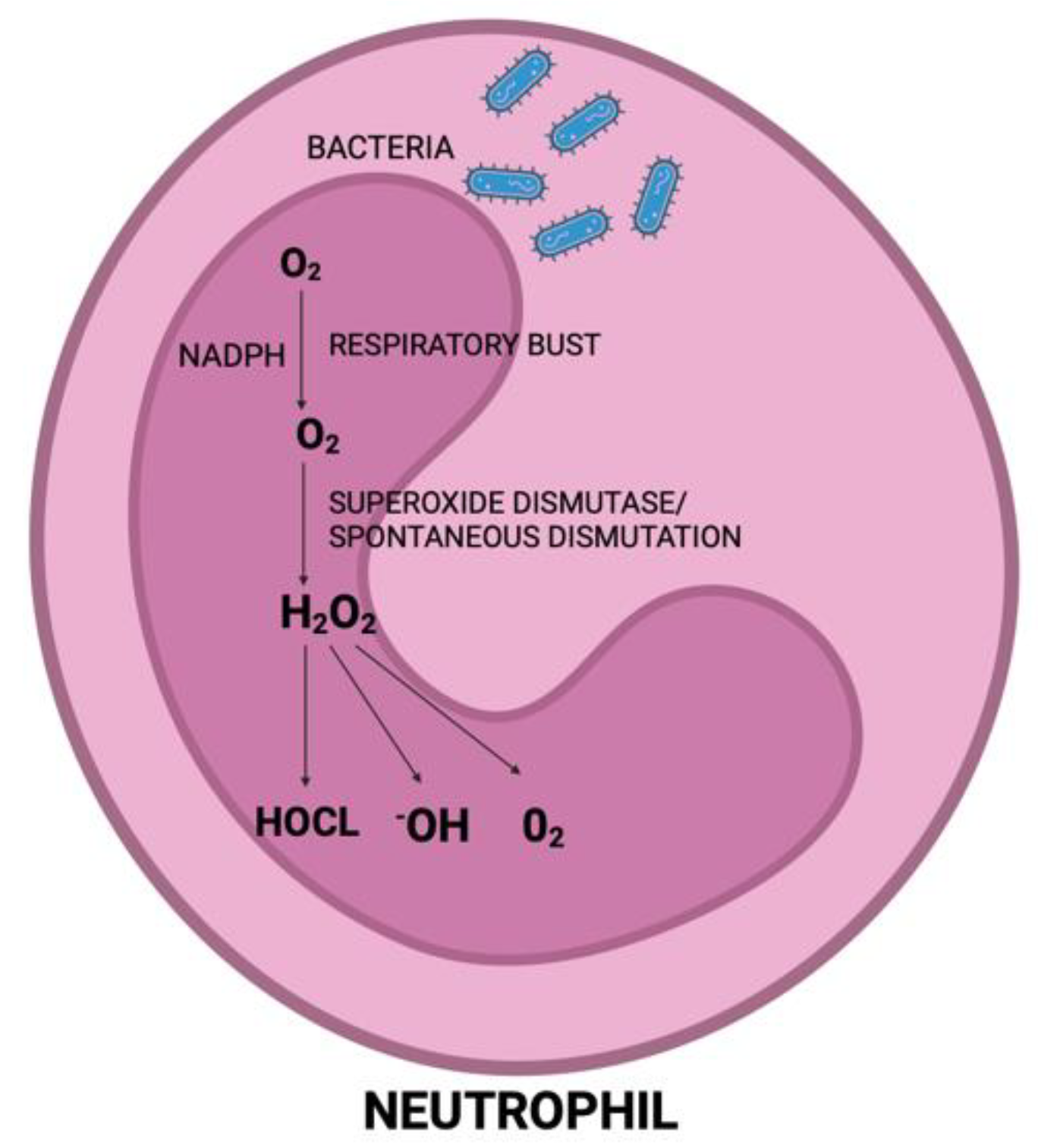
5. Impact of Periodontal Therapy on Oxidative Stress in Patients Affected by Periodontitis
5.1. Oxidative Stress Biomarkers
5.1.1. 8-Hydroxydeoxyguanosine (8-OHdG)
5.1.2. Malondialdehyde (MDA)
5.2. Arterial Stiffness and Endothelial Dysfunction Outcomes
6. Impact of Periodontal Treatment on CVD Biomarkers and Outcomes
7. Future Research on Periodontal Treatment and Cardiovascular Health
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Gasner, N.S.; Schure, R.S. Periodontal Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-De-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of periodontal disease on quality of life: A systematic review. J. Periodontal Res. 2017, 52, 651–665. [Google Scholar] [CrossRef]
- Herrera, D.; Molina, A.; Buhlin, K.; Klinge, B. Periodontal diseases and association with atherosclerotic disease. Periodontol. 2000 2020, 83, 66–89. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-Da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Martu, M.-A.; Maftei, G.-A.; Luchian, I.; Stefanescu, O.M.; Scutariu, M.M.; Solomon, S.M. The Effect of Acknowledged and Novel Anti-Rheumatic Therapies on Periodontal Tissues—A Narrative Review. Pharmaceuticals 2021, 14, 1209. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Wu, K.Y.; Leclerc, M.; Pham, H.M.; Tran, S.D. Cardiovascular Diseases and Periodontal Disease. Curr. Oral Health Rep. 2018, 5, 13–18. [Google Scholar] [CrossRef]
- Zardawi, F.; Gul, S.; Abdulkareem, A.; Sha, A.; Yates, J. Association Between Periodontal Disease and Atherosclerotic Cardiovascular Diseases: Revisited. Front. Cardiovasc. Med. 2021, 7, 625579. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Indelicato, F.; Chaurasia, A. Association among stress and depression in patients with advanced periodontitis: A cross-sectional study. Mediterr. J. Clin. Psychol. 2023, 11, 1–23. [Google Scholar] [CrossRef]
- Mustapha, I.Z.; Debrey, S.; Oladubu, M.; Ugarte, R. Markers of Systemic Bacterial Exposure in Periodontal Disease and Cardiovascular Disease Risk: A Systematic Review and Meta-Analysis. J. Periodontol. 2007, 78, 2289–2302. [Google Scholar] [CrossRef]
- Loos, B.G.; Van Dyke, T.E. The role of inflammation and genetics in periodontal disease. Periodontology 2000 2020, 83, 26–39. [Google Scholar] [CrossRef]
- Lazureanu, P.C.; Popescu, F.G.; Stef, L.; Focsa, M.; Vaida, M.A.; Mihaila, R. The Influence of Periodontal Disease on Oral Health Quality of Life in Patients with Cardiovascular Disease: A Cross-Sectional Observational Single-Center Study. Medicina 2022, 58, 584. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Hoare, A.; Hong, B.; Diaz, P.I. Microbial signatures of health, gingivitis, and periodontitis. Periodontology 2000 2021, 86, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.M.U.; Salama, R.I. Association between periodontal disease and cardiovascular disease. Pak. J. Med. Sci. 2004, 20, 151–156. [Google Scholar]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, B.A. Periodontal Therapy. Top. Companion Anim. Med. 2008, 23, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Yoshimoto, T.; Kajiya, M.; Ouhara, K.; Matsuda, S.; Takemura, T.; Akutagawa, K.; Takeda, K.; Mizuno, N.; Kurihara, H. Regulation of defensive function on gingival epithelial cells can prevent periodontal disease. Jpn. Dent. Sci. Rev. 2018, 54, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Hasturk, H.; Kantarci, A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontology 2000 2015, 69, 255–273. [Google Scholar] [CrossRef]
- Franco, C.; Patricia, H.-R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef]
- Orlandi, M.; Graziani, F.; D’Aiuto, F. Periodontal therapy and cardiovascular risk. Periodontology 2000 2020, 83, 107–124. [Google Scholar] [CrossRef]
- Madiba, T.; Bhayat, A. Periodontal disease—Risk factors and treatment options. S. Afr. Dent. J. 2018, 73, 571–575. [Google Scholar] [CrossRef]
- Buhlin, K.; Gustafsson, A.; Pockley, A.; Frostegård, J.; Klinge, B. Risk factors for cardiovascular disease in patients with periodontitis. Eur. Heart J. 2003, 24, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Persson, R.E. Cardiovascular disease and periodontitis: An update on the associations and risk. J. Clin. Periodontol. 2008, 35, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.S.; Chithra, V.; Vijayan, A.; Dinesh, R.D.; Vijayakumar, T. Altered DNA repair, oxidative stress and antioxidant status in coronary artery disease. J. Biosci. 2013, 38, 385–389. [Google Scholar] [CrossRef]
- Naderi, S.; Merchant, A.T. The Association Between Periodontitis and Cardiovascular Disease: An Update. Curr. Atheroscler. Rep. 2020, 22, 52. [Google Scholar] [CrossRef]
- Teles, R.; Wang, C. Mechanisms involved in the association between peridontal diseases and cardiovascular disease. Oral Dis. 2011, 17, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Schenkein, H.A.; Loos, B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Periodontol. 2013, 84, S51–S69. [Google Scholar] [CrossRef]
- Gurav, A.N. The implication of periodontitis in vascular endothelial dysfunction. Eur. J. Clin. Investig. 2014, 44, 1000–1009. [Google Scholar] [CrossRef]
- Li, Q.; Ouyang, X.; Lin, J. The impact of periodontitis on vascular endothelial dysfunction. Front. Cell. Infect. Microbiol. 2022, 12, 998313. [Google Scholar] [CrossRef]
- Martínez-Herrera, M.; Abad-Jiménez, Z.; Silvestre, F.J.; López-Domènech, S.; Silvestre-Rangil, J.; Márquez-Arrico, C.F.; Víctor, V.M.; Rocha, M. Effect of Non-Surgical Periodontal Treatment on Oxidative Stress Markers in Leukocytes and Their Interaction with the Endothelium in Obese Subjects with Periodontitis: A Pilot Study. J. Clin. Med. 2020, 9, 2117. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Sinning, C.; Post, F.; Warnholtz, A.; Schulz, E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008, 40, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.L.; Vita, J.A. Effects of Systemic Inflammation on Endothelium-Dependent Vasodilation. Trends Cardiovasc. Med. 2006, 16, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.X.; Ng, C.H.C.; Tsao, S.W.; Leung, W.K. Toll-Like Receptors 1/2/4/6 and Nucleotide-Binding Oligomerization Domain-Like Receptor 2 Are Key Damage-Associated Molecular Patterns Sensors on Periodontal Resident Cells. Appl. Sci. 2021, 11, 4724. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lambris, J.D. Microbial manipulation of receptor crosstalk in innate immunity. Nat. Rev. Immunol. 2011, 11, 187–200. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Tapping, R.I.; Harokopakis, E.; Nishiyama, S.-I.; Ratti, P.; Schifferle, R.E.; Lyle, E.A.; Triantafilou, M.; Triantafilou, K.; Yoshimura, F. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell. Microbiol. 2006, 8, 1557–1570. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.; Ford, P.; Cullinan, M.; Leishman, S.; Yamazaki, K. Relationship between periodontal infections and systemic disease. Clin. Microbiol. Infect. 2007, 13, 3–10. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, M.; Huang, X.; Chen, G.; Yin, Y.; Lu, X.; Feng, G.; Yu, R.; Chen, L. The Effects of Porphyromonas gingivalis on Atherosclerosis-Related Cells. Front. Immunol. 2021, 12, 766560. [Google Scholar] [CrossRef]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef]
- De Nardin, E. The Role of Inflammatory and Immunological Mediators in Periodontitis and Cardiovascular Disease. Ann. Periodontol. 2001, 6, 30–40. [Google Scholar] [CrossRef]
- Shaddox, L.; Wiedey, J.; Bimstein, E.; Magnuson, I.; Clare-Salzler, M.; Aukhil, I.; Wallet, S. Hyper-responsive Phenotype in Localized Aggressive Periodontitis. J. Dent. Res. 2010, 89, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Kang, H.; Park, H.; Kim, S.; Kim, S.; Choi, J. Immune responses to heat shock protein in Porphyromonas gingivalis-infected periodontitis and atherosclerosis patients. J. Periodontal Res. 2003, 38, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Mercanoglu, F.; Oflaz, H.; Öz, O.; Gökbuget, A.Y.; Genchellac, H.; Sezer, M.; Nişanci, Y.; Umman, S. Endothelial Dysfunction in Patients with Chronic Periodontitis and Its Improvement After Initial Periodontal Therapy. J. Periodontol. 2004, 75, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Elter, J.R.; Hinderliter, A.L.; Offenbacher, S.; Beck, J.D.; Caughey, M.; Brodala, N.; Madianos, P.N. The effects of periodontal therapy on vascular endothelial function: A pilot trial. Am. Heart J. 2006, 151, 47.e1–47.e6. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Kryuger, K.; Eizenberg, M.M.; Tatour, S.; Vigder, F.; Laster, Z.; Front, E. Periodontal care may improve endothelial function. Eur. J. Intern. Med. 2007, 18, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of Periodontitis and Endothelial Function. N. Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Cherian, D.; Peter, T.; Narayanan, A.; Madhavan, S.S.; Achammada, S.; Vynat, G.P. Malondialdehyde as a marker of oxidative stress in periodontitis patients. J. Pharm. Bioallied Sci. 2019, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Newman, H.N.; Battino, M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: A shared pathology via oxidative stress and mitochondrial dysfunction? Periodontology 2000 2014, 64, 139–153. [Google Scholar] [CrossRef]
- Miyasaki, K.T. The Neutrophil: Mechanisms of Controlling Periodontal Bacteria. J. Periodontol. 1991, 62, 761–774. [Google Scholar] [CrossRef]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2020, 84, 45–68. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.C.; Muniz, F.W.M.G.; Oballe, H.J.R.; Andrades, M.; Rösing, C.K.; Cavagni, J. The effect of periodontal therapy on oxidative stress biomarkers: A systematic review. J. Clin. Periodontol. 2018, 45, 1222–1237. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, L.; Celec, P. Oxidative Stress and Antioxidants in the Diagnosis and Therapy of Periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar] [CrossRef]
- Wang, J.; Schipper, H.M.; Velly, A.M.; Mohit, S.; Gornitsky, M. Salivary biomarkers of oxidative stress: A critical review. Free Radic Biol. Med. 2015, 85, 95–104. [Google Scholar] [CrossRef]
- Wu, L.L.; Chiou, C.-C.; Chang, P.-Y.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 2004, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baňasová, L.; Kamodyová, N.; Janšáková, K.; Tóthová, Ľ.; Stanko, P.; Turňa, J.; Celec, P. Salivary DNA and markers of oxidative stress in patients with chronic periodontitis. Clin. Oral Investig. 2015, 19, 201–207. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ngo, L.Q.; Promsudthi, A.; Surarit, R. Salivary oxidative stress biomarkers in chronic periodontitis and acute coronary syndrome. Clin. Oral Investig. 2017, 21, 2345–2353. [Google Scholar] [CrossRef]
- Fentoğlu, Ö.; Kırzıoğlu, F.Y.; Bulut, M.T.; Doğuç, D.K.; Kulaç, E.; Önder, C.; Günhan, M. Evaluation of Lipid Peroxidation and Oxidative DNA Damage in Patients with Periodontitis and Hyperlipidemia. J. Periodontol. 2015, 86, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Goriuc, A.; Cojocaru, K.-A.; Luchian, I.; Ursu, R.-G.; Butnaru, O.; Foia, L. Using 8-Hydroxy-2′-Deoxiguanosine (8-OHdG) as a Reliable Biomarker for Assessing Periodontal Disease Associated with Diabetes. Int. J. Mol. Sci. 2024, 25, 1425. [Google Scholar] [CrossRef]
- Dede, F.; Özden, F.O.; Avcı, B. 8-Hydroxy-Deoxyguanosine Levels in Gingival Crevicular Fluid and Saliva in Patients with Chronic Periodontitis After Initial Periodontal Treatment. J. Periodontol. 2013, 84, 821–828. [Google Scholar] [CrossRef]
- Viglianisi, G.; Tartaglia, G.M.; Santonocito, S.; Amato, M.; Polizzi, A.; Mascitti, M.; Isola, G. The Emerging Role of Salivary Oxidative Stress Biomarkers as Prognostic Markers of Periodontitis: New Insights for a Personalized Approach in Dentistry. J. Pers. Med. 2023, 13, 166. [Google Scholar] [CrossRef]
- Baltacıoğlu, E.; Yuva, P.; Aydın, G.; Alver, A.; Kahraman, C.; Karabulut, E.; Akalın, F.A. Lipid Peroxidation Levels and Total Oxidant/Antioxidant Status in Serum and Saliva from Patients with Chronic and Aggressive Periodontitis. Oxidative Stress Index: A New Biomarker for Periodontal Disease? J. Periodontol. 2014, 85, 1432–1441. [Google Scholar] [CrossRef]
- Veljovic, T.; Djuric, M.; Mirnic, J.; Gusic, I.; Maletin, A.; Ramic, B.; Neskovic, I.; Vukoje, K.; Brkic, S. Lipid Peroxidation Levels in Saliva and Plasma of Patients Suffering from Periodontitis. J. Clin. Med. 2022, 11, 3617. [Google Scholar] [CrossRef]
- Canakci, C.F.; Cicek, Y.; Yildirim, A.; Sezer, U.; Canakci, V. Increased Levels of 8-Hydroxydeoxyguanosine and Malondialdehyde and its Relationship with Antioxidant Enzymes in Saliva of Periodontitis Patients. Eur. J. Dent. 2009, 03, 100–106. [Google Scholar] [CrossRef]
- Trivedi, S.; Lal, N.; Mahdi, A.A.; Mittal, M.; Singh, B.; Pandey, S. Evaluation of Antioxidant Enzymes Activity and Malondialdehyde Levels in Patients with Chronic Periodontitis and Diabetes Mellitus. J. Periodontol. 2014, 85, 713–720. [Google Scholar] [CrossRef]
- Mozos, I.; Malainer, C.; Horbańczuk, J.; Gug, C.; Stoian, D.; Luca, C.T.; Atanasov, A.G. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases. Front. Immunol. 2017, 8, 1058. [Google Scholar] [CrossRef]
- Schmitt, A.; Carra, M.C.; Boutouyrie, P.; Bouchard, P. Periodontitis and arterial stiffness: A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, 977–987. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Fu, R.; Buckley, D.I.; Freeman, M.; Helfand, M. Periodontal Disease and Coronary Heart Disease Incidence: A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2008, 23, 2079–2086. [Google Scholar] [CrossRef]
- Cecelja, M.; Chowienczyk, P. Arterial stiffening: Causes and consequences. Artery Res. 2012, 7, 22. [Google Scholar] [CrossRef]
- Hayashida, H.; Saito, T.; Kawasaki, K.; Kitamura, M.; Furugen, R.; Iwasaki, T.; Hayashida, Y.; Nakazato, M.; Sekita, T.; Takamura, N.; et al. Association of periodontitis with carotid artery intima–media thickness and arterial stiffness in community-dwelling people in Japan: The Nagasaki Islands study. Atherosclerosis 2013, 229, 186–191. [Google Scholar] [CrossRef]
- Shanker, J.; Setty, P.; Arvind, P.; Nair, J.; Bhasker, D.; Balakrishna, G.; Kakkar, V.V. Relationship between Periodontal disease, Porphyromonas gingivalis, peripheral vascular resistance markers and Coronary Artery Disease in Asian Indians. Thromb. Res. 2013, 132, e8–e14. [Google Scholar] [CrossRef]
- Kapellas, K.; Jamieson, L.M.; Do, L.G.; Bartold, P.M.; Wang, H.; Maple-Brown, L.J.; Sullivan, D.; O’Dea, K.; Brown, A.; Celermajer, D.S.; et al. Associations between periodontal disease and cardiovascular surrogate measures among Indigenous Australians. Int. J. Cardiol. 2014, 173, 190–196. [Google Scholar] [CrossRef]
- A Lane, H.; Smith, J.C.; Davies, J.S. Noninvasive Assessment of Preclinical Atherosclerosis. Vasc. Health Risk Manag. 2006, 2, 19–30. [Google Scholar] [CrossRef]
- Chansawang, K.; Lertpimonchai, A.; Siripaiboonpong, N.; Thienpramuk, L.; Vathesatogkit, P.; Limpijankit, T.; Charatkulangkun, O. The severity and extent of periodontitis is associated with cardio-ankle vascular index, a novel arterial stiffness parameter. Clin. Oral Investig. 2021, 25, 3487–3495. [Google Scholar] [CrossRef]
- Darnaud, C.; Courtet, A.; Schmitt, A.; Boutouyrie, P.; Bouchard, P.; Carra, M.C. Association between periodontitis and pulse wave velocity: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 393–405. [Google Scholar] [CrossRef]
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef]
- Sanz-Miralles, E.C.; Li, R.; Momen-Heravi, F.; Mendieta, C.; Konofagou, E.E.; Papapanou, P.N. Assessment of arterial stiffness in periodontitis using a novel pulse wave imaging methodology. J. Clin. Periodontol. 2017, 44, 502–510. [Google Scholar] [CrossRef]
- Li, R.X.; Luo, J.; Balaram, S.K.; Chaudhry, F.A.; Lantis, J.C.; Shahmirzadi, D.; Konofagou, E.E. In-vivo pulse wave imaging for arterial stiffness measurement under normal and pathological conditions. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 567–570. [Google Scholar] [CrossRef]
- Jockel-Schneider, Y.; Harks, I.; Haubitz, I.; Fickl, S.; Eigenthaler, M.; Schlagenhauf, U.; Baulmann, J. Arterial Stiffness and Pulse Wave Reflection Are Increased in Patients Suffering from Severe Periodontitis. PLoS ONE 2014, 9, e103449. [Google Scholar] [CrossRef]
- Vieira, C.L.; Cury, P.R.; Miname, M.H.; Martinez, L.R.; Bortolotto, L.A.; Giuliano, I.B.; Santos, R.D.; Caramelli, B. Severe Periodontitis Is Associated with Diastolic Blood Pressure Elevation in Individuals with Heterozygous Familial Hypercholesterolemia: A Pilot Study. J. Periodontol. 2011, 82, 683–688. [Google Scholar] [CrossRef]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Iwamoto, A.; Kajikawa, M.; Matsumoto, T.; Oda, N.; et al. Endothelial Dysfunction, Increased Arterial Stiffness, and Cardiovascular Risk Prediction in Patients with Coronary Artery Disease: FMD-J (Flow-Mediated Dilation Japan) Study A. J. Am. Heart Assoc. 2018, 7, e008588. [Google Scholar] [CrossRef]
- Soltész, P.; Dér, H.; Kerekes, G.; Szodoray, P.; Szücs, G.; Dankó, K.; Shoenfeld, Y.; Szegedi, G.; Szekanecz, Z. A comparative study of arterial stiffness, flow-mediated vasodilation of the brachial artery, and the thickness of the carotid artery intima–media in patients with systemic autoimmune diseases. Clin. Rheumatol. 2009, 28, 655–662. [Google Scholar] [CrossRef]
- Seinost, G.; Wimmer, G.; Skerget, M.; Thaller, E.; Brodmann, M.; Gasser, R.; Bratschko, R.O.; Pilger, E. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am. Heart J. 2005, 149, 1050–1054. [Google Scholar] [CrossRef]
- Ding, L.; You, Q.; Jiang, Q.; Cao, S.; Jiang, S. Meta-analysis of the association between periodontal disease, periodontal treatment and carotid intima–media thickness. J. Periodontal Res. 2022, 57, 690–697. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yamanashi, H.; Kitamura, M.; Miyata, J.; Nonaka, F.; Nakamichi, S.; Saito, T.; Nagata, Y.; Maeda, T. Tooth Loss and Carotid Intima-Media Thickness in Relation to Functional Atherosclerosis: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 3993. [Google Scholar] [CrossRef]
- Hendek, M.K.; Erdemir, E.O.; Kisa, U.; Ozcan, G. Effect of Initial Periodontal Therapy on Oxidative Stress Markers in Gingival Crevicular Fluid, Saliva, and Serum in Smokers and Non-Smokers with Chronic Periodontitis. J. Periodontol. 2015, 86, 273–282. [Google Scholar] [CrossRef]
- Muthuraj, M.S.A.; Janakiram, S.; Chithresan, K.; Maradi, A.P.; Maddur, P.K.; Rangaraju, R. Effect of scaling and root planing on levels of 8-hydroxydeoxyguanosine in gingival crevicular fluid of chronic periodontitis patients with and without Type II diabetes mellitus. J. Indian Soc. Periodontol. 2017, 21, 201. [Google Scholar] [CrossRef]
- Isola, G.; Tartaglia, G.M.; Santonocito, S.; Polizzi, A.; Williams, R.C.; Iorio-Siciliano, V. Impact of N-terminal pro-B-type natriuretic peptide and related inflammatory biomarkers on periodontal treatment outcomes in patients with periodontitis: An explorative human randomized-controlled clinical trial. J. Periodontol. 2023, 94, 1414–1424. [Google Scholar] [CrossRef]
- D’aiuto, F.; Nibali, L.; Parkar, M.; Suvan, J.; Tonetti, M. Short-term Effects of Intensive Periodontal Therapy on Serum Inflammatory Markers and Cholesterol. J. Dent. Res. 2005, 84, 269–273. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Parkar, M.; Nibali, L.; Suvan, J.; Lessem, J.; Tonetti, M.S. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: Results from a randomized controlled clinical trial. Am. Heart J. 2006, 151, 977–984. [Google Scholar] [CrossRef]
- Merchant, A.T.; Virani, S.S. Evaluating Periodontal Treatment to Prevent Cardiovascular Disease: Challenges and Possible Solutions. Curr. Atheroscler. Rep. 2017, 19, 4. [Google Scholar] [CrossRef]
- Teeuw, W.J.; Slot, D.E.; Susanto, H.; Gerdes, V.E.A.; Abbas, F.; D’Aiuto, F.; Kastelein, J.J.P.; Loos, B.G. Treatment of periodontitis improves the atherosclerotic profile: A systematic review and meta-analysis. J. Clin. Periodontol. 2014, 41, 70–79. [Google Scholar] [CrossRef]
- Friedewald, V.E.; Kornman, K.S.; Beck, J.D.; Genco, R.; Goldfine, A.; Libby, P.; Offenbacher, S.; Ridker, P.M.; van Dyke, T.E.; Roberts, W.C. The American Journal of Cardiology and Journal of Periodontology Editors’ Consensus: Periodontitis and Atherosclerotic Cardiovascular Disease. J. Periodontol. 2009, 80, 1021–1032. [Google Scholar] [CrossRef]
- D’aiuto, F.; Orlandi, M.; Gunsolley, J.C. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J. Periodontol. 2013, 84, S85–S105. [Google Scholar] [CrossRef]
- Ramírez, J.H.; Arce, R.M.; Contreras, A. Periodontal treatment effects on endothelial function and cardiovascular disease biomarkers in subjects with chronic periodontitis: Protocol for a randomized clinical trial. Trials 2011, 12, 46. [Google Scholar] [CrossRef]
- Ouyang, X.Y.; Xiao, W.M.; Chu, Y.; Zhou, S.Y. Influence of periodontal intervention therapy on risk of cardiovascular disease. Periodontology 2000 2011, 56, 227–257. [Google Scholar] [CrossRef]
- Higashi, Y.; Goto, C.; Jitsuiki, D.; Umemura, T.; Nishioka, K.; Hidaka, T.; Takemoto, H.; Nakamura, S.; Soga, J.; Chayama, K.; et al. Periodontal Infection Is Associated with Endothelial Dysfunction in Healthy Subjects and Hypertensive Patients. Hypertension 2008, 51, 446–453. [Google Scholar] [CrossRef]
- Piconi, S.; Trabattoni, D.; Luraghi, C.; Perilli, E.; Borelli, M.; Pacei, M.; Rizzardini, G.; Lattuada, A.; Bray, D.H.; Catalano, M.; et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J. 2009, 23, 1196–1204. [Google Scholar] [CrossRef]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease—Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef]

| Inflammatory markers | Fibrinogen, plasma viscosity, CRP, IL-6, TNF-alpha, MMP-8, MMP-9, MMP-13, antibodies, and low-density lipoprotein |
| Markers of endothelial dysfunction | Oxidized low-density lipoprotein, brachial artery reactivity, glutathione dysfunction |
| Thrombotic markers | Tissue plasminogen activator, homocysteine, von Willebrand factor |
| Non-invasive imaging biomarkers | Ankle brachial pressure index, magnetic resonance imaging angiography, carotid intima-media thickness, CT coronary calcification |
| Invasive imaging biomarkers | Intravascular ultrasound, coronary angiography |
| Oxidative stress biomarkers | Acrolein, malonyldialdehyde (MDA), 4-hydroxynonenal (HNE), 8-hydroxydeoxyguanosine (8-OHdG), protein carbonyls, thiobarbituric acid reactive substances (TBARSs) |
| Vascular adhesion molecule (VCAM-1) | Endothelial progenitor cells (EPCs) |
| C-reactive protein (CRP) | Circulating endothelial cells (CECs) |
| Interleukin (IL18) | Osteopontin (OPN) |
| Fibrinogen | Endothelin |
| Reactive oxygen species (ROS) | Ischemia-modified albumin (IMA) |
| Tissue plasminogen activator (tPA) | Cardiac troponin T |
| Free fatty acids (FFA) | Copeptin |
| Vascular endothelial growth factor (VEGF) | Platelet-derived growth factor (PDGF) |
| Markers of Oxidative Stress | Antioxidants |
|---|---|
| Lipid peroxidation | Enzymatic |
| Malondialdehyde (MDA) | Superoxide dismutase (SOD) |
| Thiobarbituric acid reactive substances (TBARSs) | Catalase |
| 4-hydroxynonenal (HNE) | Glutathione peroxidase (GSH-Px) |
| Protein oxidation | Non-enzymatic |
| Protein carbonyls | Glutathione (GSH) |
| Nucleic acid oxidation | Vitamins (C and E) |
| 8-hydroxydeoxyguanosine (8-OHdG) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angjelova, A.; Jovanova, E.; Polizzi, A.; Laganà, L.; Santonocito, S.; Ragusa, R.; Isola, G. Impact of Periodontitis on Endothelial Risk Dysfunction and Oxidative Stress Improvement in Patients with Cardiovascular Disease. J. Clin. Med. 2024, 13, 3781. https://doi.org/10.3390/jcm13133781
Angjelova A, Jovanova E, Polizzi A, Laganà L, Santonocito S, Ragusa R, Isola G. Impact of Periodontitis on Endothelial Risk Dysfunction and Oxidative Stress Improvement in Patients with Cardiovascular Disease. Journal of Clinical Medicine. 2024; 13(13):3781. https://doi.org/10.3390/jcm13133781
Chicago/Turabian StyleAngjelova, Angela, Elena Jovanova, Alessandro Polizzi, Ludovica Laganà, Simona Santonocito, Rosalia Ragusa, and Gaetano Isola. 2024. "Impact of Periodontitis on Endothelial Risk Dysfunction and Oxidative Stress Improvement in Patients with Cardiovascular Disease" Journal of Clinical Medicine 13, no. 13: 3781. https://doi.org/10.3390/jcm13133781
APA StyleAngjelova, A., Jovanova, E., Polizzi, A., Laganà, L., Santonocito, S., Ragusa, R., & Isola, G. (2024). Impact of Periodontitis on Endothelial Risk Dysfunction and Oxidative Stress Improvement in Patients with Cardiovascular Disease. Journal of Clinical Medicine, 13(13), 3781. https://doi.org/10.3390/jcm13133781









