Reducing Left Ventricular Wall Stress through Aortic Valve Enlargement via Transcatheter Aortic Valve Implantation in Severe Aortic Stenosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Doppler Echocardiography and TAVI Procedure and Prosthesis
2.3. Outcomes of Hospital and Surveillance and Definition of Major Complications
2.4. Dimension, Volume, Mass, and Remodel Mode of Left Ventricle
2.5. Valvular Load, Arterial Load, and Global LV Afterload
2.6. Wall Stress, LV Performance, and Energetics
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Operative Results
3.2. Comparisons of Geometric and Hemodynamic Parameters following TAVI
3.3. Comparisons of Baseline and Post-TAVI between Subgroups
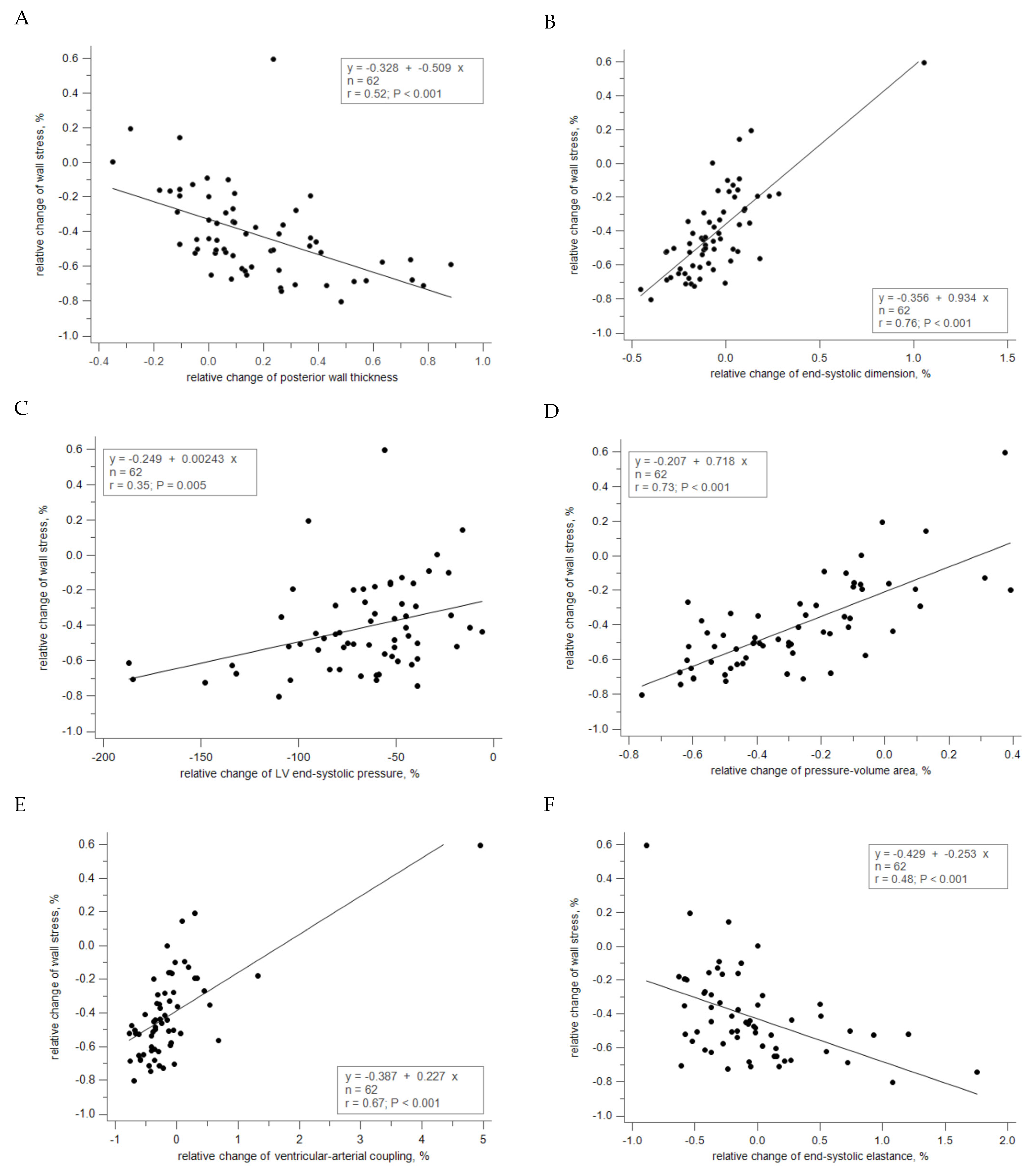
3.4. Correlation between Relative Changes of Geometry and Hemodynamics
4. Discussion
4.1. Pathophysiology of Adaptive Hypertrophy to Pressure Overload
4.2. Stratification of Adverse Remodeling by Output Using Stroke Volume Index
4.3. Diversity in Recovery Benefits by Afterload Reduction through TAVI
4.4. Correlation between Hemodynamics and Energetics
4.5. Clinical Implication
4.6. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic Valve Stenosis |
| EOAI | Effective Orifice Area Index |
| Ea | Arterial Elastance |
| Ees | End-Systolic Ventricular Elastance |
| LVESP | Left Ventricular End-Systolic Pressure |
| PVA | Pressure–Volume Area |
| RWT | Relative Wall Thickness |
| SAC | Systemic Arterial Compliance |
| SWL | Stroke Work Loss |
| SVI | Stroke Volume Index |
| SVR | Systemic Vascular Resistance |
| TAVI | Transcatheter Aortic Valve Implantation |
| VAC | Ventricular–Arterial Coupling |
| Zva | Valvular–Arterial Impedance |
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Nakayama, M.; Nevo, E.; Fetics, B.J.; Maughan, W.L.; Kass, D.A. Coupled systolic-ventricular and vascular stiffening with age: Implications for pressure regulation and cardiac reserve in the elderly. J. Am. Coll. Cardiol. 1998, 32, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Rajappan, K.; Rimoldi, O.E.; Dutka, D.P.; Ariff, B.; Pennell, D.J.; Sheridan, D.J.; Camici, P.G. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation 2002, 105, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Marcos-Alberca, P.; Almeria, C.; Feltes, G.; Rodríguez, E.; Hernández-Antolín, R.A.; Garcia, E.; Maroto, L.; Fernandez Perez, C.; Silva Cardoso, J.C.; et al. Acute left ventricle diastolic function improvement after transcatheter aortic valve implantation. Eur. J. Echocardiogr. 2011, 12, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Chiang, C.Y.; Hsu, J.C.; Huang, J.H.; Chen, J.S.; Chiu, K.M. Acute Effect in Mechanical Efficiency by Pressure-Volume Loop Analysis after Transcatheter Aortic Valve Implantation. Acta Cardiol. Sin. 2024, 40, 242–252. [Google Scholar] [CrossRef]
- Hachicha, Z.; Dumesnil, J.G.; Bogaty, P.; Pibarot, P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007, 115, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, A.; Watanabe, Y.; Kozuma, K.; Nara, Y.; Nagura, F.; Kawashima, H.; Hioki, H.; Nakashima, M.; Yamamoto, M.; Takagi, K.; et al. Prognostic Impact of Low-Flow Severe Aortic Stenosis in Small-Body Patients Undergoing TAVR: The OCEAN-TAVI Registry. JACC Cardiovasc. Imaging 2018, 11, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Petronio, A.S.; De Carlo, M.; Guarracino, F.; Benedetti, G.; Delle Donne, M.G.; Dini, F.L.; Marzilli, M.; Di Bello, V. The incremental value of valvuloarterial impedance in evaluating the results of transcatheter aortic valve implantation in symptomatic aortic stenosis. J. Am. Soc. Echocardiogr. 2012, 25, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Trivedi, D.V.; Sarkar, S.S.; Adhikari, A.S.; Sunitha, M.S.; Sutton, S.; Ruppel, K.M.; Spudich, J.A. The myosin mesa and the basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Nat. Struct. Mol. Biol. 2017, 24, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sagawa, K.; Shoukas, A.A. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ. Res. 1973, 32, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Hayashi, T.; Shirahata, M. Ventricular systolic pressure-volume area as predictor of cardiac oxygen consumption. Am. J. Physiol. 1981, 240, H39–H44. [Google Scholar] [CrossRef] [PubMed]
- Di Bello, V.; Giannini, C.; De Carlo, M.; Delle Donne, M.G.; Nardi, C.; Palagi, C.; Cucco, C.; Dini, F.L.; Guarracino, F.; Marzilli, M.; et al. Acute improvement in arterial-ventricular coupling after transcatheter aortic valve implantation (CoreValve) in patients with symptomatic aortic stenosis. Int. J. Cardiovasc. Imaging 2012, 28, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.R., Jr.; Rahimtoola, S.H.; Blundell, P.E.; Swan, H.J. Percentage of left ventricular stroke work loss. A simple hemodynamic concept for estimation of severity in valvular aortic stenosis. Circulation 1967, 35, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Burwash, I.G.; Pearlman, A.S.; Kraft, C.D.; Miyake-Hull, C.; Healy, N.L.; Otto, C.M. Flow dependence of measures of aortic stenosis severity during exercise. J. Am. Coll. Cardiol. 1994, 24, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, J.; Odreman, R.; Feijoo, J.; Moreno, M.M.; Gómez-Moreno, P.; García-Fernández, M.A. Clinical efficacy of Doppler-echocardiographic indices of aortic valve stenosis: A comparative test-based analysis of outcome. J. Am. Coll. Cardiol. 2003, 41, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Pibarot, P.; Dumesnil, J.G.; Sakr, F.; Durand, L.G. Assessment of aortic valve stenosis severity: A new index based on the energy loss concept. Circulation 2000, 101, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Briand, M.; Dumesnil, J.G.; Kadem, L.; Tongue, A.G.; Rieu, R.; Garcia, D.; Pibarot, P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment. J. Am. Coll. Cardiol. 2005, 46, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, Z.; Dumesnil, J.G.; Pibarot, P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J. Am. Coll. Cardiol. 2009, 54, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Huang, J.H.; Chiu, K.M.; Chiang, C.Y. Extent of Left Ventricular Mass Regression and Impact of Global Left Ventricular Afterload on Cardiac Events and Mortality after Aortic Valve Replacement. J. Clin. Med. 2022, 11, 7482. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Grossman, W.; Jones, D.; McLaurin, L.P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Alyono, D.; Anderson, R.W.; Parrish, D.G.; Dai, X.Z.; Bache, R.J. Alterations of myocardial blood flow associated with experimental canine left ventricular hypertrophy secondary to valvular aortic stenosis. Circ. Res. 1986, 58, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Ratshin, R.A.; Rackley, C.E.; Russell, R.O., Jr. Determination of left ventricular preload and afterload by quantitative echocardiography in man. Circ. Res. 1974, 34, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Walley, K.R. Left ventricular function: Time-varying elastance and left ventricular aortic coupling. Crit. Care 2016, 20, 270. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, F.; Herrmann, S.; Stork, S.; Niemann, M.; Frantz, S.; Lange, V.; Beer, M.; Gattenlöhner, S.; Voelker, W.; Ertl, G.; et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009, 120, 577–584. [Google Scholar] [CrossRef]
- Herrmann, S.; Stork, S.; Niemann, M.; Lange, V.; Strotmann, J.M.; Frantz, S.; Beer, M.; Gattenlöhner, S.; Voelker, W.; Ertl, G.; et al. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J. Am. Coll. Cardiol. 2011, 58, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.W.L.; Everett, R.J.; Kwiecinski, J.; Vesey, A.T.; Yeung, E.; Esson, G.; Jenkins, W.; Koo, M.; Mirsadraee, S.; White, A.C.; et al. Myocardial Fibrosis and Cardiac Decompensation in Aortic Stenosis. JACC Cardiovasc. Imaging 2017, 10, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Treibel, T.A.; Lopez, B.; Gonzalez, A.; Menacho, K.; Schofield, R.S.; Ravassa, S.; Fontana, M.; White, S.K.; DiSalvo, C.; Roberts, N.; et al. Reappraising myocardial fibrosis in severe aortic stenosis: An invasive and non-invasive study in 133 patients. Eur. Heart J. 2018, 39, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Fetics, B.; Nevo, E.; Rochitte, C.E.; Chiou, K.R.; Ding, P.A.; Kawaguchi, M.; Kass, D.A. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J. Am. Coll. Cardiol. 2001, 38, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: Assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur. J. Heart Fail. 2019, 21, 402–424. [Google Scholar] [CrossRef]
- White, S.K.; Sado, D.M.; Fontana, M.; Banypersad, S.M.; Maestrini, V.; Flett, A.S.; Piechnik, S.K.; Robson, M.D.; Hausenloy, D.J.; Sheikh, A.M.; et al. T1 mapping for myocardial extracellular volume measurement by CMR: Bolus only versus primed infusion technique. JACC Cardiovasc. Imaging 2013, 6, 955–962. [Google Scholar] [CrossRef]

| Variable | Total (n = 62) | Normal Flow (n = 53) | Low Flow (n = 9) | p |
|---|---|---|---|---|
| Age (y) | 81.4 6.5 | 81.1 6.4 | 82.7 6.8 | 0.514 |
| Body surface area | 1.56 0.17 | 1.60 0.17 | 1.53 0.16 | 0.465 |
| Sex | 29 (47) | 26 (49) | 3 (33) | 0.271 |
| Comorbidity | ||||
| Hypertension | 46 (74) | 40 (75) | 6 (67) | 0.683 |
| Coronary artery disease | 39 (63) | 36 (68) | 3 (33) | 0.066 |
| Dyslipidemia | 36 (58) | 32 (60) | 4 (44) | 0.473 |
| Diabetes | 24 (39) | 22 (42) | 2 (22) | 0.462 |
| Chronic kidney disease | 33 (53) | 29 (55) | 4 (44) | 0.722 |
| Atrial fibrillation | 14 (23) | 11 (21) | 3 (33) | 0.409 |
| Cerebrovascular accident | 7 (11) | 7 (13) | 0 (0) | 0.580 |
| Pulmonary hypertension, Gr 2 | 20 (32) | 18 (34) | 2 (22) | 0.705 |
| Dysfunction | ||||
| Systolic, ejection fraction < 55% | 12 (19) | 9 (17) | 3 (33) | 0.357 |
| Diastolic, Gr 2 | 5 (8) | 4 (8) | 1 (11) | 0.557 |
| Diastolic, Gr 2 | 31 (50) | 24 (45) | 7 (78) | 0.147 |
| Remodel mode | ||||
| Normal | 1 (2) | 1 (2) | 0 (0) | 1.000 |
| Concentric remodel | 3 (5) | 3 (6) | 0 (0) | 1.000 |
| Concentric hypertrophy | 50 (81) | 42 (79) | 8 (89) | 0.675 |
| Eccentric hypertrophy | 8 (13) | 7 (13) | 1 (11) | 1.000 |
| Total (n = 62) | |||||
|---|---|---|---|---|---|
| Baseline | Post-TAVI | p | |||
| Geometry | |||||
| PW, cm | 1.32 | 1.24, 1.35 | 1.50 | 1.40, 1.57 | <0.001 |
| RWT, % | 0.54 | 0.52, 0.60 | 0.66 | 0.60, 0.72 | <0.001 |
| ESD, cm | 2.99 | 2.86, 3.26 | 2.94 | 2.67, 3.00 | <0.001 |
| EDD, cm | 4.75 | 4.44, 5.04 | 4.49 | 4.32, 4.74 | 0.015 |
| ESVI, mLm−2 | 22.64 | 19.56, 26.23 | 20.35 | 16.75, 23.12 | <0.001 |
| EDVI, mLm−2 | 65.46 | 61.85, 74.22 | 63.12 | 53.85, 67.13 | 0.016 |
| Valvular load | |||||
| EOAI, cm2m−2 | 0.47 | 0.43, 0.52 | 1.12 | 1.06, 1.25 | <0.001 |
| MPG, mmHg | 41 | 36, 46 | 8 | 7, 9 | <0.001 |
| SWL, % | 23.79 | 21.65, 27.15 | 6.18 | 4.87, 6.93 | <0.001 |
| Arterial load | |||||
| SVR, Kdynessec−1cm−5 | 1.36 | 1.29, 1.50 | 1.14 | 1.05, 1.20 | <0.001 |
| SAC, mLmmHg−1m−2 | 0.76 | 0.67, 0.88 | 0.84 | 0.78, 0.93 | <0.001 |
| Ea, mmHgmL−1 | 2.87 | 2.65, 3.13 | 1.86 | 1.70, 2.03 | <0.001 |
| Global load | |||||
| Zva, mmHgmL−1m2 | 3.58 | 3.30, 3.99 | 2.69 | 2.46, 2.87 | <0.001 |
| ESP, mmHg | 208 | 192, 220 | 147 | 138, 151 | <0.001 |
| WS, Kdynecm−2 | 113.31 | 98.97, 129.66 | 63.20 | 58.69, 68.32 | <0.001 |
| LV performance | |||||
| SVI, mLm−2 | 47.11 | 44.27, 51.90 | 51.95 | 48.32, 54.12 | <0.001 |
| FS, % | 35.77 | 33.54, 37.61 | 36.89 | 34.27, 37.85 | 0.008 |
| EF, % | 65.35 | 62.32, 67.68 | 66.79 | 63.42, 68.39 | 0.005 |
| Ees, mmHgmL−1 | 5.64 | 4.80, 6.52 | 4.68 | 4.01, 5.07 | 0.002 |
| Energetic | |||||
| PV area, cJ | 152.99 | 145.28, 188.16 | 106.90 | 93.93, 119.40 | <0.001 |
| VA coupling, ratio | 0.50 | 0.43, 0.68 | 0.40 | 0.32, 0.47 | <0.001 |
| Normal Flow (n = 53) | Low Flow (n = 9) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-TAVI | p | Baseline | Post-TAVI | p | |||||
| Geometry | ||||||||||
| PW, cm | 1.32 | 1.25, 1.39 | 1.52 | 1.42, 1.57 | <0.001 | 1.22 | 1.05, 1.45 | 1.40 | 1.12, 1.56 | 0.176 |
| RWT, % | 0.54 | 0.52, 0.62 | 0.68 | 0.60, 0.73 | <0.001 | 0.52 | 0.42, 0.61 | 0.61 | 0.49, 0.70 | 0.164 |
| ESD, cm | 2.96 | 2.77, 3.24 | 2.95 | 2.61, 3.00 | 0.002 | 3.25 | 2.96, 3.87 | 2.92 | 2.83, 3.42 | 0.192 |
| EDD, cm | 4.73 | 4.39, 5.17 | 4.43 | 4.30, 4.74 | 0.038 | 4.80 | 4.38, 5.27 | 4.58 | 4.21, 5.02 | 0.164 |
| ESVI, mLm−2 | 22.02 | 19.03, 25.95 | 19.93 | 15.55, 23.10 | 0.001 | 27.04 | 22.46, 40.89 | 21.28 | 19.59, 30.86 | 0.301 |
| EDVI, mLm−2 | 63.96 | 57.21, 78.93 | 59.64 | 51.72, 67.89 | 0.033 | 70.65 | 65.07, 81.26 | 63.59 | 54.58, 76.05 | 0.250 |
| Valvular load | ||||||||||
| EOAI, cm2m−2 | 0.49 | 0.46, 0.53 | 1.12 | 1.06, 1.28 | <0.001 | 0.30 | 0.26, 0.37 | 1.07 | 0.88, 1.38 | 0.004 |
| MPG, mmHg | 41 | 36, 46 | 8 | 7, 9 | <0.001 | 44 | 30, 59 | 7 | 4, 15 | 0.008 |
| Stroke work loss, % | 23.08 | 21.05, 25.64 | 6.11 | 4.88, 7.00 | <0.001 | 29.73 | 20.69, 33.26 | 6.25 | 3.31, 12.42 | 0.004 |
| Arterial load | ||||||||||
| SVR, Kdynessec−1cm−5 | 1.33 | 1.17, 1.41 | 1.08 | 1.03, 1.18 | 0.002 | 1.75 | 1.63, 2.04 | 1.32 | 1.20, 1.80 | 0.060 |
| SAC, mLmmHg−1m−2 | 0.78 | 0.70, 0.91 | 0.86 | 0.79, 0.96 | <0.001 | 0.60 | 0.45, 0.97 | 0.78 | 0.61, 1.12 | 0.098 |
| Ea, mmHgmL−1 | 2.76 | 2.51, 2.94 | 1.75 | 1.65, 1.90 | <0.001 | 4.10 | 3.60, 5.22 | 2.27 | 2.23, 2.58 | 0.004 |
| Global load | ||||||||||
| Zva, mmHgmL−1m2 | 3.50 | 3.26, 3.73 | 2.52 | 2.38, 2.76 | <0.001 | 5.41 | 4.45, 7.00 | 3.22 | 3.01, 3.93 | 0.004 |
| ESP, mmHg | 210 | 202, 220 | 149 | 138, 153 | <0.001 | 185 | 168, 231 | 129 | 118, 168 | 0.004 |
| Wall stress, Kdynecm−2 | 115.00 | 91.12, 129.23 | 62.18 | 58.39, 68.01 | <0.001 | 105.17 | 90.70, 181.89 | 70.37 | 50.28, 100.05 | 0.004 |
| LV performance | ||||||||||
| SVI, mLm−2 | 50.89 | 46.36, 54.42 | 53.14 | 50.77, 57.30 | 0.010 | 28.42 | 24.46, 32.22 | 35.45 | 32.93, 49.24 | 0.004 |
| FS, % | 35.92 | 34.08, 37.65 | 37.24 | 35.29, 38.50 | 0.017 | 31.58 | 18.32, 41.47 | 32.35 | 29.87, 37.26 | 0.359 |
| EF, % | 65.52 | 63.25, 67.78 | 67.67 | 64.35, 68.73 | 0.010 | 59.47 | 37.80, 72.07 | 60.87 | 57.14, 67.27 | 0.301 |
| Ees, mmHgmL−1 | 6.16 | 4.89, 6.94 | 4.84 | 4.13, 5.57 | 0.002 | 4.65 | 2.99, 7.29 | 3.94 | 3.47, 4.76 | 0.426 |
| Energetic | ||||||||||
| PV area, cJ | 155.27 | 145.78, 189.34 | 105.21 | 94.07, 116.07 | <0.001 | 145.88 | 108.44, 202.47 | 118.76 | 77.95, 141.94 | 0.027 |
| VA coupling, ratio | 0.46 | 0.42, 0.51 | 0.36 | 0.31, 0.42 | <0.001 | 0.89 | 0.70, 1.68 | 0.61 | 0.55, 0.69 | 0.004 |
| Baseline | Post-TAVI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal Flow (n = 53) | Low Flow (n = 9) | p | Normal Flow (n = 53) | Low Flow (n = 9) | p | |||||
| Geometry | ||||||||||
| PW, cm | 1.32 | 1.25, 1.39 | 1.22 | 1.05, 1.45 | 0.379 | 1.52 | 1.42, 1.57 | 1.40 | 1.12, 1.56 | 0.093 |
| RWT, % | 0.54 | 0.52, 0.62 | 0.52 | 0.42, 0.61 | 0.418 | 0.68 | 0.60, 0.73 | 0.61 | 0.49, 0.70 | 0.153 |
| ESD, cm | 2.96 | 2.77, 3.24 | 3.25 | 2.96, 3.87 | 0.184 | 2.95 | 2.61, 3.00 | 2.92 | 2.83, 3.42 | 0.348 |
| EDD, cm | 4.73 | 4.39, 5.17 | 4.80 | 4.38, 5.27 | 0.742 | 4.43 | 4.30, 4.74 | 4.58 | 4.21, 5.02 | 0.803 |
| ESVI, mLm−2 | 22.03 | 19.03, 25.95 | 27.04 | 22.46, 40.89 | 0.212 | 19.93 | 15.55, 23.10 | 21.28 | 19.59, 30.86 | 0.171 |
| EDVI, mLm−2 | 63.96 | 57.21, 78.93 | 70.65 | 65.07, 81.26 | 0.466 | 59.64 | 51.74, 67.89 | 63.59 | 54.58, 76.05 | 0.682 |
| Valvular load | ||||||||||
| EOAI, cm2m−2 | 0.49 | 0.46, 0.53 | 0.30 | 0.26, 0.37 | <0.001 | 1.12 | 1.06, 1.28 | 1.07 | 0.88, 1.38 | 0.267 |
| MPG, mmHg | 41 | 36, 46 | 44 | 30, 59 | 0.749 | 8 | 7, 9 | 7 | 4, 15 | 0.779 |
| Stroke work loss, % | 23.08 | 21.05, 25.64 | 29.73 | 20.69, 33.26 | 0.034 | 6.11 | 4.88, 7.00 | 6.25 | 3.31, 12.42 | 0.960 |
| Arterial load | ||||||||||
| SVR, Kdynessec−1cm−5 | 1.33 | 1.17, 1.41 | 1.75 | 1.63, 2.04 | <0.001 | 1.08 | 1.03, 1.18 | 1.32 | 1.20, 1.80 | 0.004 |
| SAC, mLmmHg−1m−2 | 0.78 | 0.70, 0.91 | 0.60 | 0.45, 0.97 | 0.039 | 0.86 | 0.79, 0.96 | 0.78 | 0.61, 1.12 | 0.037 |
| Ea, mmHgmL−1 | 2.76 | 2.51, 2.94 | 4.10 | 3.60, 5.22 | <0.001 | 1.75 | 1.65, 1.90 | 2.27 | 2.23, 2.58 | <0.001 |
| Global load | ||||||||||
| Zva, mmHgmL−1m2 | 3.50 | 3.26, 3.73 | 5.41 | 4.45, 7.00 | <0.001 | 2.52 | 2.38, 2.76 | 3.22 | 3.01, 3.93 | 0.001 |
| LVESP, mmHg | 210 | 202, 220 | 185 | 168, 231 | 0.131 | 149 | 138, 153 | 129 | 118, 168 | 0.522 |
| WS, Kdynecm−2 | 115.00 | 91.12, 129.23 | 115.17 | 90.70, 181.89 | 0.803 | 62.18 | 58.39, 68.01 | 70.37 | 50.28, 100.05 | 0.034 |
| LV performance | ||||||||||
| SVI, mLm−2 | 50.89 | 46.36, 54.42 | 28.42 | 24.46, 32.22 | <0.001 | 53.14 | 50.77, 57.30 | 35.45 | 32.93, 49.24 | <0.001 |
| FS, % | 35.92 | 34.08, 37.65 | 31.58 | 18.32, 41.47 | 0.259 | 37.24 | 35.29, 38.50 | 32.35 | 29.87, 37.26 | 0.099 |
| EF, % | 65.52 | 63.25, 67.78 | 59.47 | 37.80, 72.07 | 0.259 | 67.67 | 64.35, 68.73 | 60.87 | 57.14, 67.27 | 0.103 |
| Ees, mmHgmL−1 | 6.16 | 4.89, 6.94 | 4.65 | 2.99, 7.29 | 0.029 | 4.84 | 4.13, 5.57 | 3.94 | 3.47, 4.76 | 0.031 |
| Energetic | ||||||||||
| PV area, cJ | 155.27 | 145.78, 189.34 | 155.88 | 108.44, 202.47 | 0.668 | 105.2 | 94.07, 116.07 | 118.76 | 77.95, 141.94 | 0.032 |
| VA coupling, ratio | 0.46 | 0.42, 0.51 | 0.89 | 0.70, 1.68 | 0.001 | 0.36 | 0.31, 0.42 | 0.61 | 0.55, 0.69 | 0.002 |
| EOAI, % | SAC, % | Zva, % | LVESP, % | WS, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | p | p | p | p | |||||||||||
| Geometry | |||||||||||||||
| PWT, % | 0.158 | 0.04 | 0.770 | −0.277 | 0.15 | 0.220 | 0.120 | 0.15 | 0.254 | 0.045 | 0.10 | 0.455 | −0.509 | 0.52 | <0.001 |
| RWT, % | −0.059 | 0.02 | 0.873 | −0.165 | 0.14 | 0.283 | 0.115 | 0.21 | 0.105 | 0.027 | 0.09 | 0.505 | −0.461 | 0.69 | <0.001 |
| ESD, % | 0.079 | 0.02 | 0.906 | −0.058 | 0.03 | 0.837 | −0.054 | 0.05 | 0.680 | −0.020 | 0.03 | 0.788 | 0.934 | 0.76 | <0.001 |
| EDD, % | 0.329 | 0.04 | 0.744 | −0.088 | 0.03 | 0.836 | −0.157 | 0.10 | 0.424 | −0.012 | 0.01 | 0.913 | 1.307 | 0.71 | <0.001 |
| ESVI, % | 0.013 | 0.01 | 0.944 | −0.005 | 0.01 | 0.942 | −0.006 | 0.02 | 0.870 | −0.003 | 0.02 | 0.894 | 0.238 | 0.70 | <0.001 |
| EDVI, % | 0.127 | 0.04 | 0.763 | −0.003 | 0.00 | 0.988 | −0.062 | 0.10 | 0.447 | −0.001 | 0.00 | 0.991 | 0.545 | 0.71 | <0.001 |
| Valvular load | |||||||||||||||
| EOAI, % | 0.156 | 0.37 | 0.003 | −0.090 | 0.46 | <0.001 | −0.039 | 0.35 | 0.005 | −0.033 | 0.14 | 0.283 | |||
| MPG, % | −2.360 | 0.37 | 0.003 | −0.125 | 0.05 | 0.719 | 0.351 | 0.28 | 0.027 | 0.377 | 0.53 | <0.001 | 0.276 | 0.18 | 0.155 |
| SWL, % | −2.207 | 0.37 | 0.003 | 0.149 | 0.06 | 0.647 | 0.243 | 0.21 | 0.103 | 0.279 | 0.42 | <0.001 | 0.193 | 0.14 | 0.290 |
| Arterial load | |||||||||||||||
| SVR, % | −0.796 | 0.23 | 0.072 | −0.887 | 0.61 | <0.001 | 0.384 | 0.57 | <0.001 | 0.040 | 0.10 | 0.421 | −0.013 | 0.02 | 0.903 |
| SAC, % | 0.881 | 0.37 | 0.003 | −0.366 | 0.79 | <0.001 | −0.090 | 0.34 | 0.007 | −0.050 | 0.09 | 0.491 | |||
| Ea, % | −2.742 | 0.52 | <0.001 | −1.651 | 0.74 | <0.001 | 1.016 | 0.98 | <0.001 | 0.332 | 0.56 | <0.001 | 0.145 | 0.11 | 0.374 |
| Global load | |||||||||||||||
| Zva, % | −2.358 | 0.46 | <0.001 | −1.697 | 0.79 | <0.001 | 0.270 | 0.47 | <0.001 | 0.091 | 0.07 | 0.563 | |||
| ESP, % | −3.155 | 0.35 | 0.005 | −0.128 | 0.34 | 0.007 | 0.833 | 0.47 | <0.001 | 0.706 | 0.33 | 0.009 | |||
| WS, % | −0.584 | 0.14 | 0.283 | −0.158 | 0.09 | 0.491 | 0.062 | 0.07 | 0.563 | 0.155 | 0.33 | 0.009 | |||
| Systolic | |||||||||||||||
| SVI, % | 1.548 | 0.46 | <0.001 | 1.035 | 0.73 | <0.001 | −0.478 | 0.72 | <0.001 | 0.006 | 0.02 | 0.900 | 0.011 | 0.01 | 0.917 |
| FS, % | 0.037 | 0.01 | 0.938 | 0.012 | 0.01 | 0.953 | 0.002 | 0.00 | 0.980 | 0.038 | 0.09 | 0.463 | −0.301 | 0.35 | 0.005 |
| EF, % | −0.150 | 0.03 | 0.790 | −0.075 | 0.04 | 0.750 | 0.115 | 0.14 | 0.295 | 0.055 | 0.11 | 0.374 | −0.400 | 0.39 | 0.002 |
| Ees, % | −0.431 | 0.19 | 0.129 | −0.166 | 0.18 | 0.166 | 0.151 | 0.35 | 0.006 | 0.093 | 0.38 | 0.002 | −0.253 | 0.48 | <0.001 |
| Energetic | |||||||||||||||
| PV area, % | −0.219 | 0.05 | 0.681 | −0.143 | 0.08 | 0.523 | 0.048 | 0.06 | 0.644 | 0.155 | 0.33 | 0.009 | 0.718 | 0.73 | <0.001 |
| VA coupling, % | −0.098 | 0.07 | 0.599 | −0.106 | 0.18 | 0.172 | 0.043 | 0.15 | 0.236 | −0.004 | 0.02 | 0.858 | 0.227 | 0.67 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-Y.; Lin, S.-C.; Hsu, J.-C.; Chen, J.-S.; Huang, J.-H.; Chiu, K.-M. Reducing Left Ventricular Wall Stress through Aortic Valve Enlargement via Transcatheter Aortic Valve Implantation in Severe Aortic Stenosis. J. Clin. Med. 2024, 13, 3777. https://doi.org/10.3390/jcm13133777
Chiang C-Y, Lin S-C, Hsu J-C, Chen J-S, Huang J-H, Chiu K-M. Reducing Left Ventricular Wall Stress through Aortic Valve Enlargement via Transcatheter Aortic Valve Implantation in Severe Aortic Stenosis. Journal of Clinical Medicine. 2024; 13(13):3777. https://doi.org/10.3390/jcm13133777
Chicago/Turabian StyleChiang, Chih-Yao, Shen-Che Lin, Jung-Cheng Hsu, Jer-Shen Chen, Jih-Hsin Huang, and Kuan-Ming Chiu. 2024. "Reducing Left Ventricular Wall Stress through Aortic Valve Enlargement via Transcatheter Aortic Valve Implantation in Severe Aortic Stenosis" Journal of Clinical Medicine 13, no. 13: 3777. https://doi.org/10.3390/jcm13133777
APA StyleChiang, C.-Y., Lin, S.-C., Hsu, J.-C., Chen, J.-S., Huang, J.-H., & Chiu, K.-M. (2024). Reducing Left Ventricular Wall Stress through Aortic Valve Enlargement via Transcatheter Aortic Valve Implantation in Severe Aortic Stenosis. Journal of Clinical Medicine, 13(13), 3777. https://doi.org/10.3390/jcm13133777




