Unraveling the Clinical Features and Outcomes of IgG4-Related Ophthalmic Disease
Abstract
1. Introduction
2. Patients and Methods
3. Results
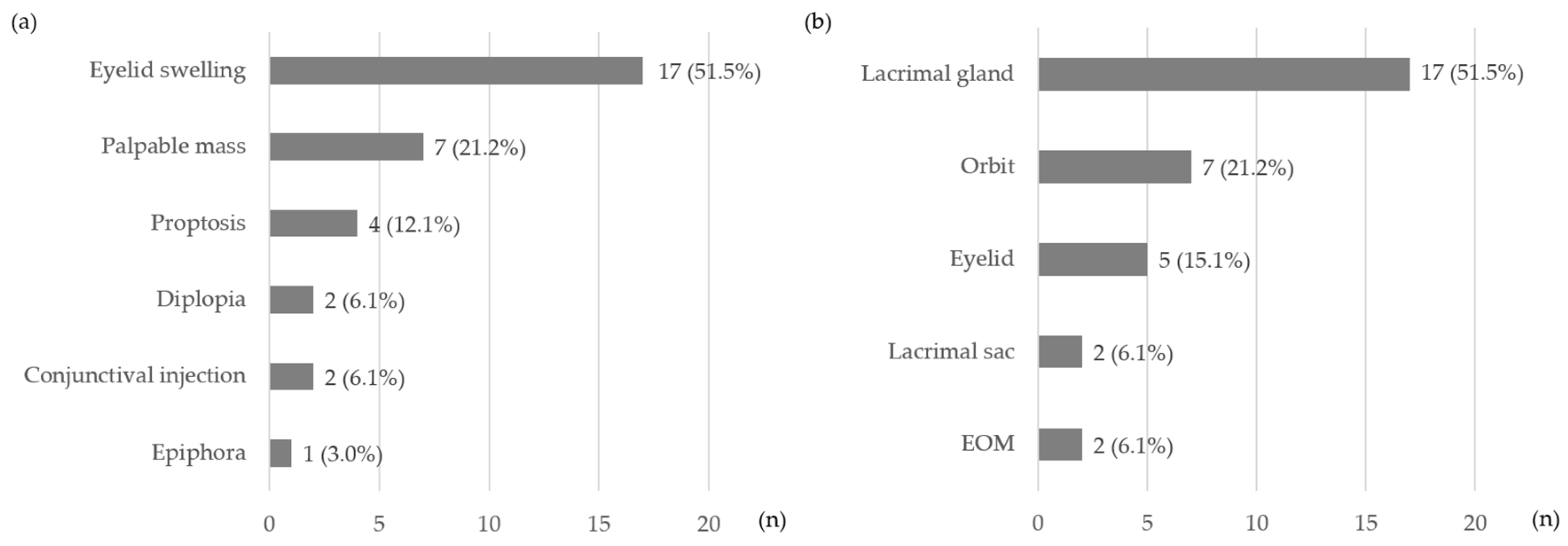
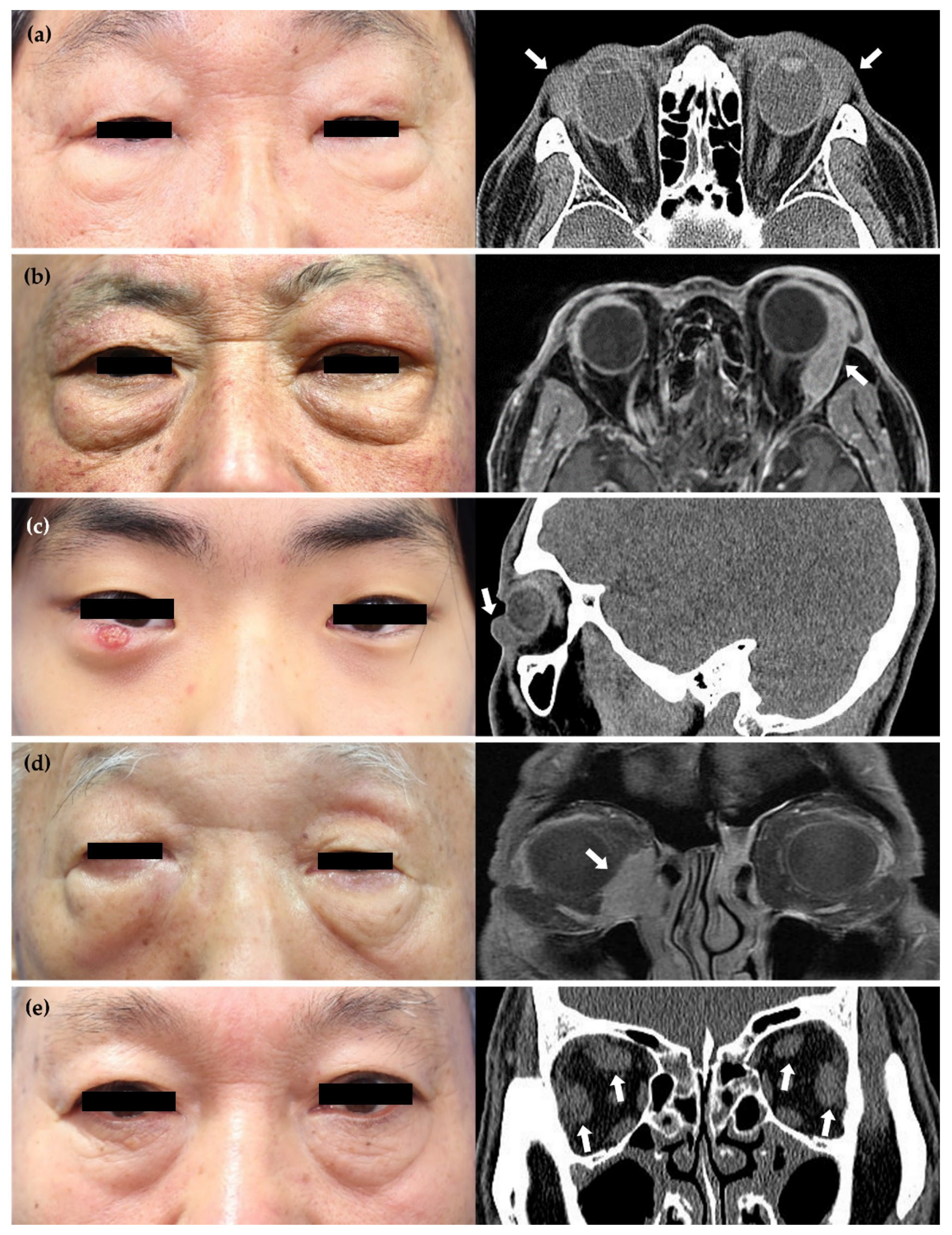
3.1. Baseline Characteristics of Patients with IgG4-nonROD and IgG4-ROD
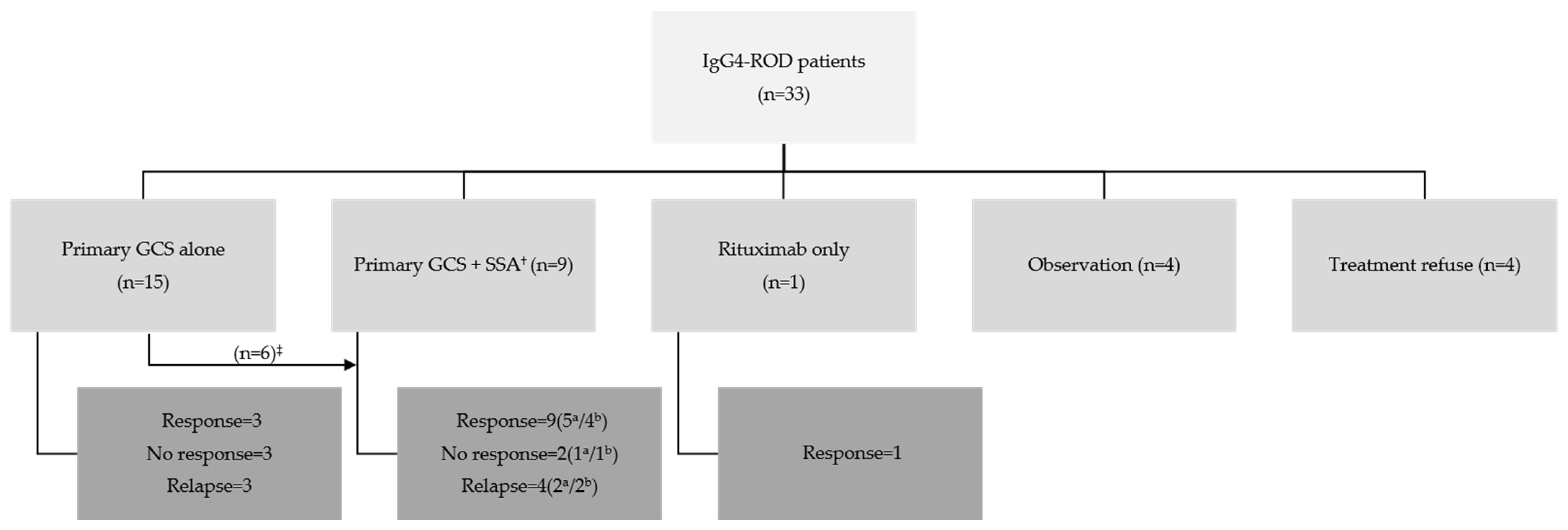
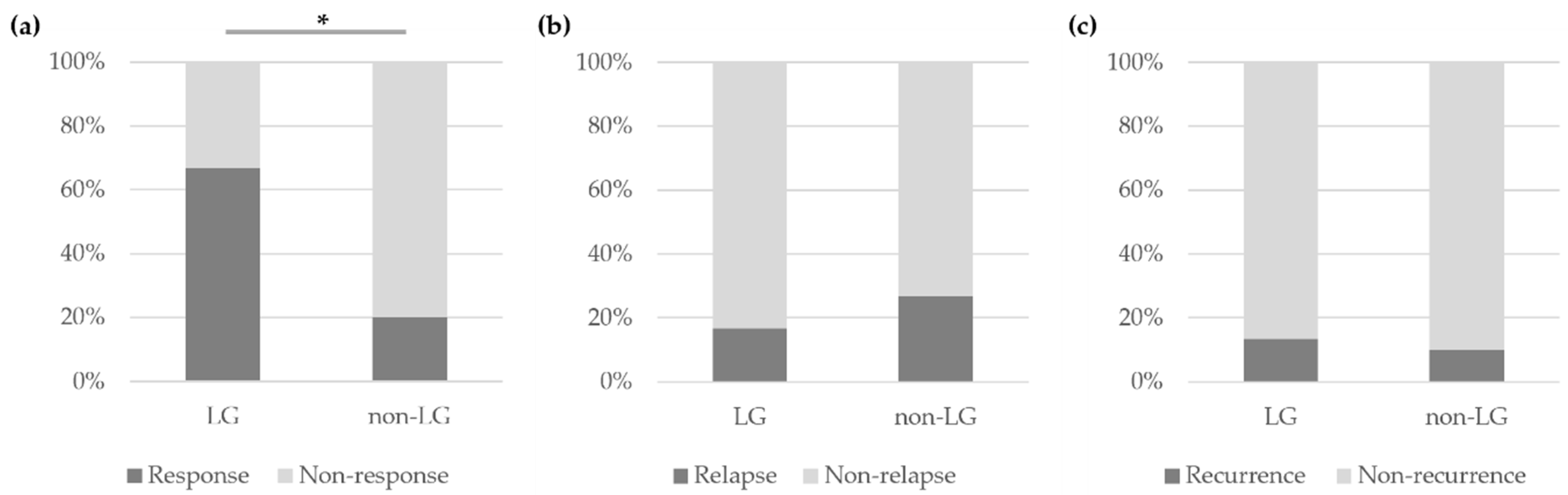
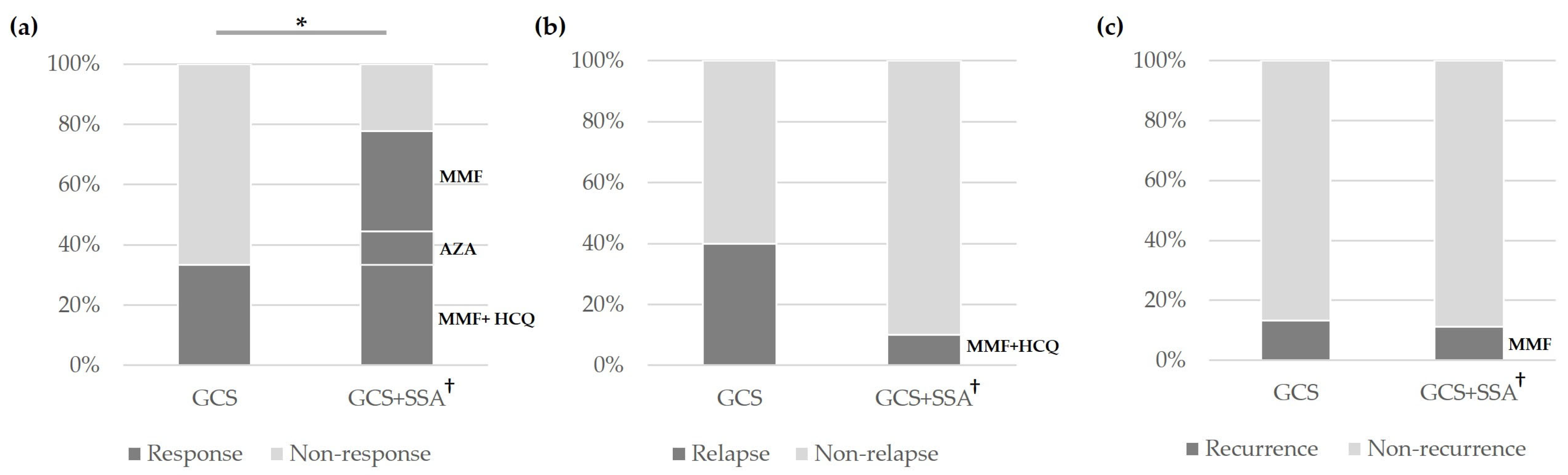
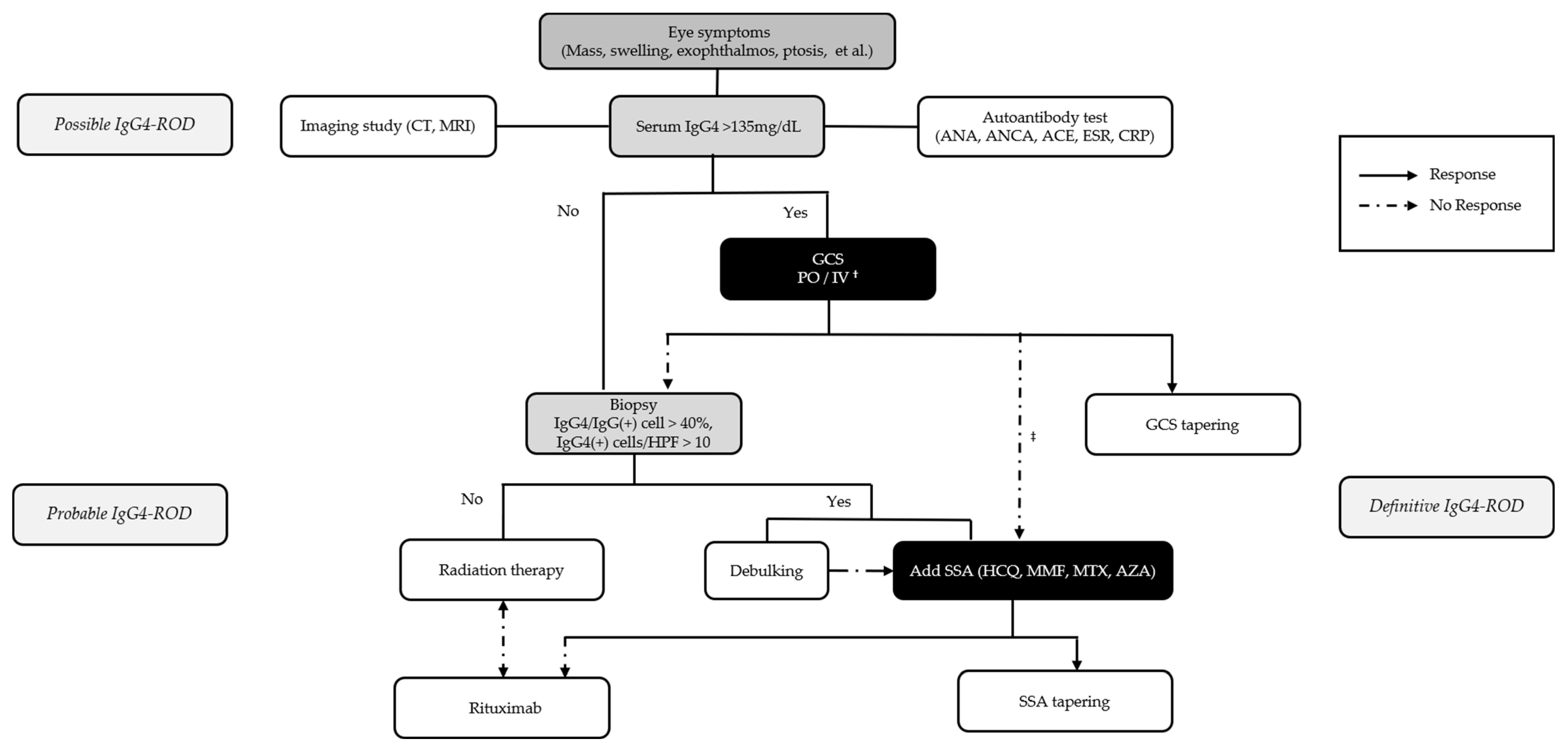
3.2. Treatment and Outcomes of Patients with IgG4-ROD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umehara, H.; Okazaki, K.; Kawa, S.; Takahashi, H.; Goto, H.; Matsui, S.; Ishizaka, N.; Akamizu, T.; Sato, Y.; Kawano, M.; et al. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod. Rheumatol. 2021, 31, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Wallace, Z.S.; Deshpande, V.; Stone, J.H. Ophthalmic manifestations of IgG4-related disease: Single-center experience and literature review. Semin. Arthritis Rheum. 2014, 43, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Perugino, C.A.; Stone, J.H. IgG4-related disease: An update on pathophysiology and implications for clinical care. Nat. Rev. Rheumatol. 2020, 16, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Hayashi, H.; Takahashi, K.; Koide, S.; Sato, W.; Hasegawa, M.; Yamaguchi, Y.; Aten, J.; Ito, Y.; Yuzawa, Y. Tubulointerstitial fibrosis in patients with IgG4-related kidney disease: Pathological findings on repeat renal biopsy. Rheumatol. Int. 2015, 35, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Song, T.J.; Park, J.S.; Yoon, J.H.; Yang, M.J.; Yoon, S.B.; Lee, J.M.; Lee, Y.N.; Kim, S.H.; Choi, E.K.; et al. Comparison of the long-term outcomes between proximal and distal IgG4-related sclerosing cholangitis: A multicenter cohort study. J. Gastroenterol. Hepatol. 2023, 38, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.K.H.; Li, E.Y.M.; Chan, R.Y.C.; Chu, W.C.W.; Cheng, A.C.O.; Chan, K.K.W.; Chin, J.K.Y.; Kwok, J.S.W.; Io, I.Y.F.; Yip, N.K.F.; et al. Treatment outcomes and their determinants of IgG4-related ophthalmic disease: A territory-wide cohort study. Br. J. Ophthalmol. 2023, 107, 1920–1924. [Google Scholar] [CrossRef]
- Cai, S.; Hu, Z.; Chen, Y.; Zhong, J.; Dong, L. Potential roles of non-lymphocytic cells in the pathogenesis of IgG4-related disease. Front. Immunol. 2022, 13, 940581. [Google Scholar] [CrossRef] [PubMed]
- Wallace, Z.S.; Zhang, Y.; Perugino, C.A.; Naden, R.; Choi, H.K.; Stone, J.H.; ACR/EULAR IgG4-RD Classification Criteria Committee. Clinical phenotypes of IgG4-related disease: An analysis of two international cross-sectional cohorts. Ann. Rheum. Dis. 2019, 78, 406–412. [Google Scholar] [CrossRef]
- Wallace, Z.S.; Naden, R.P.; Chari, S.; Choi, H.K.; Della-Torre, E.; Dicaire, J.F.; Hart, P.A.; Inoue, D.; Kawano, M.; Khosroshahi, A.; et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann. Rheum. Dis. 2020, 79, 77–87. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P.; Ye, H.; Xiao, W.; Chen, R.; Mao, Y.; Ai, S.; Liu, Z.; Tang, L.; Yang, H. Clinical features and outcomes of IgG4-related idiopathic orbital inflammatory disease: From a large southern China-based cohort. Eye 2021, 35, 1248–1255. [Google Scholar] [CrossRef]
- Detiger, S.E.; Karim, A.F.; Verdijk, R.M.; van Hagen, P.M.; van Laar, J.A.M.; Paridaens, D. The treatment outcomes in IgG4-related orbital disease: A systematic review of the literature. Acta Ophthalmol. 2019, 97, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chang, J.; Chen, H.; Chen, Y.; Yang, H.; Fei, Y.; Zhang, P.; Zeng, X.; Zhang, F.; Zhang, W. Efficacy between high and medium doses of glucocorticoid therapy in remission induction of IgG4-related diseases: A preliminary randomized controlled trial. Int. J. Rheum. Dis. 2017, 20, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Masaki, Y.; Matsui, S.; Saeki, T.; Tsuboi, H.; Hirata, S.; Izumi, Y.; Miyashita, T.; Fujikawa, K.; Dobashi, H.; Susaki, K.; et al. A multicenter phase II prospective clinical trial of glucocorticoid for patients with untreated IgG4-related disease. Mod. Rheumatol. 2017, 27, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Son, K.Y.; Woo, K.I.; Kim, Y.D. Clinical Outcomes of IgG4-Related Ophthalmic Disease and Idiopathic Sclerosing Orbital Inflammation. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nojima, M.; Takahashi, H.; Yokoyama, Y.; Ishigami, K.; Yajima, H.; Shimizu, Y.; Tabeya, T.; Matsui, M.; Suzuki, C.; et al. Identification of relapse predictors in IgG4-related disease using multivariate analysis of clinical data at the first visit and initial treatment. Rheumatology 2015, 54, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Ebbo, M.; Patient, M.; Grados, A.; Groh, M.; Desblaches, J.; Hachulla, E.; Saadoun, D.; Audia, S.; Rigolet, A.; Terrier, B.; et al. Ophthalmic manifestations in IgG4-related disease: Clinical presentation and response to treatment in a French case-series. Medicine 2017, 96, e6205. [Google Scholar] [CrossRef] [PubMed]
- Andrew, N.; Sladden, N.; Kearney, D.; Crompton, J.; Selva, D. Sequential biopsies from immunoglobulin G4-related orbital disease demonstrate progressive fibrosis. Clin. Exp. Ophthalmol. 2014, 42, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Katz, G.; Stone, J.H. Clinical Perspectives on IgG4-Related Disease and Its Classification. Annu. Rev. Med. 2022, 73, 545–562. [Google Scholar] [CrossRef]
- Sanchez-Oro, R.; Alonso-Munoz, E.M.; Marti Romero, L. Review of IgG4-related disease. Gastroenterol. Hepatol. 2019, 42, 638–647. [Google Scholar] [CrossRef]
- Maritati, F.; Peyronel, F.; Vaglio, A. IgG4-related disease: A clinical perspective. Rheumatology 2020, 59, iii123–iii131. [Google Scholar] [CrossRef]
- Adam, Z.; Zeman, D.; Cermak, A.; Dastych, M.; Doubkova, M.; Horvath, T.; Skorkovska, S.; Adamova, Z.; Rehak, Z.; Koukalova, R.; et al. IgG4-related disease. Clinical manifestation differential diagnosis and recent International Diagnostic Criteria for IgG4-related disease. Vnitr. Lek. 2022, 68, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Martín-Nares, E.; Hernández-Molina, G.; Lima, G.; Hernández-Ramírez, D.F.; Chan-Campos, I.; Saavedra-González, V.; Llorente, L. Tear levels of IL-7, IL-1α, and IL-1β may differentiate between IgG4-related disease and Sjögren’s syndrome. Clin. Rheumatol. 2023, 42, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ohshima, K.; Ichimura, K.; Sato, M.; Yamadori, I.; Tanaka, T.; Takata, K.; Morito, T.; Kondo, E.; Yoshino, T. Ocular adnexal IgG4-related disease has uniform clinicopathology. Pathol. Int. 2008, 58, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Sogabe, Y.; Ohshima, K.; Azumi, A.; Takahira, M.; Kase, S.; Tsuji, H.; Yoshikawa, H.; Nakamura, T. Location and frequency of lesions in patients with IgG4-related ophthalmic diseases. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.M.; Kim, N.; Paik, J.H. Immunoglobulin G4-related Ophthalmic Disease of the Caruncle: A Case Report. Korean J. Ophthalmol. 2022, 36, 568–569. [Google Scholar] [CrossRef] [PubMed]
- Yoo, R.E.; Park, S.W.; Rhim, J.H.; Kim, J.E.; Kim, S.C.; Choe, J.Y.; Choung, H.K.; Khwarg, S.I.; Kim, J.H.; Lee, J.H.; et al. CT and MR imaging findings of ocular adnexal mucosa-associated lymphoid tissue lymphoma associated with IgG4-related disease: Multi-institutional case series. Int. J. Ophthalmol. 2020, 13, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Meng, F.; Ren, H.; Yue, H.; Xue, K.; Zhang, R. Pathological count of IgG4-positive plasmacytes suggests extraophthalmic involvement and relapse in patients with IgG4-related ophthalmic disease: A retrospective study. Arthritis Res. Ther. 2022, 24, 80. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Ahn, S.M.; Oh, J.S.; Hong, S.; Lee, C.K.; Yoo, B.; Kim, Y.G. Serum IgG4 level during initial treatment as a predictor of relapse in IgG4-related disease. PLoS ONE 2023, 18, e0282852. [Google Scholar] [CrossRef]
- Kamisawa, T.; Okazaki, K.; Kawa, S.; Ito, T.; Inui, K.; Irie, H.; Nishino, T.; Notohara, K.; Nishimori, I.; Tanaka, S.; et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 III. Treatment and prognosis of autoimmune pancreatitis. J. Gastroenterol. 2014, 49, 961–970. [Google Scholar] [CrossRef]
- Kubota, T.; Moritani, S.; Katayama, M.; Terasaki, H. Ocular adnexal IgG4-related lymphoplasmacytic infiltrative disorder. Arch. Ophthalmol. 2010, 128, 577–584. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.Q.; Zhang, P.P.; Zhang, X.; Peng, L.Y.; Chen, H.; Zhou, J.X.; Zhang, S.Z.; Yang, H.X.; Liu, J.J.; et al. Clinical outcomes and predictive relapse factors of IgG4-related disease following treatment: A long-term cohort study. J. Intern. Med. 2019, 286, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Mou, D.; Wang, Z.; Zeng, Q.; Wang, Z.; Xue, J.; Ren, L.; Liu, Y.; Su, Y. Clinical features and relapse risks of IgG4-related ophthalmic disease: A single-center experience in China. Arthritis Res. Ther. 2021, 23, 98. [Google Scholar] [CrossRef]
- Gupta, N.; Mathew, J.; Mohan, H.; Chowdhury, S.D.; Kurien, R.T.; Christopher, D.J.; Thangakunam, B.; Alexander, M.; Sivadasan, A.; Tamilarasi, V.; et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of Immunoglobulin G4-related disease (IgG4-RD): A series from a tertiary care teaching hospital in South India. Rheumatol. Int. 2018, 38, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ominato, J.; Oyama, T.; Cho, H.; Shiozaki, N.; Umezu, H.; Takizawa, J.; Fukuchi, T. The natural course of IgG4-related ophthalmic disease after debulking surgery: A single-centre retrospective study. BMJ Open Ophthalmol. 2019, 4, e000295. [Google Scholar] [CrossRef]
- Carruthers, M.N.; Topazian, M.D.; Khosroshahi, A.; Witzig, T.E.; Wallace, Z.S.; Hart, P.A.; Deshpande, V.; Smyrk, T.C.; Chari, S.; Stone, J.H. Rituximab for IgG4-related disease: A prospective, open-label trial. Ann. Rheum. Dis. 2015, 74, 1171–1177. [Google Scholar] [CrossRef]
- Ebbo, M.; Grados, A.; Samson, M.; Groh, M.; Loundou, A.; Rigolet, A.; Terrier, B.; Guillaud, C.; Carra-Dalliere, C.; Renou, F.; et al. Long-term efficacy and safety of rituximab in IgG4-related disease: Data from a French nationwide study of thirty-three patients. PLoS ONE 2017, 12, e0183844. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, A.; Carruthers, M.N.; Deshpande, V.; Unizony, S.; Bloch, D.B.; Stone, J.H. Rituximab for the treatment of IgG4-related disease: Lessons from 10 consecutive patients. Medicine 2012, 91, 57–66. [Google Scholar] [CrossRef]
- Campochiaro, C.; Della-Torre, E.; Lanzillotta, M.; Bozzolo, E.; Baldissera, E.; Milani, R.; Arcidiacono, P.G.; Crippa, S.; Falconi, M.; Dagna, L. Long-term efficacy of maintenance therapy with Rituximab for IgG4-related disease. Eur. J. Intern. Med. 2020, 74, 92–98. [Google Scholar] [CrossRef]
- Karim, A.F.; Bansie, R.D.; Rombach, S.M.; Paridaens, D.; Verdijk, R.M.; van Hagen, P.M.; Van Laar, J.A.M. The treatment outcomes in IgG4-related disease. Neth. J. Med. 2018, 76, 275–285. [Google Scholar]
- Muller-Calleja, N.; Manukyan, D.; Canisius, A.; Strand, D.; Lackner, K.J. Hydroxychloroquine inhibits proinflammatory signalling pathways by targeting endosomal NADPH oxidase. Ann. Rheum. Dis. 2017, 76, 891–897. [Google Scholar] [CrossRef]
- Browning, D.J. Hydroxychloroquine and chloroquine retinopathy: Screening for drug toxicity. Am. J. Ophthalmol. 2002, 133, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Luo, X.; Fei, Y.; Peng, L.; Zhou, J.; Li, J.; Lu, H.; Liu, Z.; Zhang, P.; Liu, X.; et al. Long-Term Outcomes of IgG4-Related Ophthalmic Disease in a Chinese IgG4-Related Disease Cohort. Front. Med. 2021, 8, 784520. [Google Scholar] [CrossRef] [PubMed]
| IgG4-nonROD (n = 37) | IgG4-ROD (n = 33) | p-Value | |
|---|---|---|---|
| Age, years ± SD | 59.24 ± 14.65 | 47.52 ± 19.50 | 0.006 * |
| Male, n (%) | 26 (70.27) | 19 (57.58) | 0.27 † |
| Serum IgG4 level, mg/dL ± SD | 215.81 ± 97.60 | 292.37 ± 250.22 | 0.79 * |
| Elevated pre-treatment serum IgG4 level, n (%) | 31 (83.78) | 28 (84.85) | 1.00 ‡ |
| Follow-up duration, months ± SD | 11.49 ± 6.61 | 16.64 ± 11.79 | 0.06 * |
| Diagnostic criteria | |||
| Definitive a | 8 (21.62) | 9 (27.27) | 0.78 ‡ |
| Probable b | 6 (16.22) | 5 (15.15) | 1.00 ‡ |
| Possible c | 23 (62.16) | 19 (57.58) | 0.81 ‡ |
| No Response (n = 5) | Response (n = 20) | p-Value | |
|---|---|---|---|
| Age, years ± SD | 30.40 ± 22.40 | 54.40 ± 13.84 | 0.006 * |
| Male, n (%) | 3 (60.0) | 13 (65.0) | 1.00 † |
| Serum IgG4 level, mg/dL ± SD | 137.46 ± 56.88 | 333.23 ± 305.06 | 0.53 * |
| Elevated pre-treatment serum IgG4 level, n (%) | 5 (100.00) | 17 (85.00) | 1.00 ‡ |
| Follow-up duration, months ± SD | 19.43 ± 7.77 | 18.23 ± 13.89 | 0.41 * |
| Diagnostic criteria, n (%) | |||
| Definitive a | 1 (20.00) | 8 (40.00) | 0.62 ‡ |
| Probable b | 0 (0.00) | 1 (5.00) | 1.00 ‡ |
| Possible c | 4 (80.00) | 11 (55.00) | 0.62 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Jeong, S.; Lew, H. Unraveling the Clinical Features and Outcomes of IgG4-Related Ophthalmic Disease. J. Clin. Med. 2024, 13, 3780. https://doi.org/10.3390/jcm13133780
Kim D, Jeong S, Lew H. Unraveling the Clinical Features and Outcomes of IgG4-Related Ophthalmic Disease. Journal of Clinical Medicine. 2024; 13(13):3780. https://doi.org/10.3390/jcm13133780
Chicago/Turabian StyleKim, Doah, SangYoon Jeong, and Helen Lew. 2024. "Unraveling the Clinical Features and Outcomes of IgG4-Related Ophthalmic Disease" Journal of Clinical Medicine 13, no. 13: 3780. https://doi.org/10.3390/jcm13133780
APA StyleKim, D., Jeong, S., & Lew, H. (2024). Unraveling the Clinical Features and Outcomes of IgG4-Related Ophthalmic Disease. Journal of Clinical Medicine, 13(13), 3780. https://doi.org/10.3390/jcm13133780







