Clinical Outcomes of Aspirin and Clopidogrel among Patients with Chronic Obstructive Lung Disease: Insights from a Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Studies and Data Sources
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Statistical Methods
2.5. Tools to Assess Quality of Studies
3. Results
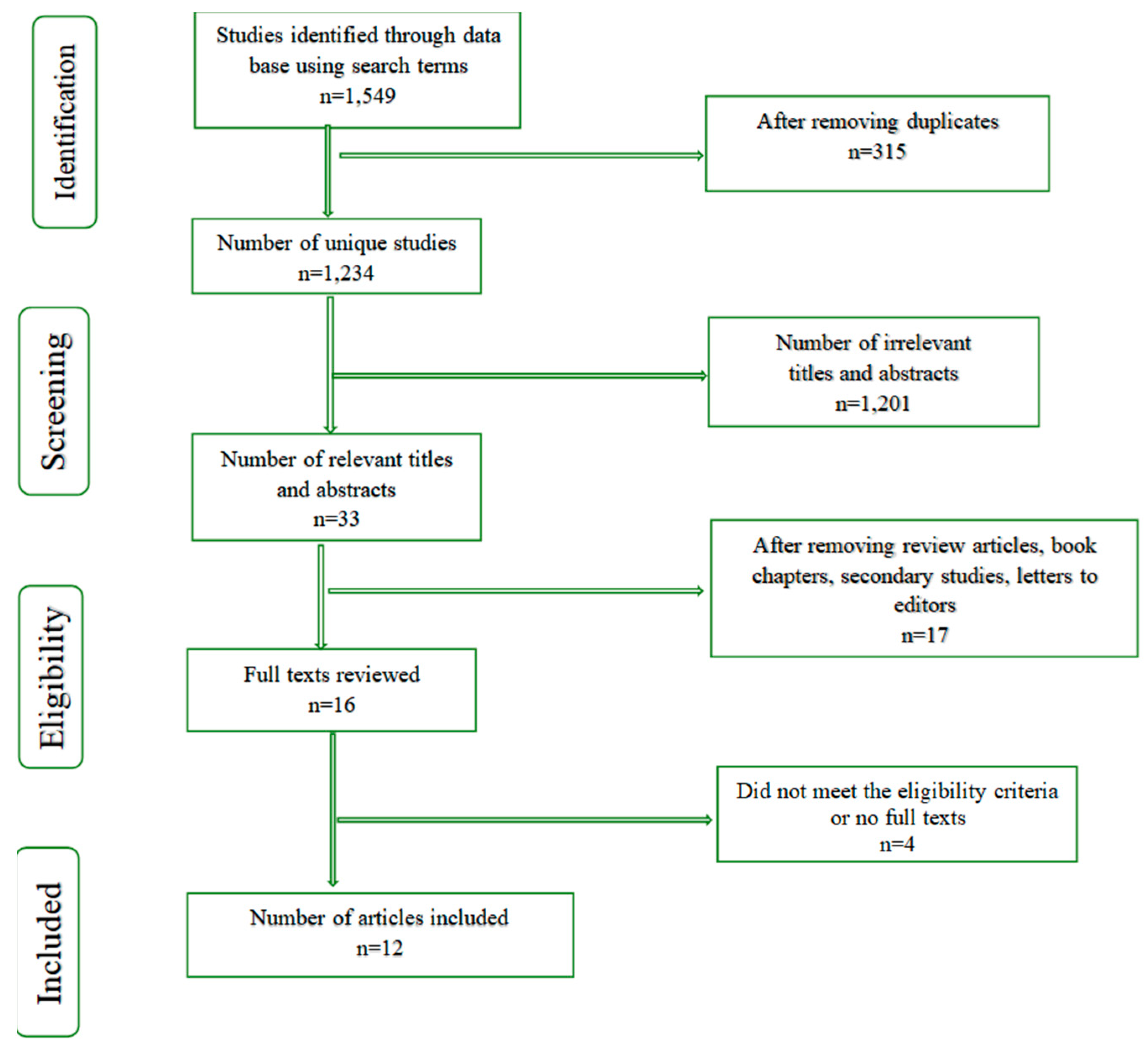
3.1. Search Strategy
3.2. Features of the Identified Research Studies
3.3. Main Findings of Each Study
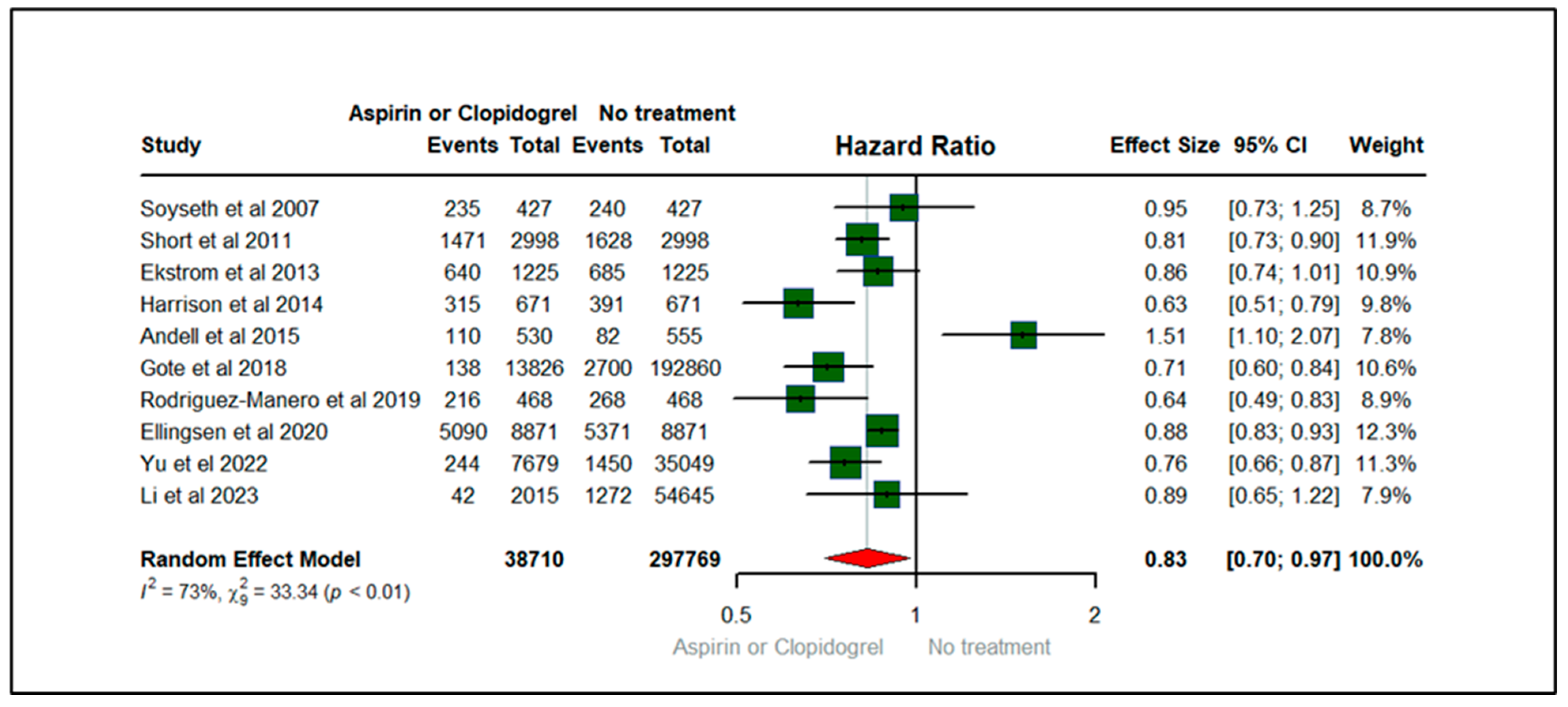
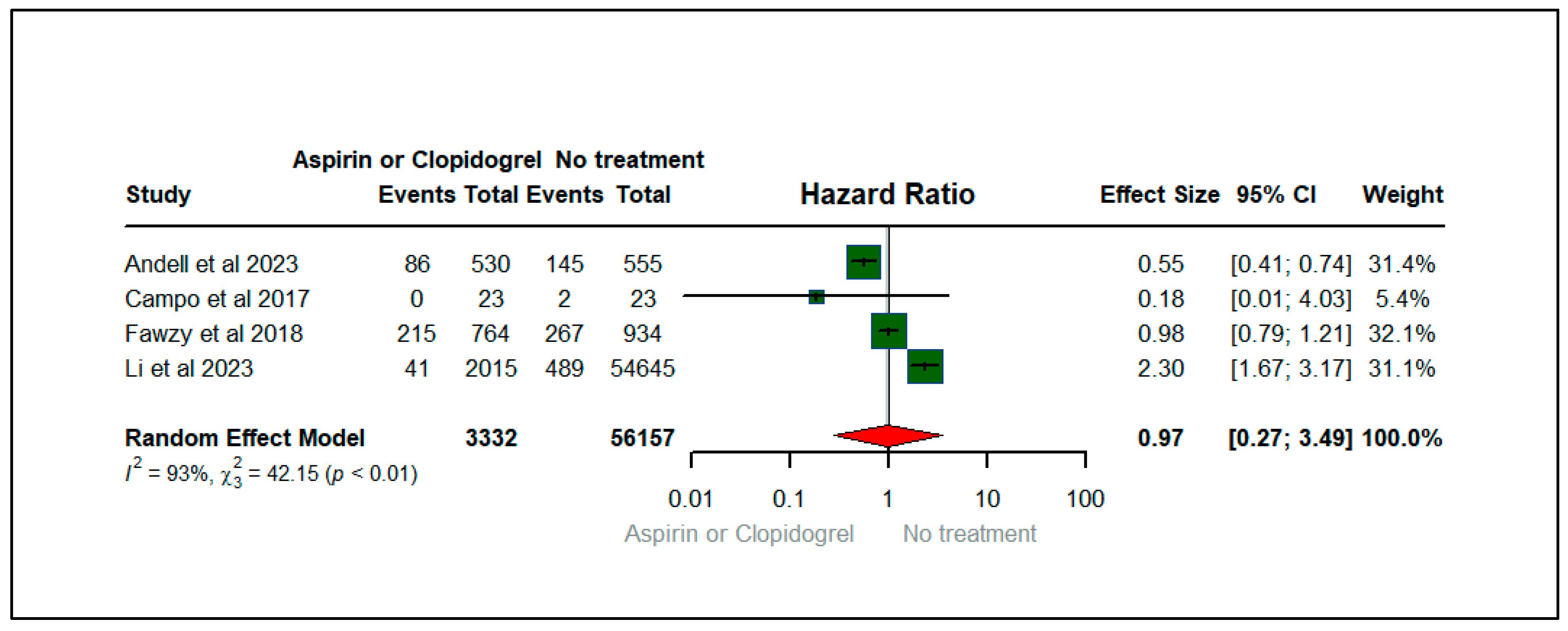
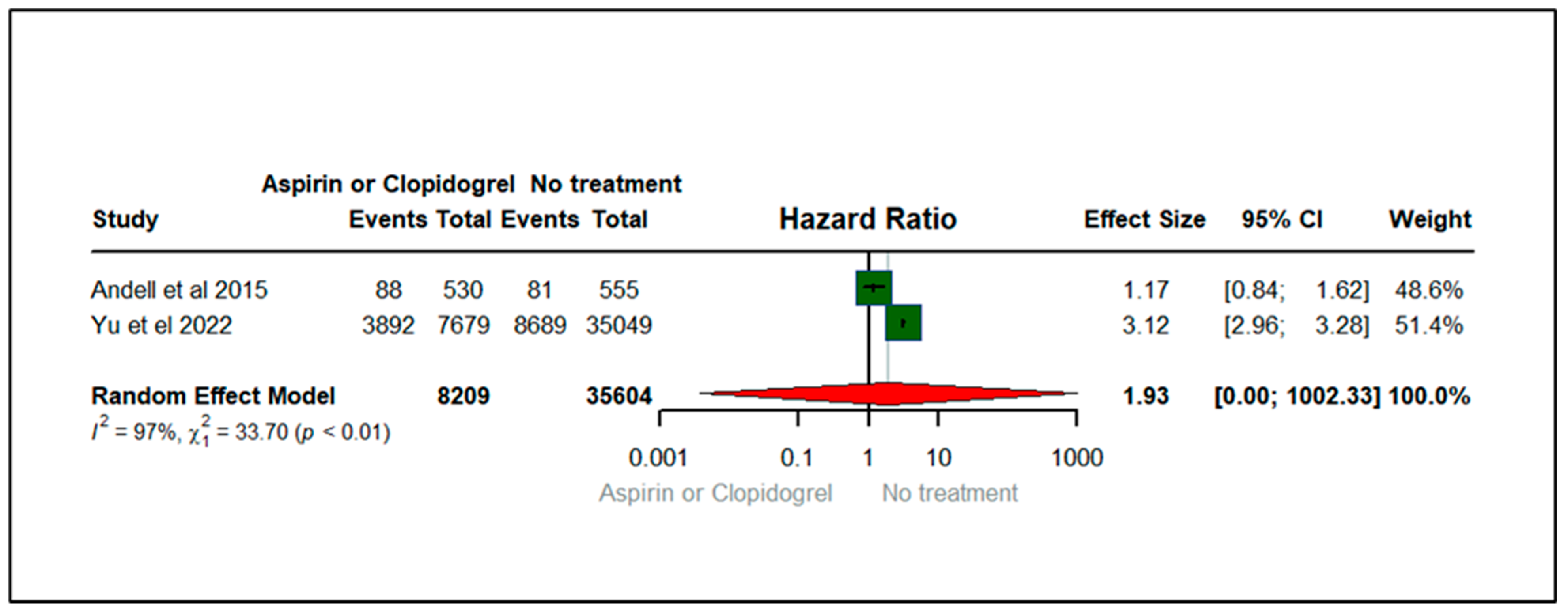
3.4. Forest Plots: Clinical Outcomes
3.5. Results of Publication Bias
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Venkatesan, P. GOLD COPD report: 2024 update. Lancet Respir. Med. 2024, 12, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Abraham, J.; Ali, M.K.; Alvarado, M.; Atkinson, C.; Baddour, L.M.; Bartels, D.H.; Benjamin, E.J.; Bhalla, K.; Birbeck, G. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–606. [Google Scholar] [CrossRef]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.; Han, M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Zysman, M.; Mahay, G.; Guibert, N.; Barnig, C.; Leroy, S.; Guilleminault, L. Impact of pharmacological and non-pharmacological interventions on mortality in chronic obstructive pulmonary disease (COPD) patients. Respir. Med. Res. 2023, 84, 101035. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, F.H.; Wilkinson, M.; Keevil, B.; Niven, R.; Fowler, S.J. Short-and medium-term effect of inhaled corticosteroids on exhaled breath biomarkers in severe asthma. J. Breath Res. 2022, 16, 047101. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fujita, Y.; Chen, H.; Somekawa, K.; Kashizaki, F.; Koizumi, H.; Takahashi, K.; Horita, N.; Hara, Y.; Muro, S. The Effectiveness and Safety of Long-Term Macrolide Therapy for COPD in Stable Status: A Systematic Review and Meta-Analysis. Diseases 2023, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.K.; Connett, J.; Bailey, W.C.; Casaburi, R.; Cooper, J.A.D., Jr.; Criner, G.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; Lazarus, S.C. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011, 365, 689–698. [Google Scholar] [CrossRef]
- Herath, S.C.; Normansell, R.; Maisey, S.; Poole, P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2018, 10, CD009764. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.K.; Kim, D.K. Role of phosphodiesterase-4 inhibitors in chronic obstructive pulmonary disease. Korean J. Intern. Med. 2020, 35, 276. [Google Scholar] [CrossRef]
- Schick, M.A.; Schlegel, N. Clinical implication of phosphodiesterase-4-inhibition. Int. J. Mol. Sci. 2022, 23, 1209. [Google Scholar] [CrossRef] [PubMed]
- Criner, G.J.; Connett, J.E.; Aaron, S.D.; Albert, R.K.; Bailey, W.C.; Casaburi, R.; Cooper, J.A.D., Jr.; Curtis, J.L.; Dransfield, M.T.; Han, M.K. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N. Engl. J. Med. 2014, 370, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Badimon, J. Overview of aspirin and platelet biology. Am. J. Cardiol. 2021, 144, S2–S9. [Google Scholar] [CrossRef]
- Ekstrom, M.P.; Hermansson, A.B.; Strom, K.E. Effect of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease: A time-dependent analysis. In A101. Pharmacological Treatment of Copd: Old and New; American Thoracic Society: New York, NY, USA, 2012; A2263p. [Google Scholar]
- Harrison, M.T.; Short, P.; Williamson, P.A.; Singanayagam, A.; Chalmers, J.D.; Schembri, S. Thrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: A role for antiplatelet therapy? Thorax 2014, 69, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Aaron, C.P.; Schwartz, J.E.; Hoffman, E.A.; Angelini, E.; Austin, J.H.; Cushman, M.; Jacobs Jr, D.R.; Kaufman, J.D.; Laine, A.; Smith, L.J. A longitudinal cohort study of aspirin use and progression of emphysema-like lung characteristics on CT imaging: The MESA lung study. Chest 2018, 154, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D. Doing Meta-Analysis with R: A Hands-On Guide; Chapman and Hall/CRC: Boca Raton, FL, USA, 2021. [Google Scholar]
- Søyseth, V.; Brekke, P.; Smith, P.; Omland, T. Statin use is associated with reduced mortality in COPD. Eur. Respir. J. 2007, 29, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Short, P.M.; Lipworth, S.I.; Elder, D.H.; Schembri, S.; Lipworth, B.J. Effect of β blockers in treatment of chronic obstructive pulmonary disease: A retrospective cohort study. BMJ 2011, 342, d2549. [Google Scholar] [CrossRef] [PubMed]
- Andell, P.; James, S.K.; Cannon, C.P.; Cyr, D.D.; Himmelmann, A.; Husted, S.; Keltai, M.; Koul, S.; Santoso, A.; Steg, P.G. Ticagrelor versus clopidogrel in patients with acute coronary syndromes and chronic obstructive pulmonary disease: An analysis from the Platelet Inhibition and Patient Outcomes (PLATO) trial. J. Am. Heart Assoc. 2015, 4, e002490. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.; Dalla Sega, F.V.; Pavasini, R.; Aquila, G.; Gallo, F.; Fortini, F.; Tonet, E.; Cimaglia, P.; Del Franco, A.; Pestelli, G. Biological effects of ticagrelor over clopidogrel in patients with stable coronary artery disease and chronic obstructive pulmonary disease. Thromb. Haemost. 2017, 117, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Faridi, M.K.; Camargo, C.A.; Hasegawa, K. The association of aspirin use with severity of acute exacerbation of chronic obstructive pulmonary disease: A retrospective cohort study. NPJ Prim. Care Respir. Med. 2018, 28, 7. [Google Scholar] [CrossRef]
- Fawzy, A.; Putcha, N.; Aaron, C.P.; Bowler, R.P.; Comellas, A.P.; Cooper, C.B.; Dransfield, M.T.; Han, M.K.; Hoffman, E.A.; Kanner, R.E. Aspirin use and respiratory morbidity in COPD: A propensity score-matched analysis in subpopulations and intermediate outcome measures in COPD study. Chest 2019, 155, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mañero, M.; López-Pardo, E.; Cordero, A.; Ruano-Ravina, A.; Novo-Platas, J.; Pereira-Vázquez, M.; Martínez-Gómez, Á.; García-Seara, J.; Martínez-Sande, J.-L.; Peña-Gil, C. A prospective study of the clinical outcomes and prognosis associated with comorbid COPD in the atrial fibrillation population. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, J.; Johansson, G.; Larsson, K.; Lisspers, K.; Malinovschi, A.; Ställberg, B.; Thuresson, M.; Janson, C. Impact of Comorbidities and Commonly Used Drugs on Mortality in COPD–Real-World Data from a Primary Care Setting. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 235–245. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Ip, M.S.-M.; Li, X.; Cheung, K.-S.; Ren, Q.-W.; Wu, M.-Z.; Li, H.-L.; Wong, P.-F.; Tse, H.-F.; Yiu, K.-H. Low-dose aspirin and incidence of lung carcinoma in patients with chronic obstructive pulmonary disease in Hong Kong: A cohort study. PLoS Med. 2022, 19, e1003880. [Google Scholar] [CrossRef]
- Li, X.; Dai, B.; Han, Q.; Wu, Y.; Ran, B.; Wang, T.; Wen, F.; Chen, J. High risks adverse events associated with usage of aspirin in chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2023, 17, 1285–1295. [Google Scholar] [CrossRef]
- Pavasini, R.; Biscaglia, S.; d’Ascenzo, F.; Del Franco, A.; Contoli, M.; Zaraket, F.; Guerra, F.; Ferrari, R.; Campo, G. Antiplatelet Treatment Reduces All-Cause Mortality in COPD Patients: A Systematic Review and Meta-Analysis. Copd 2016, 13, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Gando, S. Microvascular thrombosis and multiple organ dysfunction syndrome. Crit. Care Med. 2010, 38, S35–S42. [Google Scholar] [CrossRef]
- Toner, P.; McAuley, D.F.; Shyamsundar, M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit. Care 2015, 19, 374. [Google Scholar] [CrossRef]
- Davi, G.; Basili, S.; Vieri, M.; Cipollone, F.; Santarone, S.; Alessandri, C.; GAZZANIGA, P.; Cordova, C.; Violi, F.; Bronchitis, C.O.; et al. Enhanced thromboxane biosynthesis in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1997, 156, 1794–1799. [Google Scholar] [CrossRef]
- Patrignani, P.; Filabozzi, P.; Patrono, C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J. Clin. Investig. 1982, 69, 1366–1372. [Google Scholar] [CrossRef]
- Agustí, A.; Edwards, L.D.; Rennard, S.I.; MacNee, W.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Lomas, D.A.; Calverley, P.M.; Wouters, E. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: A novel phenotype. PLoS ONE 2012, 7, e37483. [Google Scholar] [CrossRef] [PubMed]
- Hamid, U.; Krasnodembskaya, A.; Fitzgerald, M.; Shyamsundar, M.; Kissenpfennig, A.; Scott, C.; Lefrancais, E.; Looney, M.; Verghis, R.; Scott, J. Aspirin reduces lipopolysaccharide-induced pulmonary inflammation in human models of ARDS. Thorax 2017, 72, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L. P2Y12 inhibitors: Differences in properties and mechanisms of action and potential consequences for clinical use. Eur. Heart J. 2009, 30, 1964–1977. [Google Scholar] [CrossRef] [PubMed]

| Study | Year | Country | Study Design | Sample Size | Patients | Intervention | Control | Intervention Duration | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Soyseth et al., 2007 [18] | 2007 | Norway | Hospital-based study | 854 | COPD | Aspirin | No Aspirin | 1.9 years | Mortality |
| Short et al., 2011 [19] | 2011 | Scotland | Hospital-based study | 5977 | COPD | Aspirin | No Aspirin | 4.35 years | Mortality |
| Ekstrom et al., 2013 [14] | 2013 | Sweden | Time-dependent analysis | 2249 | COPD | Aspirin | No Aspirin | 1.1 years | Mortality |
| Harrison et al., 2014 [15] | 2014 | UK | Observational cohort study | 1343 | COPD | Aspirin | No Aspirin | 1 year | Mortality |
| Andell et al., 2015 [20] | 2015 | Multi-country | Double-blinded RCT | 1085 | Diagnosed with COPD and Acute coronary syndrome | Clopidogrel | Ticagrelor | one year | Death from any cause, major bleeding, dyspnea |
| Campo et al., 2017 [21] | 2017 | Italy | RCT | 46 | COPD | Aspirin | No Aspirin | One month | Dyspnea |
| Gote et al., 2018 [22] | 2018 | United States | Retrospective cohort study | 206,686 | Acute exacerbation of COPD | Aspirin | No Aspirin | 3 months | In-hospital death, length of hospital stays, invasive mechanical ventilation |
| Fawzy et al., 2018 [23] | 2018 | United States | Observational cohort study | 1698 | COPD | Aspirin | No Aspirin | One month | Dyspnea |
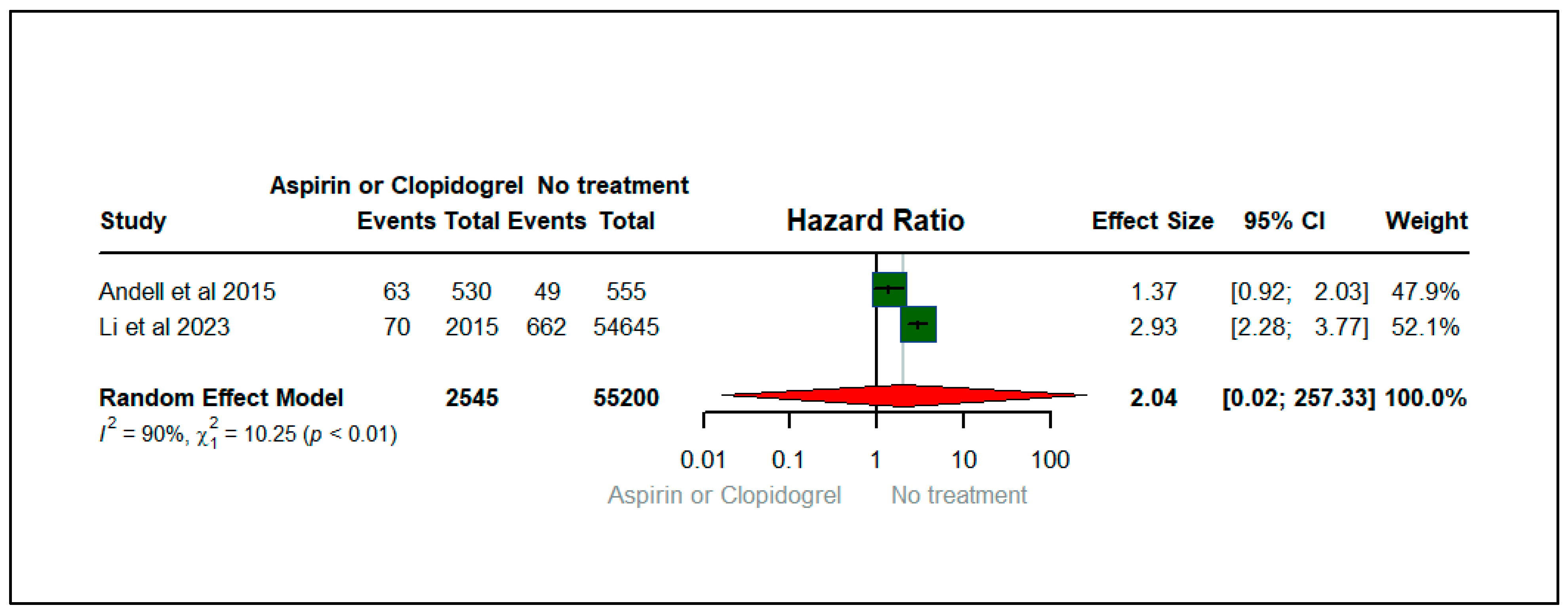
| Rodríguez-Mañero et al., 2019 [24] | 2019 | Spain | Prospective analysis | 937 | COPD | Aspirin and Clopidogrel | No treatment | NR | Mortality |
| Ellingsen et al., 2020 [25] | 2020 | Sweden | Observational retrospective cohort study | 17,745 | COPD | Aspirin | No Aspirin | 3.62 years | Mortality |
| Yu et al., 2022 [26] | 2022 | China | Retrospective cohort study | 42,728 | COPD | Aspirin | No Aspirin | 2.6 years | Death, major bleeding |
| Li et al., 2023 [27] | 2023 | China | Retrospective cohort study | 56,660 | COPD | Aspirin | No Aspirin | NR | MI, dyspnea, death |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhawiti, N.M.; Ismaeil, T.T.; Fouda, S.; Alotaibi, B.A.; El-Metwally, A.; Barhoumi, T.; Alotaibi, T.F. Clinical Outcomes of Aspirin and Clopidogrel among Patients with Chronic Obstructive Lung Disease: Insights from a Meta-Analysis. J. Clin. Med. 2024, 13, 3715. https://doi.org/10.3390/jcm13133715
Alhawiti NM, Ismaeil TT, Fouda S, Alotaibi BA, El-Metwally A, Barhoumi T, Alotaibi TF. Clinical Outcomes of Aspirin and Clopidogrel among Patients with Chronic Obstructive Lung Disease: Insights from a Meta-Analysis. Journal of Clinical Medicine. 2024; 13(13):3715. https://doi.org/10.3390/jcm13133715
Chicago/Turabian StyleAlhawiti, Naif M., Taha T. Ismaeil, Sherouk Fouda, Badi A. Alotaibi, Ashraf El-Metwally, Tlili Barhoumi, and Tareq F. Alotaibi. 2024. "Clinical Outcomes of Aspirin and Clopidogrel among Patients with Chronic Obstructive Lung Disease: Insights from a Meta-Analysis" Journal of Clinical Medicine 13, no. 13: 3715. https://doi.org/10.3390/jcm13133715
APA StyleAlhawiti, N. M., Ismaeil, T. T., Fouda, S., Alotaibi, B. A., El-Metwally, A., Barhoumi, T., & Alotaibi, T. F. (2024). Clinical Outcomes of Aspirin and Clopidogrel among Patients with Chronic Obstructive Lung Disease: Insights from a Meta-Analysis. Journal of Clinical Medicine, 13(13), 3715. https://doi.org/10.3390/jcm13133715







