Chemotherapy Related Cardiotoxicity Evaluation—A Contemporary Review with a Focus on Cardiac Imaging
Abstract
1. Key Points
2. Introduction
3. Cancer Therapy-Related Cardiac Dysfunction (CTRCD)
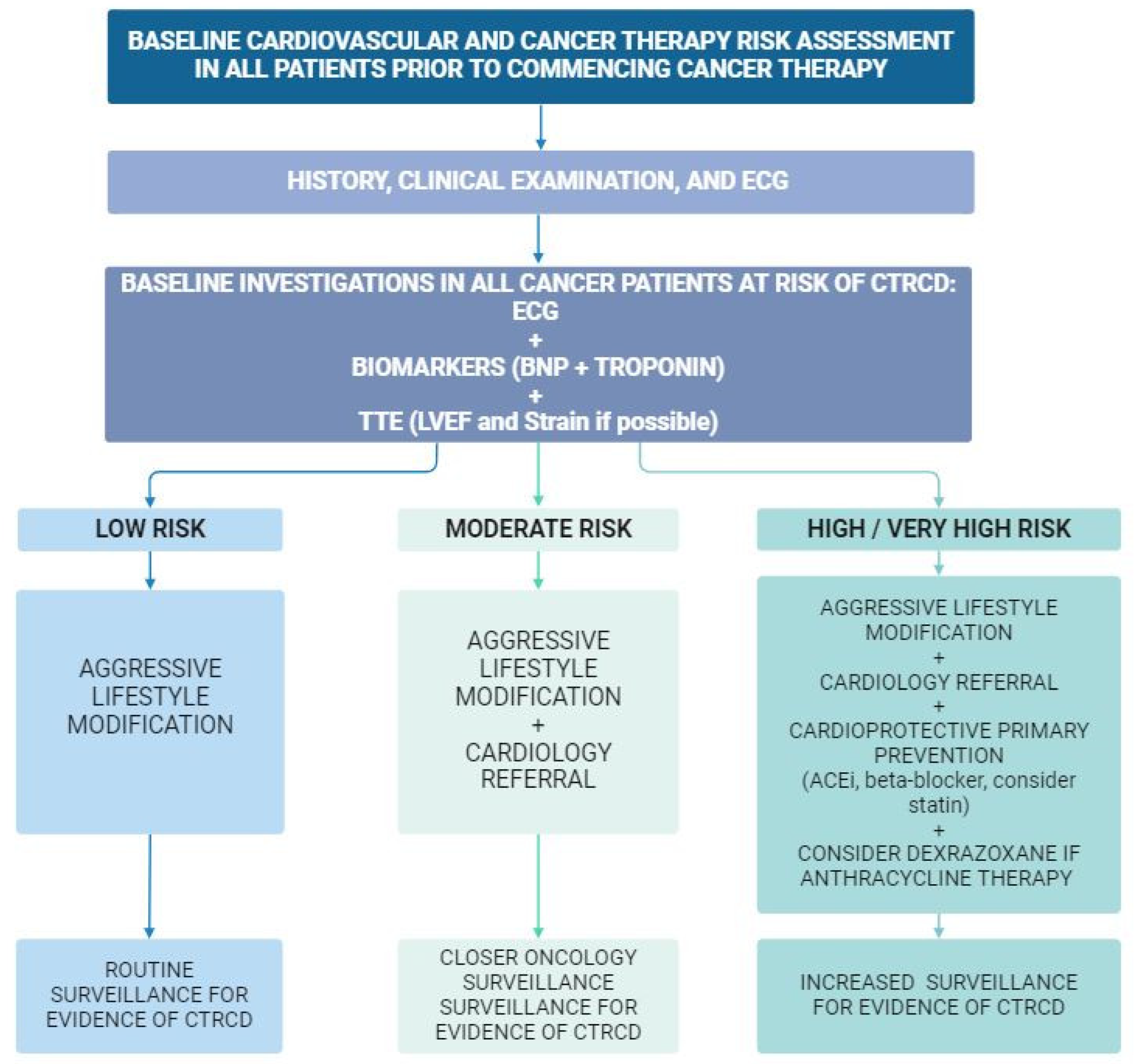
4. Risk Assessment
4.1. Patient-Related Factors for CTRCD
4.2. Cardiac-Specific Biomarkers
4.3. Cancer Treatment-Related Risk Factors for CTRCD
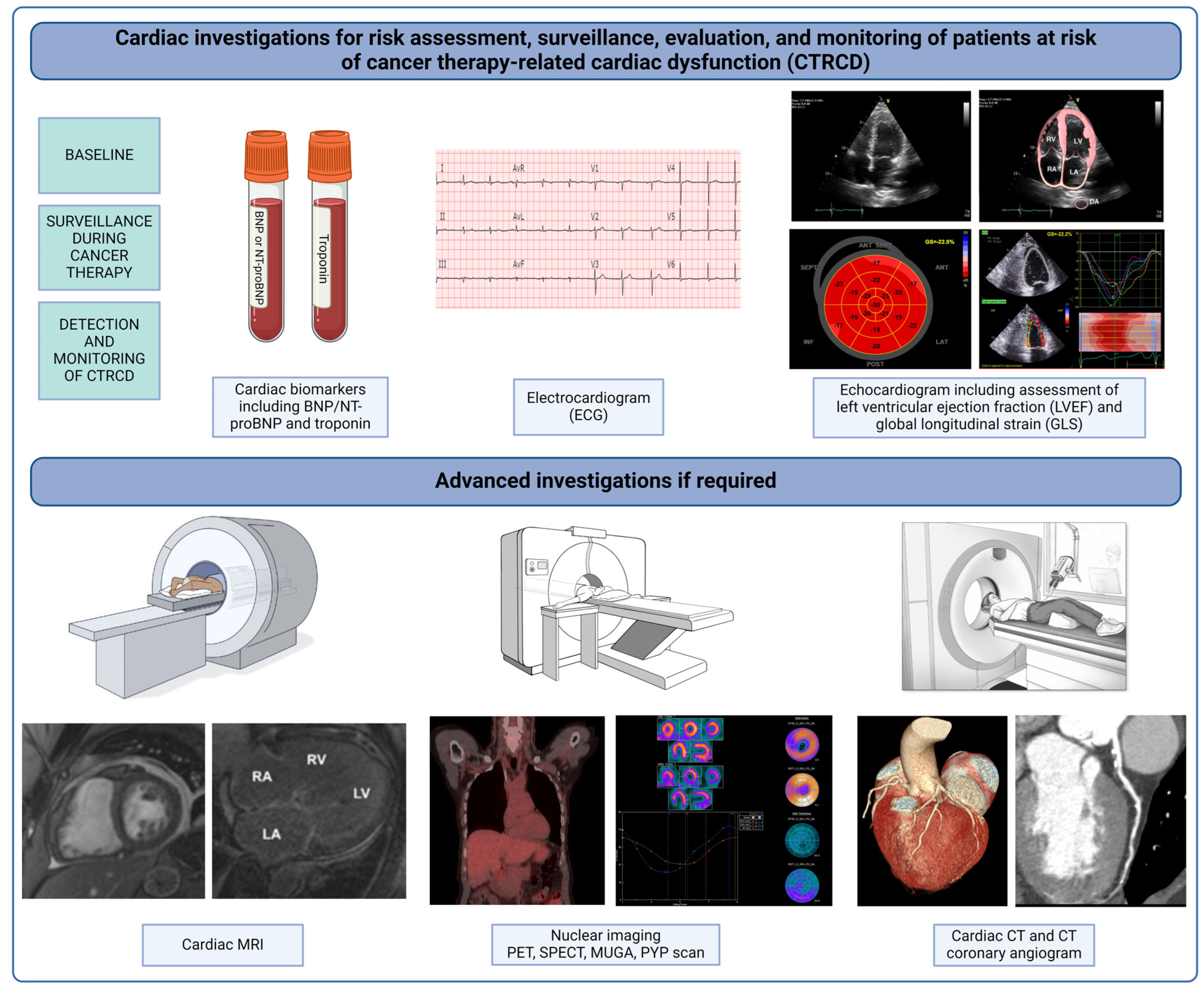
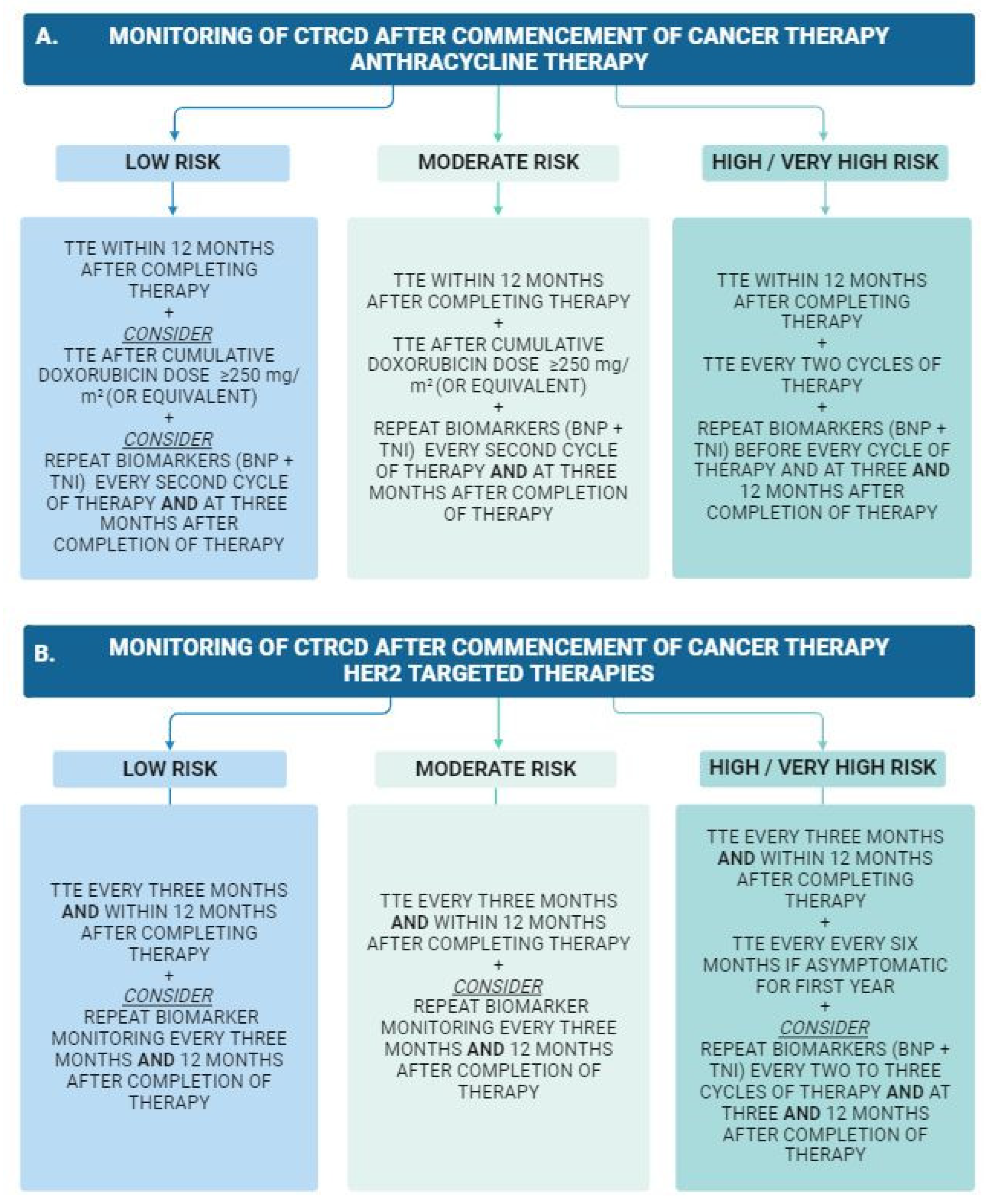
5. Cardiac Surveillance during and after Cancer Therapy
Serum Biomarkers
6. Cardiac Imaging
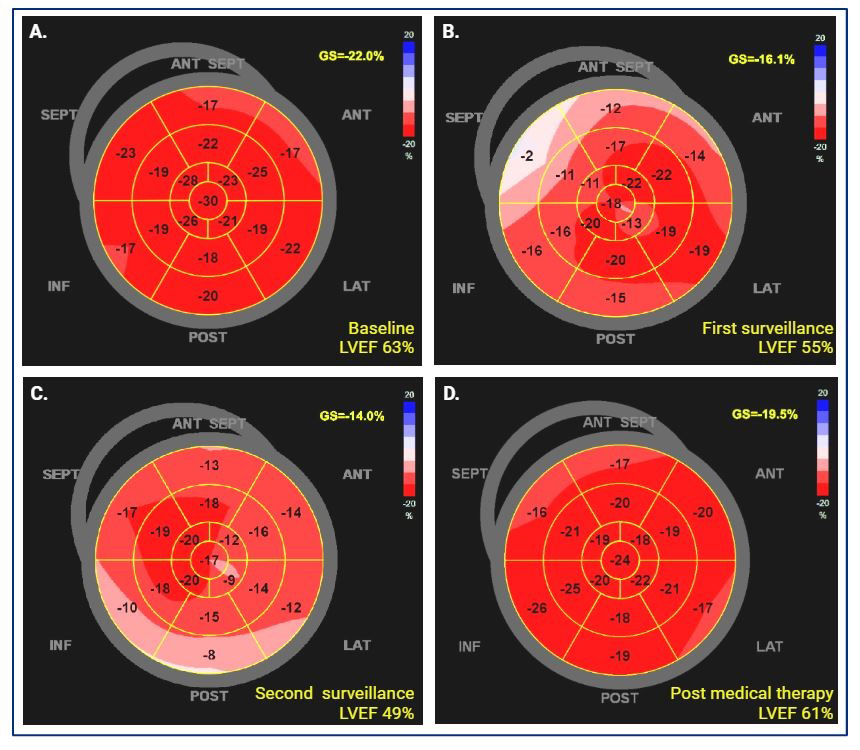
Echocardiography
7. Cardiac Magnet Resonance Imaging
8. Nuclear Imaging
9. Cardiac Computed Tomography (CT)
Artificial Intelligence (AI)
10. Prevention and Management of Left Ventricular Cardiotoxicity
10.1. Prevention
10.2. Management
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Parry, C.; Kent, E.E.; Mariotto, A.B.; Alfano, C.M.; Rowland, J.H. Cancer Survivors: A Booming Population. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Ghosh, A.K.; Ky, B.; Marwick, T.; Stout, M.; Harkness, A.; Steeds, R.; Robinson, S.; Oxborough, D.; Adlam, D.; et al. BSE and BCOS Guideline for Transthoracic Echocardiographic Assessment of Adult Cancer Patients Receiving Anthracyclines and/or Trastuzumab. JACC CardioOncol. 2021, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.P. Cancer survival: Global surveillance will stimulate health policy and improve equity. Lancet 2014, 383, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Celutkiene, J.; Pudil, R.; Lopez-Fernandez, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; von Haehling, S.; et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: A position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 1504–1524. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, L.A.; Ganatra, S.; Lopez-Mattei, J.; Yang, E.H.; Zaha, V.G.; Wong, T.C.; Ayoub, C.; DeCara, J.M.; Dent, S.; Deswal, A.; et al. Advances in Multimodality Imaging in Cardio-Oncology: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 1560–1578. [Google Scholar] [CrossRef]
- Martin Garcia, A.; Mitroi, C.; Mazon Ramos, P.; Garcia Sanz, R.; Virizuela, J.A.; Arenas, M.; Egocheaga Cabello, I.; Albert, D.; Anguita Sanchez, M.; Arrarte Esteban, V.I.; et al. Stratification and management of cardiovascular risk in cancer patients. A consensus document of the SEC, FEC, SEOM, SEOR, SEHH, SEMG, AEEMT, AEEC, and AECC. Rev. Esp. Cardiol. 2021, 74, 438–448. [Google Scholar] [CrossRef]
- Herrmann, J.; Lerman, A.; Sandhu, N.P.; Villarraga, H.R.; Mulvagh, S.L.; Kohli, M. Evaluation and management of patients with heart disease and cancer: Cardio-oncology. Mayo Clin. Proc. 2014, 89, 1287–1306. [Google Scholar] [CrossRef]
- Hershman, D.L.; Till, C.; Shen, S.; Wright, J.D.; Ramsey, S.D.; Barlow, W.E.; Unger, J.M. Association of Cardiovascular Risk Factors With Cardiac Events and Survival Outcomes Among Patients With Breast Cancer Enrolled in SWOG Clinical Trials. J. Clin. Oncol. 2018, 36, 2710–2717. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.; Baudet, M.; Cohen-Solal, A. Subclinical Left Ventricular Dysfunction During Chemotherapy. Card. Fail. Rev. 2019, 5, 31–36. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-Induced Cardiomyopathy: Clinical Relevance and Response to Pharmacologic Therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Lippman, S.M. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J. Clin. Oncol. 2005, 23, 2900–2902. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Rossello, X.; Dorresteijn, J.A.; Janssen, A.; Lambrinou, E.; Scherrenberg, M.; Bonnefoy-Cudraz, E.; Cobain, M.; Piepoli, M.F.; Visseren, F.L.; Dendale, P.; et al. Risk prediction tools in cardiovascular disease prevention: A report from the ESC Prevention of CVD Programme led by the European Association of Preventive Cardiology (EAPC) in collaboration with the Acute Cardiovascular Care Association (ACCA) and the Association of Cardiovascular Nursing and Allied Professions (ACNAP). Eur. J. Prev. Cardiol. 2019, 26, 1534–1544. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Kim, Y.; Restrepo-Cordoba, M.A.; Lunde, I.G.; Wakimoto, H.; Smith, A.M.; Toepfer, C.N.; Getz, K.; Gorham, J.; Patel, P.; et al. Genetic Variants Associated With Cancer Therapy-Induced Cardiomyopathy. Circulation 2019, 140, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Pennesi, G.; Donatelli, F.; Cammarota, R.; De Flora, S.; Noonan, D.M. Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. J. Natl. Cancer Inst. 2010, 102, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Caro-Codon, J.; Lopez-Fernandez, T.; Alvarez-Ortega, C.; Zamora Aunon, P.; Rodriguez, I.R.; Gomez Prieto, P.; Buno Soto, A.; Canales Albendea, M.; Albaladejo, A.; Mediavilla, G.; et al. Cardiovascular risk factors during cancer treatment. Prevalence and prognostic relevance: Insights from the CARDIOTOX registry. Eur. J. Prev. Cardiol. 2022, 29, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Suntheralingam, S.; Fan, C.S.; Calvillo-Arguelles, O.; Abdel-Qadir, H.; Amir, E.; Thavendiranathan, P. Evaluation of Risk Prediction Models to Identify Cancer Therapeutics Related Cardiac Dysfunction in Women with HER2+ Breast Cancer. J. Clin. Med. 2022, 11, 847. [Google Scholar] [CrossRef] [PubMed]
- Ezaz, G.; Long, J.B.; Gross, C.P.; Chen, J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J. Am. Heart Assoc. 2014, 3, e000472. [Google Scholar] [CrossRef] [PubMed]
- Addison, D.; Neilan, T.G.; Barac, A.; Scherrer-Crosbie, M.; Okwuosa, T.M.; Plana, J.C.; Reding, K.W.; Taqueti, V.R.; Yang, E.H.; Zaha, V.G.; et al. Cardiovascular Imaging in Contemporary Cardio-Oncology: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Pudil, R.; Mueller, C.; Celutkiene, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.F.; Ky, B.; Weiss, B.M.; Dahm, C.N.; Gupta, D.K.; Du, L.; Carver, J.R.; Cohen, A.D.; Engelhardt, B.G.; Garfall, A.L.; et al. Prospective Study of Cardiac Events During Proteasome Inhibitor Therapy for Relapsed Multiple Myeloma. J. Clin. Oncol. 2019, 37, 1946–1955. [Google Scholar] [CrossRef]
- Pavo, N.; Raderer, M.; Hulsmann, M.; Neuhold, S.; Adlbrecht, C.; Strunk, G.; Goliasch, G.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015, 101, 1874–1880. [Google Scholar] [CrossRef]
- Lopez-Sendon, J.; Alvarez-Ortega, C.; Zamora Aunon, P.; Buno Soto, A.; Lyon, A.R.; Farmakis, D.; Cardinale, D.; Canales Albendea, M.; Feliu Batlle, J.; Rodriguez Rodriguez, I.; et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: The CARDIOTOX registry. Eur. Heart J. 2020, 41, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Colombo, N.; Boeri, M.; Lamantia, G.; Civelli, M.; Peccatori, F.; Martinelli, G.; Fiorentini, C.; et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004, 109, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Olenchock, B.A.; Salem, J.E.; Wiviott, S.D.; Ederhy, S.; Cohen, A.; Stewart, G.C.; Choueiri, T.K.; Di Carli, M.; Allenbach, Y.; et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation 2019, 140, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Cardinale, D.; Dent, S.; Criscitiello, C.; Aseyev, O.; Lenihan, D.; Cipolla, C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Monsuez, J.J.; Charniot, J.C.; Vignat, N.; Artigou, J.Y. Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 2010, 144, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.T.; Bickford, C.L. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Chari, A.; Stewart, A.K.; Russell, S.D.; Moreau, P.; Herrmann, J.; Banchs, J.; Hajek, R.; Groarke, J.; Lyon, A.R.; Batty, G.N.; et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018, 2, 1633–1644. [Google Scholar] [CrossRef]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Alejandre, R.; Ruiz-Fernandez, I.; Martin, P. Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis. Cancers 2022, 14, 4494. [Google Scholar] [CrossRef] [PubMed]
- Alvi, R.M.; Frigault, M.J.; Fradley, M.G.; Jain, M.D.; Mahmood, S.S.; Awadalla, M.; Lee, D.H.; Zlotoff, D.A.; Zhang, L.; Drobni, Z.D.; et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J. Am. Coll. Cardiol. 2019, 74, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.A.; Sridharan, A.; Pimentel, R.C.; Markowitz, S.M.; Rosenfeld, L.E.; Fradley, M.G.; Yang, E.H. Ventricular Arrhythmia in Cancer Patients: Mechanisms, Treatment Strategies and Future Avenues. Arrhythm. Electrophysiol. Rev. 2023, 12, e16. [Google Scholar] [CrossRef] [PubMed]
- Asnani, A.; Manning, A.; Mansour, M.; Ruskin, J.; Hochberg, E.P.; Ptaszek, L.M. Management of atrial fibrillation in patients taking targeted cancer therapies. Cardiooncology 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Huang, C.C.; Tsai, Y.F.; Lin, Y.S.; Feng, C.J.; Chen, Y.J.; Lai, J.I.; Chao, T.C.; Liu, C.Y.; Tseng, L.M. The association of trastuzumab with atrial fibrillation and heart failure in breast cancer patients in routine clinical practice: A population-based propensity score matching and competing risk model analysis. Breast Cancer Res. Treat. 2023, 198, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Levicki, R.; Lovric Bencic, M.; Ivanac Vranesic, I.; Bradic, L.; Begovac, M.; Jug, J.; Dedic Plavetic, N. Effects of trastuzumab and trastuzumab emtansine on corrected QT interval and left ventricular ejection fraction in patients with metastatic (HER2+) breast cancer. Egypt. Heart J. 2023, 75, 11. [Google Scholar] [CrossRef] [PubMed]
- Buza, V.; Rajagopalan, B.; Curtis, A.B. Cancer Treatment-Induced Arrhythmias: Focus on Chemotherapy and Targeted Therapies. Circ. Arrhythm. Electrophysiol. 2017, 10, e005443. [Google Scholar] [CrossRef]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef]
- Toma, M.; Rrapaj, E.; Spallarossa, P.; Guerra, F.; Ameri, P. Patterns of anticoagulation for atrial fibrillation in cancer patients referred to cardio-oncological evaluation. Eur. J. Intern. Med. 2020, 82, 128–129. [Google Scholar] [CrossRef]
- Banke, A.; Fosbol, E.L.; Moller, J.E.; Gislason, G.H.; Andersen, M.; Bernsdorf, M.; Jensen, M.B.; Schou, M.; Ejlertsen, B. Long-term effect of epirubicin on incidence of heart failure in women with breast cancer: Insight from a randomized clinical trial. Eur. J. Heart Fail. 2018, 20, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Sandri, M.T.; Martinoni, A.; Borghini, E.; Civelli, M.; Lamantia, G.; Cinieri, S.; Martinelli, G.; Fiorentini, C.; Cipolla, C.M. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann. Oncol. 2002, 13, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L., Jr.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Banchs, J.; Carver, J.R.; et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J. Am. Coll. Cardiol. 2014, 63, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Mincu, R.I.; Mahabadi, A.A.; Settelmeier, S.; Al-Rashid, F.; Rassaf, T.; Totzeck, M. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: A meta-analysis. Eur. J. Heart Fail. 2020, 22, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.H.; Heckmann, M.B.; Bailly, G.; Finke, D.; Procureur, A.; Power, J.R.; Stein, F.; Bretagne, M.; Ederhy, S.; Fenioux, C.; et al. Cardiomuscular Biomarkers in the Diagnosis and Prognostication of Immune Checkpoint Inhibitor Myocarditis. Circulation 2023, 148, 473–486. [Google Scholar] [CrossRef]
- Sandri, M.T.; Salvatici, M.; Cardinale, D.; Zorzino, L.; Passerini, R.; Lentati, P.; Leon, M.; Civelli, M.; Martinelli, G.; Cipolla, C.M. N-terminal pro-B-type natriuretic peptide after high-dose chemotherapy: A marker predictive of cardiac dysfunction? Clin. Chem. 2005, 51, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, D.; Hasbak, P.; Kjaer, A. BNP predicts chemotherapy-related cardiotoxicity and death: Comparison with gated equilibrium radionuclide ventriculography. PLoS ONE 2014, 9, e96736. [Google Scholar] [CrossRef]
- Villarraga, H.R.; Herrmann, J.; Nkomo, V.T. Cardio-oncology: Role of echocardiography. Prog. Cardiovasc. Dis. 2014, 57, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Barletta, G.; von Bardeleben, S.; Vanoverschelde, J.L.; Kasprzak, J.; Greis, C.; Becher, H. Analysis of left ventricular volumes and function: A multicenter comparison of cardiac magnetic resonance imaging, cine ventriculography, and unenhanced and contrast-enhanced two-dimensional and three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 292–301. [Google Scholar] [CrossRef]
- Slawinski, G.; Hawryszko, M.; Lizewska-Springer, A.; Nabialek-Trojanowska, I.; Lewicka, E. Global Longitudinal Strain in Cardio-Oncology: A Review. Cancers 2023, 15, 986. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and Incremental Value of Deformation Indices for Prediction of Trastuzumab-Induced Cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.W.; Richards, D.A.B.; Boyd, A.; Hui, R.; Harnett, P.R.; Meikle, S.R.; Byth, K.; Stuart, K.; Clarke, J.L.; Thomas, L. Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: A comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. Eur. J. Cancer 2013, 49, 3396–3403. [Google Scholar] [CrossRef]
- Cocco, L.D.; Chiaparini, A.F.; Saffi, M.A.L.; Leiria, T.L.L. Global Longitudinal Strain for the Early Detection of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. Clin. Oncol. 2022, 34, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Gutierrez, R.; Chitturi, K.R.; Xu, J.; Wang, Y.; Kinder, E.; Senapati, A.; Chebrolu, L.B.; Kassi, M.; Trachtenberg, B.H. Baseline global longitudinal strain predictive of anthracycline-induced cardiotoxicity. Cardiooncology 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Marzlin, N.; Hays, A.G.; Peters, M.; Kaminski, A.; Roemer, S.; O’Leary, P.; Kroboth, S.; Harland, D.R.; Khandheria, B.K.; Tajik, A.J.; et al. Myocardial Work in Echocardiography. Circ. Cardiovasc. Imaging 2023, 16, e014419. [Google Scholar] [CrossRef]
- van der Bijl, P.; Kostyukevich, M.; El Mahdiui, M.; Hansen, G.; Samset, E.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. A Roadmap to Assess Myocardial Work: From Theory to Clinical Practice. JACC Cardiovasc. Imaging 2019, 12, 2549–2554. [Google Scholar] [CrossRef]
- Calvillo-Arguelles, O.; Thampinathan, B.; Somerset, E.; Shalmon, T.; Amir, E.; Steve Fan, C.P.; Moon, S.; Abdel-Qadir, H.; Thevakumaran, Y.; Day, J.; et al. Diagnostic and Prognostic Value of Myocardial Work Indices for Identification of Cancer Therapy-Related Cardiotoxicity. JACC Cardiovasc. Imaging 2022, 15, 1361–1376. [Google Scholar] [CrossRef]
- Di Lisi, D.; Manno, G.; Novo, G. Subclinical Cardiotoxicity: The Emerging Role of Myocardial Work and Other Imaging Techniques. Curr. Probl. Cardiol. 2021, 46, 100818. [Google Scholar] [CrossRef]
- Argulian, E.; Narula, J. Myocardial Work in Cardio-Oncology: How Well Does it Work? JACC Cardiovasc. Imaging 2022, 15, 1377–1379. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.W.; Hamo, C.E.; Cardinale, D.; Ky, B.; Nohria, A.; Baer, L.; Skopicki, H.; Lenihan, D.J.; Gheorghiade, M.; Lyon, A.R.; et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ. Heart Fail. 2016, 9, e002661. [Google Scholar] [CrossRef] [PubMed]
- Oreto, L.; Todaro, M.C.; Umland, M.M.; Kramer, C.; Qamar, R.; Carerj, S.; Khandheria, B.K.; Paterick, T.E. Use of Echocardiography to Evaluate the Cardiac Effects of Therapies Used in Cancer Treatment: What Do We Know? J. Am. Soc. Echocardiogr. 2012, 25, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.C.; Scherrer-Crosbie, M. Cardiac complications of chemotherapy: Role of imaging. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Shu, F.; Zhang, C.; Song, F.; Xu, Y.; Guo, Y.; Xue, K.; Lin, J.; Shu, X.; Hsi, D.H.; et al. Early Detection and Prediction of Anthracycline-Induced Right Ventricular Cardiotoxicity by 3-Dimensional Echocardiography. JACC CardioOncol. 2020, 2, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Mondillo, S. Echocardiography In the Prediction of Atrial Fibrillation Recurrence: A Review. J. Atr. Fibrillation 2012, 5, 675. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Youn, H.J. Role of echocardiography in atrial fibrillation. J. Cardiovasc. Ultrasound 2011, 19, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. Europace 2018, 20, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Galli, E.; Schnell, F. Left Atrial Strain: A Must or a Plus for Routine Clinical Practice? Circ. Cardiovasc. Imaging 2017, 10, e007023. [Google Scholar] [CrossRef]
- Motoc, A.; Luchian, M.L.; Scheirlynck, E.; Roosens, B.; Chameleva, H.; Gevers, M.; Galloo, X.; von Kemp, B.; Ramak, R.; Sieira, J.; et al. Incremental value of left atrial strain to predict atrial fibrillation recurrence after cryoballoon ablation. PLoS ONE 2021, 16, e0259999. [Google Scholar] [CrossRef]
- Ma, X.X.; Boldt, L.H.; Zhang, Y.L.; Zhu, M.R.; Hu, B.; Parwani, A.; Belyavskiy, E.; Radha Krishnan, A.K.; Krisper, M.; Kohncke, C.; et al. Clinical Relevance of Left Atrial Strain to Predict Recurrence of Atrial Fibrillation after Catheter Ablation: A Meta-Analysis. Echocardiography 2016, 33, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Di Lisi, D.; Moreo, A.; Casavecchia, G.; Cadeddu Dessalvi, C.; Bergamini, C.; Zito, C.; Madaudo, C.; Madonna, R.; Cameli, M.; Novo, G. Atrial Strain Assessment for the Early Detection of Cancer Therapy-Related Cardiac Dysfunction in Breast Cancer Women (The STRANO STUDY: Atrial Strain in Cardio-Oncology). J. Clin. Med. 2023, 12, 7127. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Souza, T.; Quinaglia, T.; Neilan, T.G.; Coelho-Filho, O.R. Assessment of Cardiotoxicity of Cancer Chemotherapy: The Value of Cardiac MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2019, 27, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Lin, L.; Zhang, G.; Zhou, X. Cardiovascular Magnetic Resonance Imaging in the Early Detection of Cardiotoxicity Induced by Cancer Therapies. Diagnostics 2022, 12, 1846. [Google Scholar] [CrossRef] [PubMed]
- O’Quinn, R.; Ferrari, V.A.; Daly, R.; Hundley, G.; Baldassarre, L.A.; Han, Y.; Barac, A.; Arnold, A. Cardiac Magnetic Resonance in Cardio-Oncology: Advantages, Importance of Expediency, and Considerations to Navigate Pre-Authorization. JACC CardioOncol. 2021, 3, 191–200. [Google Scholar] [CrossRef]
- Bottinor, W.; Trankle, C.R.; Hundley, W.G. The Role of Cardiovascular MRI in Cardio-Oncology. Heart Fail. Clin. 2021, 17, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Farhad, H.; Staziaki, P.V.; Addison, D.; Coelho-Filho, O.R.; Shah, R.V.; Mitchell, R.N.; Szilveszter, B.; Abbasi, S.A.; Kwong, R.Y.; Scherrer-Crosbie, M.; et al. Characterization of the Changes in Cardiac Structure and Function in Mice Treated With Anthracyclines Using Serial Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2016, 9, e003584. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, M.T.; Inserra, M.C.; Passaniti, G.; Celona, A.; D’Angelo, T.; Romeo, P.; Basile, A. Role of advanced cardiovascular imaging in chemotherapy-induced cardiotoxicity. Heliyon 2023, 9, e15226. [Google Scholar] [CrossRef] [PubMed]
- Muehlberg, F.; Funk, S.; Zange, L.; von Knobelsdorff-Brenkenhoff, F.; Blaszczyk, E.; Schulz, A.; Ghani, S.; Reichardt, A.; Reichardt, P.; Schulz-Menger, J. Native myocardial T1 time can predict development of subsequent anthracycline-induced cardiomyopathy. ESC Heart Fail. 2018, 5, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Park, H.S.; Park, J.K.; Han, K.; Park, C.H.; Kim, T.K.; Yoo, S.J.; Lee, J.Y.; Kim, P.K.; Hur, J.; et al. Early Detection and Serial Monitoring of Anthracycline-Induced Cardiotoxicity Using T1-mapping Cardiac Magnetic Resonance Imaging: An Animal Study. Sci. Rep. 2017, 7, 2663. [Google Scholar] [CrossRef]
- Galan-Arriola, C.; Lobo, M.; Vilchez-Tschischke, J.P.; Lopez, G.J.; de Molina-Iracheta, A.; Perez-Martinez, C.; Aguero, J.; Fernandez-Jimenez, R.; Martin-Garcia, A.; Oliver, E.; et al. Serial Magnetic Resonance Imaging to Identify Early Stages of Anthracycline-Induced Cardiotoxicity. J. Am. Coll. Cardiol. 2019, 73, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Grant, A.D.; Negishi, T.; Plana, J.C.; Popovic, Z.B.; Marwick, T.H. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J. Am. Coll. Cardiol. 2013, 61, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Loffler, A.I.; Salerno, M. Cardiac MRI for the evaluation of oncologic cardiotoxicity. J. Nucl. Cardiol. 2018, 25, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Fallah-Rad, N.; Lytwyn, M.; Fang, T.; Kirkpatrick, I.; Jassal, D.S. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J. Cardiovasc. Magn. Reson. 2008, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.H.; D’Agostino, R.B.; Hamilton, C.A.; Vasu, S.; Hall, M.E.; Kitzman, D.W.; Thohan, V.; Lawrence, J.A.; Ellis, L.R.; Lash, T.L.; et al. Longitudinal Assessment of Concurrent Changes in Left Ventricular Ejection Fraction and Left Ventricular Myocardial Tissue Characteristics After Administration of Cardiotoxic Chemotherapies Using T1-Weighted and T2-Weighted Cardiovascular Magnetic Resonan. Circulation. Cardiovasc. Imaging 2014, 7, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.M.; Ayoub, C.; Chetrit, M.; Kwon, D.H.; Jellis, C.L.; Cremer, P.C.; Bolen, M.A.; Flamm, S.D.; Klein, A.L. Cardiac Magnetic Resonance Imaging Techniques and Applications for Pericardial Diseases. Circ. Cardiovasc. Imaging 2022, 15, e014283. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; von Knobelsdorff-Brenkenhoff, F.; Bratland, Å.; Storås, T.H.; et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016, 37, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Coelho-Filho, O.R.; Pena-Herrera, D.; Shah, R.V.; Jerosch-Herold, M.; Francis, S.A.; Moslehi, J.; Kwong, R.Y. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am. J. Cardiol. 2012, 110, 1679–1686. [Google Scholar] [CrossRef]
- Wassmuth, R.; Lentzsch, S.; Erdbruegger, U.; Schulz-Menger, J.; Doerken, B.; Dietz, R.; Friedrich, M.G. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging—A pilot study. Am. Heart J. 2001, 141, 1007–1013. [Google Scholar] [CrossRef]
- Taylor, A.J.; Cerqueira, M.; Hodgson, J.M.; Mark, D.; Min, J.; O’Gara, P.; Rubin, G.D.; Kramer, C.M.; Taylor, A.J.; Berman, D.; et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2010, 4, 407.e1–407.e33. [Google Scholar] [CrossRef]
- Huang, H.; Nijjar, P.S.; Misialek, J.R.; Blaes, A.; Derrico, N.P.; Kazmirczak, F.; Klem, I.; Farzaneh-Far, A.; Shenoy, C. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: Comparison with cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2017, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Printezi, M.I.; Yousif, L.I.E.; Kamphuis, J.A.M.; van Laake, L.W.; Cramer, M.J.; Hobbelink, M.G.G.; Asselbergs, F.W.; Teske, A.J. LVEF by Multigated Acquisition Scan Compared to Other Imaging Modalities in Cardio-Oncology: A Systematic Review. Curr. Heart Fail. Rep. 2022, 19, 136–145. [Google Scholar] [CrossRef] [PubMed]
- de Geus-Oei, L.-F.; Mavinkurve-Groothuis, A.M.C.; Bellersen, L.; Gotthardt, M.; Oyen, W.J.G.; Kapusta, L.; van Laarhoven, H.W.M. Scintigraphic Techniques for Early Detection of Cancer Treatment-Induced Cardiotoxicity. J. Nucl. Med. Technol. 2013, 41, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.A.; Abbas, M.T.; Kanaan, C.N.; Awad, K.A.; Baba Ali, N.; Scalia, I.G.; Farina, J.M.; Pereyra, M.; Mahmoud, A.K.; Steidley, D.E.; et al. How Artificial Intelligence Can Enhance the Diagnosis of Cardiac Amyloidosis: A Review of Recent Advances and Challenges. J. Cardiovasc. Dev. Dis. 2024, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Juni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Mattei, J.; Yang, E.H.; Baldassarre, L.A.; Agha, A.; Blankstein, R.; Choi, A.D.; Chen, M.Y.; Meyersohn, N.; Daly, R.; Slim, A.; et al. Cardiac computed tomographic imaging in cardio-oncology: An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). Endorsed by the International Cardio-Oncology Society (ICOS). J. Cardiovasc. Comput. Tomogr. 2023, 17, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.D.; Fergestrom, N.; Gage, B.F.; Paisley, R.; Moon, P.; Novak, E.; Cheezum, M.; Shaw, L.J.; Villines, T.C. Impact of Statins on Cardiovascular Outcomes Following Coronary Artery Calcium Scoring. J. Am. Coll. Cardiol. 2018, 72, 3233–3242. [Google Scholar] [CrossRef]
- Martinez, D.S.; Noseworthy, P.A.; Akbilgic, O.; Herrmann, J.; Ruddy, K.J.; Hamid, A.; Maddula, R.; Singh, A.; Davis, R.; Gunturkun, F.; et al. Artificial intelligence opportunities in cardio-oncology: Overview with spotlight on electrocardiography. Am. Heart J. Plus 2022, 15, 100129. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.M.; Sehrawat, O.; Maanja, M.; Wight, J.; Noseworthy, P.A. Artificial Intelligence for the Detection and Treatment of Atrial Fibrillation. Arrhythm. Electrophysiol. Rev. 2023, 12, e12. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, M.; Giallauria, F.; Carrizzo, A.; Visco, V.; Silverio, A.; Cesaro, A.; Calabro, P.; De Luca, N.; Mancusi, C.; Masarone, D.; et al. Artificial intelligence in cardiovascular prevention: New ways will open new doors. J. Cardiovasc. Med. 2023, 24, e106–e115. [Google Scholar] [CrossRef]
- Madan, N.; Lucas, J.; Akhter, N.; Collier, P.; Cheng, F.; Guha, A.; Zhang, L.; Sharma, A.; Hamid, A.; Ndiokho, I.; et al. Artificial intelligence and imaging: Opportunities in cardio-oncology. Am. Heart J. Plus 2022, 15, 100126. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Liu, C.F.; Feng, Y.H.; Liao, C.T.; Wang, J.J.; Chen, Z.C.; Lee, H.C.; Shih, J.Y. An artificial intelligence approach for predicting cardiotoxicity in breast cancer patients receiving anthracycline. Arch. Toxicol. 2022, 96, 2731–2737. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, Y.; Hussain, M.; Brown, S.A.; Budd, T.; Tang, W.H.W.; Abraham, J.; Xu, B.; Shah, C.; Moudgil, R.; et al. Machine Learning-Based Risk Assessment for Cancer Therapy-Related Cardiac Dysfunction in 4300 Longitudinal Oncology Patients. J. Am. Heart Assoc. 2020, 9, e019628. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, L.; Chou, C.; Ngorsuraches, S.; Qian, J. Using Machine Learning Approaches to Predict Short-Term Risk of Cardiotoxicity Among Patients with Colorectal Cancer After Starting Fluoropyrimidine-Based Chemotherapy. Cardiovasc. Toxicol. 2022, 22, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Yagi, R.; Goto, S.; Himeno, Y.; Katsumata, Y.; Hashimoto, M.; MacRae, C.A.; Deo, R.C. Artificial intelligence-enabled prediction of chemotherapy-induced cardiotoxicity from baseline electrocardiograms. Nat. Commun. 2024, 15, 2536. [Google Scholar] [CrossRef]
- Acar, Z.; Kale, A.; Turgut, M.; Demircan, S.; Durna, K.; Demir, S.; Meriç, M.; Ağaç, M.T. Efficiency of Atorvastatin in the Protection of Anthracycline-Induced Cardiomyopathy. J. Am. Coll. Cardiol. 2011, 58, 988–989. [Google Scholar] [CrossRef]
- Gilchrist, S.C.; Barac, A.; Ades, P.A.; Alfano, C.M.; Franklin, B.A.; Jones, L.W.; La Gerche, A.; Ligibel, J.A.; Lopez, G.; Madan, K.; et al. Cardio-Oncology Rehabilitation to Manage Cardiovascular Outcomes in Cancer Patients and Survivors: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e997–e1012. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Anselmi, F.; Fiorentini, C.; Mannucci, R.; Bonifazi, M.; Mondillo, S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur. J. Prev. Cardiol. 2021, 28, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Bennett, H.; Bezak, E.; Perry, R. The role of exercise in the prevention of cancer therapy-related cardiac dysfunction in breast cancer patients undergoing chemotherapy: Systematic review. Eur. J. Prev. Cardiol. 2022, 29, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Zabor, E.C.; Schwitzer, E.; Koelwyn, G.J.; Adams, S.C.; Nilsen, T.S.; Moskowitz, C.S.; Matsoukas, K.; Iyengar, N.M.; Dang, C.T.; et al. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients With Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2018, 36, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Caspani, F.; Tralongo, A.C.; Campiotti, L.; Asteggiano, R.; Guasti, L.; Squizzato, A. Prevention of anthracycline-induced cardiotoxicity: A systematic review and meta-analysis. Intern. Emerg. Med. 2021, 16, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Zhang, T.; Xiong, X.; Liu, N.; Pang, B.; Ruan, Y.; Gao, Y.; Shang, H.; Xing, Y. Role of cardioprotective agents on chemotherapy-induced heart failure: A systematic review and network meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 151, 104577. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Laborante, R.; Bianchini, E.; Galli, M.; Ciliberti, G.; Mennuni, M.; Patti, G. Statins as preventive therapy for anthracycline cardiotoxicity: A meta-analysis of randomized controlled trials. Int. J. Cardiol. 2023, 391, 131219. [Google Scholar] [CrossRef]
- Hundley, W.G.; D’Agostino, R., Jr.; Crotts, T.; Craver, K.; Hackney, M.H.; Jordan, J.H.; Ky, B.; Wagner, L.I.; Herrington, D.M.; Yeboah, J.; et al. Statins and Left Ventricular Ejection Fraction Following Doxorubicin Treatment. NEJM Evid. 2022, 1, EVIDoa2200097. [Google Scholar] [CrossRef]
- Neilan, T.G.; Quinaglia, T.; Onoue, T.; Mahmood, S.S.; Drobni, Z.D.; Gilman, H.K.; Smith, A.; Heemelaar, J.C.; Brahmbhatt, P.; Ho, J.S.; et al. Atorvastatin for Anthracycline-Associated Cardiac Dysfunction: The STOP-CA Randomized Clinical Trial. JAMA 2023, 330, 528–536. [Google Scholar] [CrossRef]
- Negishi, T.; Thavendiranathan, P.; Penicka, M.; Lemieux, J.; Murbraech, K.; Miyazaki, S.; Shirazi, M.; Santoro, C.; Cho, G.Y.; Popescu, B.A.; et al. Cardioprotection Using Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy: 3-Year Results of the SUCCOUR Trial. JACC Cardiovasc. Imaging 2023, 16, 269–278. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Gregorietti, V.; Fernandez, T.L.; Costa, D.; Chahla, E.O.; Daniele, A.J. Use of Sacubitril/valsartan in patients with cardio toxicity and heart failure due to chemotherapy. Cardiooncology 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jang, G.; Hwang, J.; Wei, X.; Kim, H.; Son, J.; Rhee, S.J.; Yun, K.H.; Oh, S.K.; Oh, C.M.; et al. Combined Therapy of Low-Dose Angiotensin Receptor-Neprilysin Inhibitor and Sodium-Glucose Cotransporter-2 Inhibitor Prevents Doxorubicin-Induced Cardiac Dysfunction in Rodent Model with Minimal Adverse Effects. Pharmaceutics 2022, 14, 2629. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.; Carrasco, R.; Austin, P.C.; Chen, Y.; Zhou, L.; Fang, J.; Su, H.M.H.; Lega, I.C.; Kaul, P.; Neilan, T.G.; et al. The Association of Sodium-Glucose Cotransporter 2 Inhibitors With Cardiovascular Outcomes in Anthracycline-Treated Patients With Cancer. JACC CardioOncol. 2023, 5, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Vafa, R.G.; Sabahizadeh, A.; Mofarrah, R. Guarding the heart: How SGLT-2 inhibitors protect against chemotherapy-induced cardiotoxicity: SGLT-2 inhibitors and chemotherapy-induced cardiotoxicity. Curr. Probl. Cardiol. 2024, 49, 102350. [Google Scholar] [CrossRef] [PubMed]
| Grading | ESC 2022 [15] | ACC CardioOncology and Imaging Councils 2022 [8] | ICOS 2021 [14] | ESMO 2020 [7] | ASE 2014 [12] | |
|---|---|---|---|---|---|---|
| Symptomatic | Mild | Mild heart failure symptoms | Mild heart failure symptoms | Asymptomatic | ||
| Moderate | Heart failure symptoms requiring outpatient therapy | Heart failure symptoms requiring outpatient therapy | Heart failure symptoms regardless of LVEF | |||
| Severe | Heart failure symptoms requiring hospitalization | Heart failure symptoms requiring hospitalization | ||||
| Very severe | Heart failure symptoms requiring hospitalization AND inotropic support or mechanical circulatory support | Heart failure symptoms requiring hospitalization AND inotropic support or mechanical circulatory support | ||||
| Asymptomatic | Mild | Baseline normal LVEF (≥50%) New relative decline in GLS > 15% from baseline OR Elevated cardiac biomarkers from baseline | Possible: Reduction in LVEF ≥ 10% to overall LVEF 50–55% OR Reduction in LVEF by <10% to overall LVEF <50% OR Relative reduction in by GLS ≥ 15% with or without change in LVEF | Baseline normal LVEF (≥50%) New relative decline in GLS > 15% from baseline OR Elevated cardiac biomarkers from baseline | Baseline normal LVEF (≥50%) with decline in LVEF > 15% | Reduction in LVEF > 10% to overall LVEF < 53% OR Relative reduction in GLS > 15% from baseline is suggestive of CTRCD |
| Moderate | Reduction in LVEF ≥ 10% from baseline (to LVEF 40–49%) OR Reduction in LVEF < 10% (to LVEF 40–49%) AND relative decline in GLS > 15% from baseline OR rise in cardiac biomarkers | Definite: Reduction in LVEF ≥ 10% to overall LVEF <50% | Reduction in LVEF ≥ 10% from baseline (to LVEF 40–49%) OR Reduction in LVEF < 10% (to LVEF 40–49%) AND relative decline in GLS > 15% from baseline OR rise in cardiac biomarkers | Reduction in LVEF ≥10% from baseline OR Reduction in LVEF to <50% (but ≥40%) | ||
| Severe | New decline in LVEF to <40% | New decline in LVEF to <40% | LVEF to <40% |
Patient Factors:
|
Baseline Cardiac Parameters and Biomarkers:
|
Cancer Treatment Factors:
|
| Anthracyclines | Doxorubicin Duanorubicin Epirubicin Idrarubicin Mitoxanthrone |
| Alkylating Agents | Cyclophosphamide Ifosfamide Mitomycin |
| HER2 a targeted therapies | Trastuzumab/Pertuzumab Lapatanib Neratinib |
| Antimetabolites | Clofarabine 5-fluorouracil Capecitabine |
| Small molecule TKI | Sunitinib b Pazopanib b Sorefenib b Dasatinib c Imatininb c Nilotinib c |
| MEK Inhibitors | Trametinib |
| Proteasome Inhibitors | Carfilzomib |
| Other Agents | Interferon alpha Bevacizumab d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalia, I.G.; Gheyath, B.; Tamarappoo, B.K.; Moudgil, R.; Otton, J.; Pereyra, M.; Narayanasamy, H.; Larsen, C.; Herrmann, J.; Arsanjani, R.; et al. Chemotherapy Related Cardiotoxicity Evaluation—A Contemporary Review with a Focus on Cardiac Imaging. J. Clin. Med. 2024, 13, 3714. https://doi.org/10.3390/jcm13133714
Scalia IG, Gheyath B, Tamarappoo BK, Moudgil R, Otton J, Pereyra M, Narayanasamy H, Larsen C, Herrmann J, Arsanjani R, et al. Chemotherapy Related Cardiotoxicity Evaluation—A Contemporary Review with a Focus on Cardiac Imaging. Journal of Clinical Medicine. 2024; 13(13):3714. https://doi.org/10.3390/jcm13133714
Chicago/Turabian StyleScalia, Isabel G., Bashaer Gheyath, Balaji K. Tamarappoo, Rohit Moudgil, James Otton, Milagros Pereyra, Hema Narayanasamy, Carolyn Larsen, Joerg Herrmann, Reza Arsanjani, and et al. 2024. "Chemotherapy Related Cardiotoxicity Evaluation—A Contemporary Review with a Focus on Cardiac Imaging" Journal of Clinical Medicine 13, no. 13: 3714. https://doi.org/10.3390/jcm13133714
APA StyleScalia, I. G., Gheyath, B., Tamarappoo, B. K., Moudgil, R., Otton, J., Pereyra, M., Narayanasamy, H., Larsen, C., Herrmann, J., Arsanjani, R., & Ayoub, C. (2024). Chemotherapy Related Cardiotoxicity Evaluation—A Contemporary Review with a Focus on Cardiac Imaging. Journal of Clinical Medicine, 13(13), 3714. https://doi.org/10.3390/jcm13133714









