Treating Trigeminal Schwannoma through a Transorbital Approach: A Systematic Review
Abstract
1. Introduction
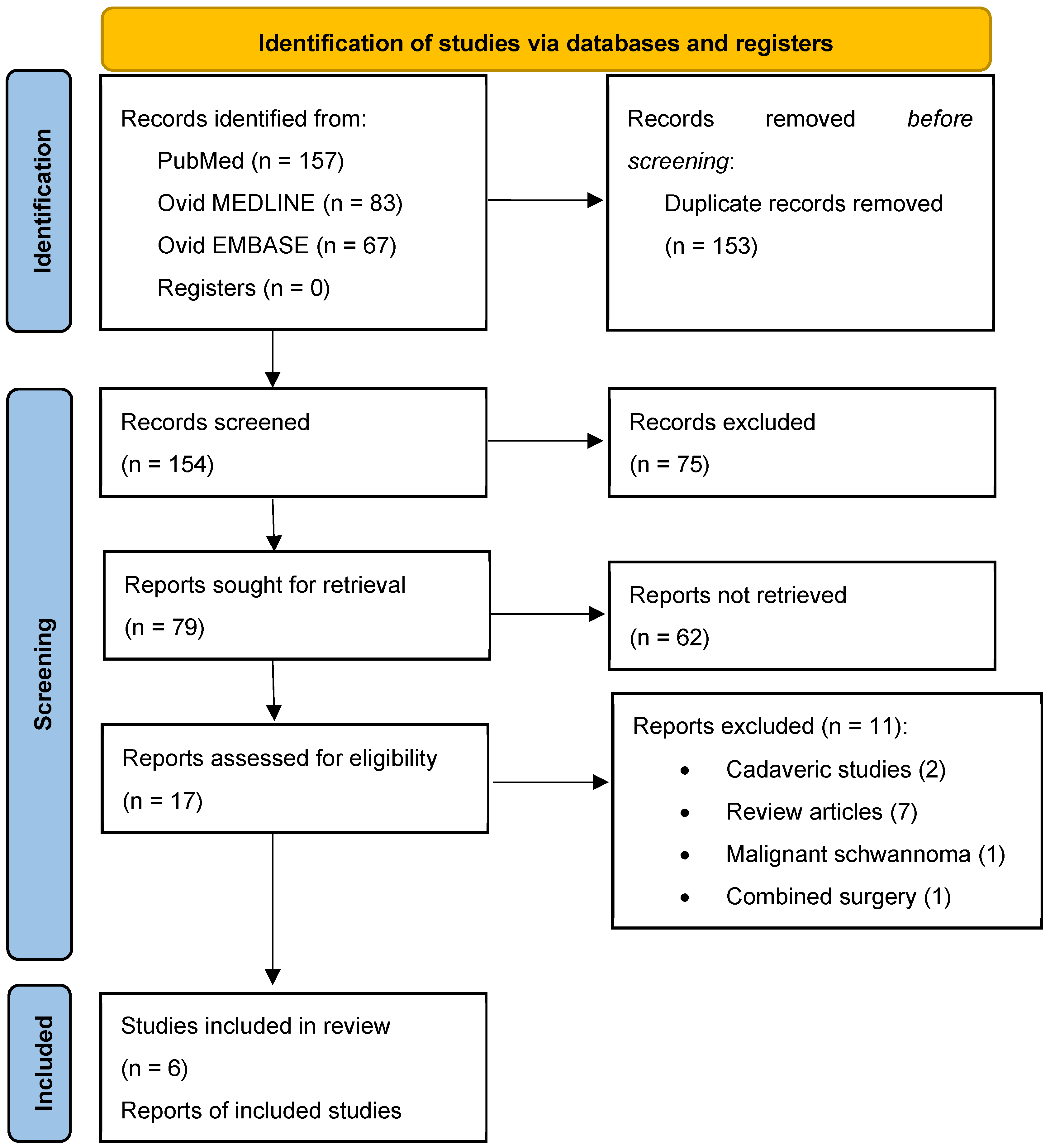
2. Materials and Methods
2.1. Literature Search
2.2. Risk of Bias Assessment
Statistical Analysis
3. Results
3.1. Literature Review
3.2. Study Population and Clinical Data
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Argenyi, Z.B.; Cooper, P.H.; Santa Cruz, D. Plexiform and other unusual variants of palisaded encapsulated neuroma. J. Cutan. Pathol. 1993, 20, 34–39. [Google Scholar] [CrossRef]
- MacCollin, M.; Woodfin, W.; Kronn, D.; Short, M.P. Schwannomatosis: A clinical and pathologic study. Neurology 1996, 46, 1072–1079. [Google Scholar] [CrossRef]
- Greene, J.; Al-Dhahir, M.A. Acoustic Neuroma. 2023 Aug 17. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Agarwal, A. Intracranial trigeminal schwannoma. Neuroradiol. J. 2015, 28, 36–41. [Google Scholar] [CrossRef]
- Aftahy, A.K.; Groll, M.; Barz, M.; Wagner, A.; Lange, N.; Butenschön, V.M.; Delbridge, C.; Bernhardt, D.; Meyer, B.; Negwer, C.; et al. Surgical Outcome of Trigeminal Schwannomas. Cancers 2021, 13, 1310. [Google Scholar] [CrossRef]
- Moualed, D.; Wong, J.; Thomas, O.; Heal, C.; Saqib, R.; Choi, C.; Lloyd, S.; Rutherford, S.; Stapleton, E.; Hammerbeck-Ward, C.; et al. Prevalence and natural history of schwannomas in neurofibromatosis type 2 (NF2): The influence of pathogenic variants. Eur. J. Hum. Genet. EJHG 2022, 30, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ramina, R.; Mattei, T.A.; Sória, M.G.; da Silva, E.B., Jr.; Leal, A.G.; Neto, M.C.; Fernandes, Y.B. Surgical management of trigeminal schwannomas. Neurosurg. Focus 2008, 25, E6; discussion E6. [Google Scholar] [CrossRef] [PubMed]
- De Simone, M.; Conti, V.; Palermo, G.; De Maria, L.; Iaconetta, G. Advancements in Glioma Care: Focus on Emerging Neurosurgical Techniques. Biomedicines 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Simone, M.; Fontanella, M.M.; Choucha, A.; Schaller, K.; Machi, P.; Lanzino, G.; Bijlenga, P.; Kurz, F.T.; Lövblad, K.-O.; De Maria, L. Current and Future Applications of Arterial Spin Labeling MRI in Cerebral Arteriovenous Malformations. Biomedicines 2024, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Joo, W.; Yoshioka, F.; Funaki, T.; Mizokami, K.; Rhoton, A.L., Jr. Microsurgical anatomy of the trigeminal nerve. Clin. Anat. 2014, 27, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Yasargil, M.G. Microsurgical pterional approach to the aneurysms of the basilar bifurcation. Surg. Neurol. 1976, 6, 83–91. [Google Scholar]
- van Furth, W.R.; Agur, A.M.R.; Woolridge, N.; Cusimano, M.D. The Orbitozygomatic Approach. Neurosurgery 2006, 58, ONS103–ONS107. [Google Scholar] [PubMed]
- Kawase, T.; Toya, S.; Shiobara, R.; Mine, T. Transpetrosal Approach for Aneurysms of the Lower Basilar Artery. J. Neurosurg. 1985, 63, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Dolenc, V. Direct Microsurgical Repair of Intracavernous Vascular Lesions. J. Neurosurg. 1983, 58, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Metwali, H.; Samii, A.; Gerganov, V. Retrosigmoid Intradural Inframeatal Approach: Indications and Technique. Neurosurgery 2013, 73, ons53–ons59; discussion ons60. [Google Scholar] [CrossRef] [PubMed]
- Samii, A.; Giordano, M.; Samii, M. Retrosigmoid Intradural Suprameatal Approach: 2-Dimensional Operative Video. Oper. Neurosurg. 2023, 26, 599. [Google Scholar] [CrossRef] [PubMed]
- Peciu-Florianu, I.; Régis, J.; Levivier, M.; Dedeciusova, M.; Reyns, N.; Tuleasca, C. Tumor control and trigeminal dysfunction improvement after stereotactic radiosurgery for trigeminal schwannomas: A systematic review and meta-analysis. Neurosurg. Rev. 2021, 44, 2391–2403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jeon, C.; Hong, C.K.; Woo, K.I.; Hong, S.D.; Nam, D.H.; Lee, J.I.; Choi, J.W.; Seol, H.J.; Kong, D.S. Endoscopic transorbital surgery for Meckel’s cave and middle cranial fossa tumors: Surgical technique and early results. J. Neurosurg. 2018, 131, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80–84. [Google Scholar] [CrossRef]
- Park, H.H.; Hong, S.D.; Kim, Y.H.; Hong, C.K.; Woo, K.I.; Yun, I.S.; Kong, D.S. Endoscopic transorbital and endonasal approach for trigeminal schwannomas: A retrospective multicenter analysis (KOSEN-005). J. Neurosurg. 2020, 133, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.; Hong, S.D.; Woo, K.I.; Seol, H.J.; Nam, D.H.; Lee, J.I.; Kong, D.S. Use of endoscopic transorbital and endonasal approaches for 360° circumferential access to orbital tumors. J. Neurosurg. 2020, 135, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, H.; Wang, Z.; Li, L.; Li, C.; Han, S.; Wu, A. Endoscopic transorbital approach for skull base lesions: A report of 16 clinical cases. Neurosurg. Rev. 2023, 46, 74. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.S.; Kim, Y.H.; Lee, W.J.; Kim, Y.H.; Hong, C.K. Indications and outcomes of endoscopic transorbital surgery for trigeminal schwannoma based on tumor classification: A multicenter study with 50 cases. J. Neurosurg. 2022, 138, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, A.; Guizzardi, G.; Sanchez España, J.C.; Matas Fassi, J.; Topczewski, T.E.; Ferres, A.; Mosteiro, A.; Reyes, L.; Tercero, J.; Lopez, M.; et al. Complications of the Superior Eyelid Endoscopic Transorbital Approach to the Skull Base: Preliminary Experience With Specific Focus on Orbital Outcome. J. Neuroophthalmol. 2024, 44, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kawase, T. Trigeminal neurinomas extending into multiple fossae: Surgical methods and review of the literature. J. Neurosurg. 1999, 91, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Meningiomas. Their Classification, Regional Behaviour, Life History, and Surgical End Results. Bull. Med. Libr. Assoc. 1938, 27, 185.
- Krayenbuhl, H. Das Neurinom des Nervus trigeminus [Neurinoma of tirgeminal nerve]. Bull. Schweiz. Akad. Med. Wiss. 1959, 15, 89–100. [Google Scholar] [PubMed]
- Samii, M.; Migliori, M.M.; Tatagiba, M.; Babu, R. Surgical treatment of trigeminal schwannomas. J. Neurosurg. 1995, 82, 711–718. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Chen, G.; Liang, J.; Guo, H.; Song, G.; Bao, Y. Trigeminal schwannoma: A single-center experience with 43 cases and review of literature. Br. J. Neurosurg. 2021, 35, 49–56. [Google Scholar] [CrossRef]
- Sindou, M.; Pelissou, I. Trigeminal neurinomas. A special type of cavernous sinus tumors. In The Cavernous Sinus; Dolenc, V.V., Ed.; Springer: Vienna, Austria, 1987; pp. 355–376. [Google Scholar] [CrossRef]
- Yin, J.; Wu, Y.; Zhang, Z.; Zhang, Y.; He, J.; Yang, Z.; Wang, B.; Wang, X.; Liu, G.; Bie, Z.; et al. Operative management of trigeminal schwannomas: Based on a modified classification in a study of 93 cases. Acta Neurochir. 2023, 165, 4157–4168. [Google Scholar] [CrossRef]
- Zanin, L.; Agosti, E.; Ebner, F.; de Maria, L.; Belotti, F.; Buffoli, B.; Rezzani, R.; Hirt, B.; Ravanelli, M.; Ius, T.; et al. Quantitative Anatomical Comparison of Surgical Approaches to Meckel’s Cave. J. Clin. Med. 2023, 12, 6847. [Google Scholar] [CrossRef]
- Kassam, A.B.; Prevedello, D.M.; Carrau, R.L.; Snyderman, C.H.; Gardner, P.; Osawa, S.; Seker, A.; Rhoton, A.L., Jr. The front door to meckel’s cave: An anteromedial corridor via expanded endoscopic endonasal approach—Technical considerations and clinical series. Oper. Neurosurg. 2009, 64, ons71–ons83. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Gardner, P.A.; Stefko, S.T.; Madhok, R.; Fernandez-Miranda, J.C.; Snyderman, C.H. Endoscopic Endonasal Approach for Nonvestibular Schwannomas. Neurosurgery 2011, 69, 1046–1057. [Google Scholar] [CrossRef]
- Raza, S.M.; Donaldson, A.M.; Mehta, A.; Tsiouris, A.J.; Anand, V.K.; Schwartz, T.H. Surgical management of trigeminal schwannomas: Defining the role for endoscopic endonasal approaches. FOC 2014, 37, E17. [Google Scholar] [CrossRef]
- Van Rompaey, J.; Bush, C.; Khabbaz, E.; Vender, J.; Panizza, B.; Solares, C.A. What is the Best Route to the Meckel Cave? Anatomical Comparison between the Endoscopic Endonasal Approach and a Lateral Approach. J. Neurol. Surg. Part B Skull Base 2013, 74, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Van Rompaey, J.; Suruliraj, A.; Carrau, R.; Panizza, B.; Solares, C.A. Meckel’s cave access: Anatomic study comparing the endoscopic transantral and endonasal approaches. Eur. Arch. Otorhinolaryngol. 2014, 271, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.S.; Bergeron, C.M.; Ellenbogen, R.G. Transorbital neuroendoscopic surgery. Neurosurgery 2010, 67 (Suppl. S3), ons16–ons28. [Google Scholar] [CrossRef] [PubMed]
- Dallan, I.; Castelnuovo, P.; Locatelli, D.; Turri-Zanoni, M.; AlQahtani, A.; Battaglia, P.; Hirt, B.; Sellari-Franceschini, S. Multiportal Combined Transorbital Transnasal Endoscopic Approach for the Management of Selected Skull Base Lesions: Preliminary Experience. World Neurosurg. 2015, 84, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wanibuchi, M.; Fukushima, T.; Zomordi, A.R.; Nonaka, Y.; Friedman, A.H. Trigeminal Schwannomas: Skull Base Approaches and Operative Results in 105 Patients. Oper. Neurosurg. 2012, 70 (Suppl. S1), ons132–ons144. [Google Scholar] [CrossRef]
- De Simone, M.; Zoia, C.; Choucha, A.; Kong, D.S.; De Maria, L. The Transorbital Approach: A Comprehensive Review of Targets, Surgical Techniques, and Multiportal Variants. J. Clin. Med. 2024, 13, 2712. [Google Scholar] [CrossRef] [PubMed]
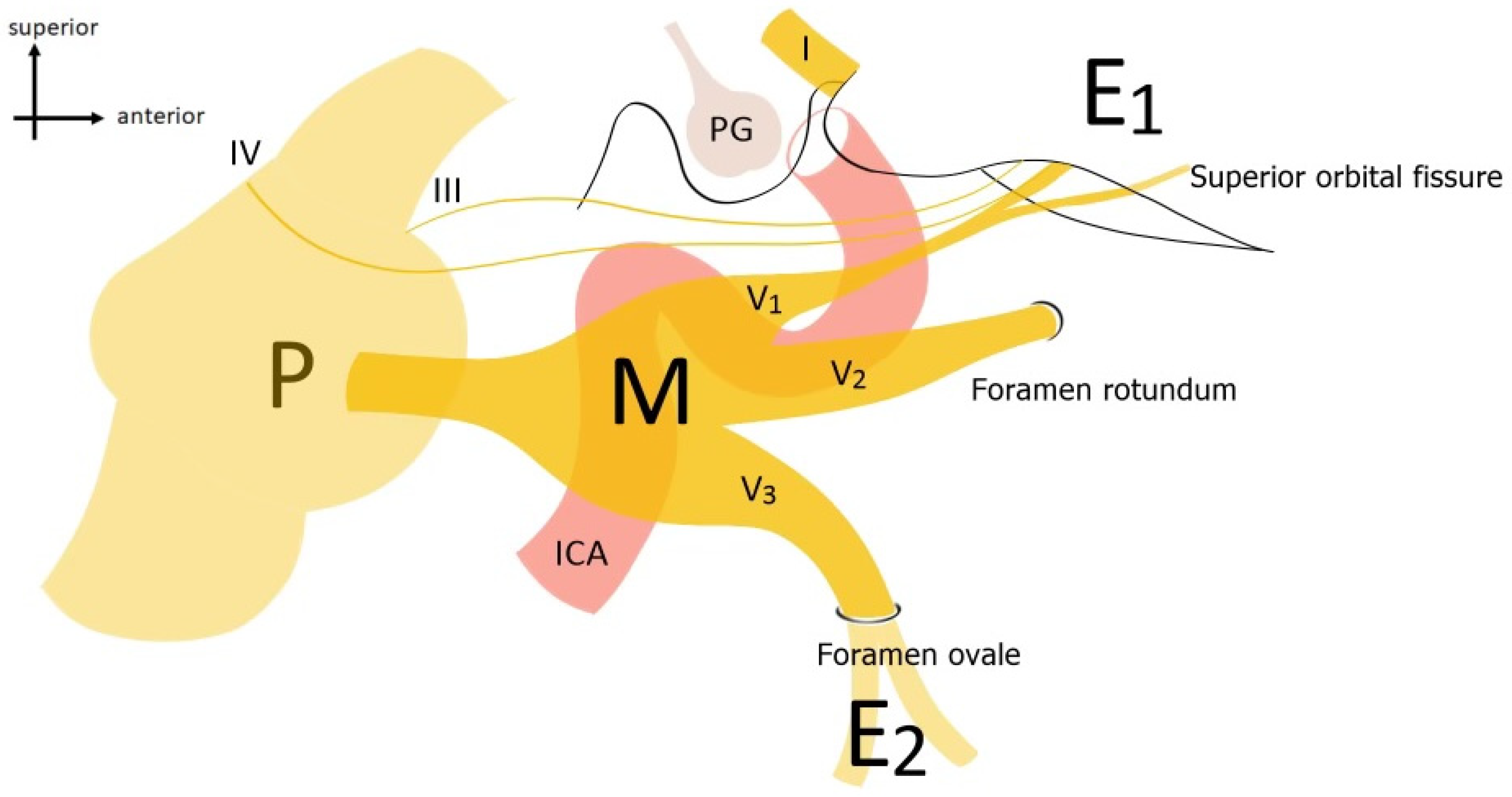

| Category | Approach | Description |
|---|---|---|
| Anterolateral | Pterional Approach (PTA) | Described by Yasargil et al. [11], provides access to the anterolateral region of Meckel’s cave. |
| Anterolateral | Fronto-temporal-orbito-zygomatic Approach (FTOZA) | Following Van Furth et al. [12], allows wide lateral exposure of the skull and the ophthalmic nerve (V1). |
| Lateral | Kawase Approach (KWA) | Proposed by Kawase et al. [13], offers extensive lateral exposure of the trigeminal nerve. |
| Lateral | Subtemporal Approach (STA) | Based on Dolenc et al. [14], provides lateral access to the posterolateral region of Meckel’s cave. |
| Posterolateral | Retrosigmoid Approach (RSA) | According to Samii et al. [15], offers posterolateral access to the trigeminal nerve and the Gasserian ganglion. |
| Posterolateral | Retrosigmoid Approach with Suprameatal Extension (RSAS) | Following Samii et al. [16], provides wide posterolateral exposure of the trigeminal nerve trunk. Best for tumors in the cerebellopontine angle extending toward the Meckel cave and supratentorial regions. |
| Study | Year | Study’s Nature | Case Number | Age (Mean) | Sex | Follow-Up (m) |
|---|---|---|---|---|---|---|
| Jeon et al. [18] | 2019 | Retrospective interventional case series | 4 | 59.5 (52–66) | 3F 1M | 24 |
| Park et al. [22] | 2020 | Retrospective multicenter | 12 | 45.7 (21–68) | 7F 5M | NA |
| Jeon et al. [23] | 2021 | Retrospective case series | 1 | 66 | 1F | 28 |
| Han et al. [24] | 2023 | Retrospective case series | 1 | 40 | 1F | 6 |
| Doo-Sik Kong et al. [25] | 2023 | Retrospective | 50 | 46.9 | 35F 15M | 21.9 (range 1–61.7) |
| Di Somma et al. [26] | 2024 | Retrospective (consecutive cohort) | 2 | 56 | 1F 1M | 2 |
| Overall | 70 | 55 | 48F 22M |
| Author, Year | Location | Size | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | P | MP | E | ME | MPE | Total | <3 cm | 3–6 cm | >6 cm | |
| Jeon et al., 2019 [18] | 1 | 0 | 1 | 0 | 1 | 1 | 4 | 0 | 3 | 1 |
| Park et al., 2020 [22] | 4 | 0 | 5 | 2 | 1 | 0 | 12 | 3 | 5 | 4 |
| Jeon et al., 2021 [23] | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Han et al., 2023 [24] | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Doo-Sik Kong et al., 2023 [25] | 17 | 0 | 20 | 0 | 3 | 10 | 50 | - | 50 (average 3.11) | - |
| Di Somma et al., 2024 [26] | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 0 |
| Overall | 24 | 0 | 28 | 2 | 5 | 11 | 70 | 4 | 61 (87.2%) | 5 |
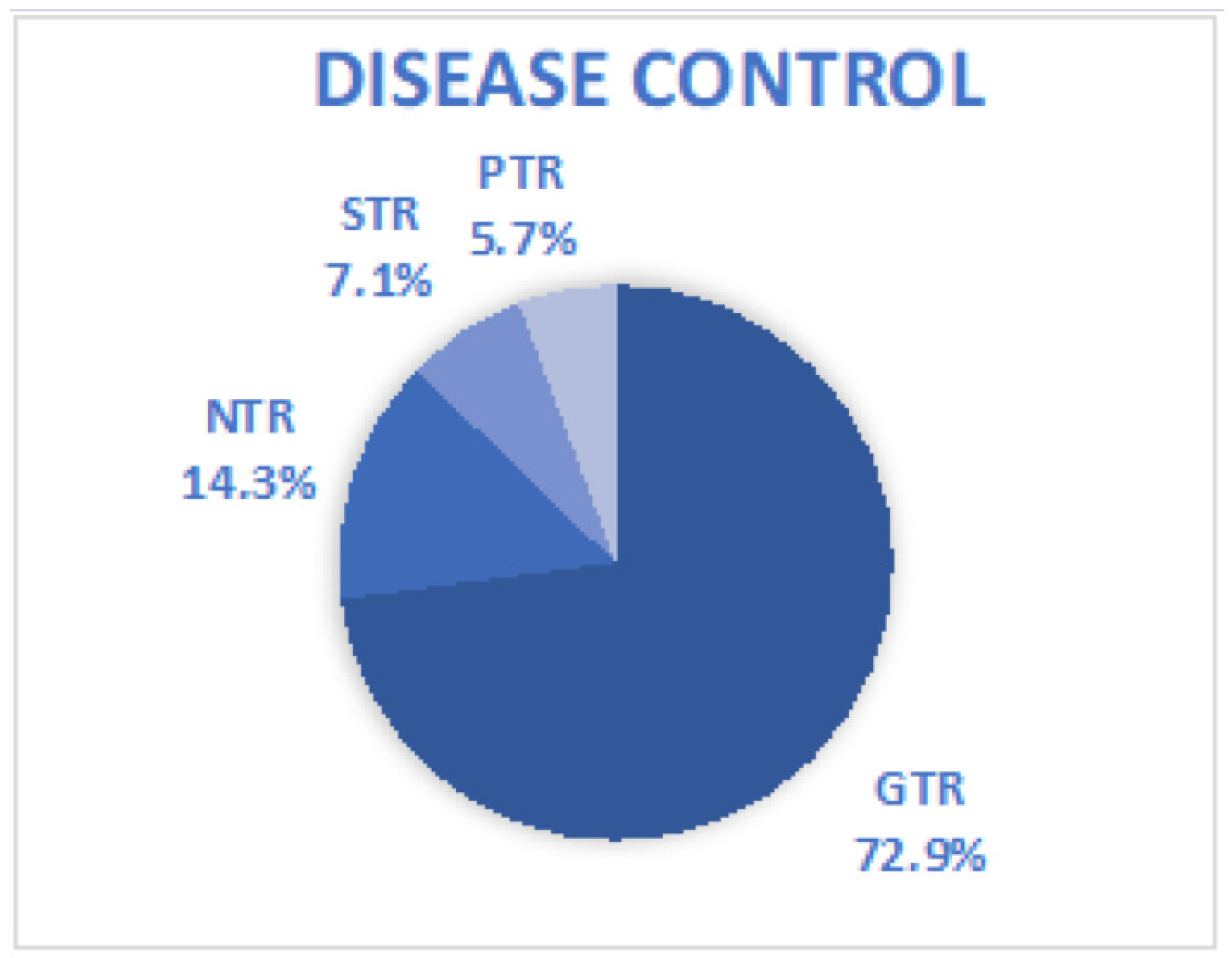
| Study | EOR | Preoperative Symptoms | Case Number | Postoperative Symptoms | Access (Intradural vs. Extradural) | Surgical Approach | Recurrency |
|---|---|---|---|---|---|---|---|
| Jeon et al., 2019 [18] | 3 GTR 1 Intended PTR | 1 Proptosis 2 Sensory changes (facial numbness) | 4 | 1 complete ptosis | 2 vs. 2 | 2 ETOA 1 ETOA plus SOC 1 extended ETOA | 0 |
| Park et al., 2020 [22] | 9 GTR 1 NTR 2 STR | 9 Sensory changes (numbness) 3 Proptosis 1 Diplopia | 12 | 5 Sensory changes (numbness) 1 Diplopia medial gaze | 10 vs. 2 | 11 ETOA 1 ETOA plus RLS | 0 |
| Jeon et al., 2021 [23] | 1 GTR | Proptosis | 1 | Sensory changes (Transient numbness) | 1 vs. 0 | ETOA | 0 |
| Han et al., 2023 [24] | 1 GTR | Facial numbness | 1 | None | NA | Extended ETOA | |
| Doo-Sik Kong et al., 2023 [25] | 35 GTR 9 NTR 3 STR 3 PTR | 42 Sensory changes 18 Diplopia 5 Proptosis 7 Neuralgic pain | 50 | 7/1 Diplopia (transient/permanent) 4/1 Ptosis (transient/permanent) 2 Wound infection 7 Corneal keratopathy 5 Mastication difficulty 14 Neuralgia requiring medication | NA | 47 ETOA 2 ETOA plus EEA 1 extended ETOA (lateral canthotomy) | 0 |
| Di Somma et al., 2024 [26] | 2 GTR | None | 2 | Upper eyelid edema, diplopia lateral gaze, | NA | 2 ETOA | 0 |
| sensory changes (V2–3 numbness) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Simone, M.; Choucha, A.; Dannhoff, G.; Kong, D.-S.; Zoia, C.; Iaconetta, G. Treating Trigeminal Schwannoma through a Transorbital Approach: A Systematic Review. J. Clin. Med. 2024, 13, 3701. https://doi.org/10.3390/jcm13133701
De Simone M, Choucha A, Dannhoff G, Kong D-S, Zoia C, Iaconetta G. Treating Trigeminal Schwannoma through a Transorbital Approach: A Systematic Review. Journal of Clinical Medicine. 2024; 13(13):3701. https://doi.org/10.3390/jcm13133701
Chicago/Turabian StyleDe Simone, Matteo, Anis Choucha, Guillaume Dannhoff, Doo-Sik Kong, Cesare Zoia, and Giorgio Iaconetta. 2024. "Treating Trigeminal Schwannoma through a Transorbital Approach: A Systematic Review" Journal of Clinical Medicine 13, no. 13: 3701. https://doi.org/10.3390/jcm13133701
APA StyleDe Simone, M., Choucha, A., Dannhoff, G., Kong, D.-S., Zoia, C., & Iaconetta, G. (2024). Treating Trigeminal Schwannoma through a Transorbital Approach: A Systematic Review. Journal of Clinical Medicine, 13(13), 3701. https://doi.org/10.3390/jcm13133701








