Robotic-Assisted Pelvic Exenteration for Cervical Cancer: A Systematic Review and Novel Insights into Compartment-Based Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objectives and PICO Process
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction
2.5. Risk of Bias
3. Results
4. Discussion
5. Prognostic Factors
6. Preoperative Imaging for Planning Pelvic Exenteration
6.1. Magnetic Resonance Imaging
6.2. Compartment-Based Imaging
6.3. Positron-Emission Tomography-Computerized Tomography
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Plante, M.; Kwon, J.S.; Ferguson, S.; Samouëlian, V.; Ferron, G.; Maulard, A.; de Kroon, C.; Van Driel, W.; Tidy, J.; Williamson, K.; et al. Simple versus Radical Hysterectomy in Women with Low-Risk Cervical Cancer. N. Engl. J. Med. 2024, 390, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Taliento, C.; Scutiero, G.; Arcieri, M.; Pellecchia, G.; Tius, V.; Bogani, G.; Petrillo, M.; Pavone, M.; Bizzarri, N.; Driul, L.; et al. Simple hysterectomy versus radical hysterectomy in early-stage cervical cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2024, 50, 108252. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. NCCN Guidelines® Insights: Cervical Cancer, Version 1.2024. J. Natl. Compr. Cancer Netw. 2023, 21, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Sardain, H.; Lavoué, V.; Foucher, F.; Levêque, J. Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review. J. Gynecol. Obstet. Biol. Reprod. 2016, 45, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Iavazzo, C.; Gkegkes, I.D. Robotic technology for pelvic exenteration in cases of cervical cancer. Int. J. Gynaecol. Obstet. 2014, 125, 15–17. [Google Scholar] [CrossRef]
- Schneider, A.; Köhler, C.; Erdemoglu, E. Current developments for pelvic exenteration in gynecologic oncology. Curr. Opin. Obstet. Gynecol. 2009, 21, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Marnitz, S.; Köhler, C.; Müller, M.; Behrens, K.; Hasenbein, K.; Schneider, A. Indications for primary and secondary exenterations in patients with cervical cancer. Gynecol. Oncol. 2006, 103, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Puntambekar, S.; Lawande, A.; Desai, R.; Puntambekar, S.; Joshi, G.A.; Joshi, S.N. Initial experience of robotic anterior pelvic exenteration at a single institute. Int. J. Gynaecol. Obstet. 2014, 126, 41–44. [Google Scholar] [CrossRef]
- Matsuo, K.; Matsuzaki, S.; Mandelbaum, R.S.; Kanao, H.; Chang, E.J.; Klar, M.; Roman, L.D.; Wright, J.D. Utilization and perioperative outcome of minimally invasive pelvic exenteration in gynecologic malignancies: A national study in the United States. Gynecol. Oncol. 2021, 161, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Pomel, C.; Rouzier, R.; Pocard, M.; Thoury, A.; Sideris, L.; Morice, P.; Duvillard, P.; Bourgain, J.L.; Castaigne, D. Laparoscopic total pelvic exenteration for cervical cancer relapse. Gynecol. Oncol. 2003, 91, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.C. Robotic assisted total pelvic exenteration: A case report. Gynecol. Oncol. 2009, 115, 310–311. [Google Scholar] [CrossRef]
- Zapardiel, I.; Ceccaroni, M.; Minig, L.; Halaska, M.J.; Fujii, S. Avascular spaces in radical hysterectomy. Int. J. Gynecol. Cancer 2023, 33, 285–292. [Google Scholar] [CrossRef]
- Bergerot, C.D.; Philip, E.J.; Bergerot, P.G.; Hsu, J.; Dizman, N.; Salgia, M.; Salgia, N.; Vaishampayan, U.; Battle, D.; Loscalzo, M.; et al. Discrepancies between genitourinary cancer patients’ and clinicians’ characterization of the Eastern Cooperative Oncology Group performance status. Cancer 2021, 127, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Sardain, H.; Lavoue, V.; Redpath, M.; Bertheuil, N.; Foucher, F.; Levêque, J. Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review. Eur. J. Surg. Oncol. 2015, 41, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Cianci, S.; Arcieri, M.; Vizzielli, G.; Martinelli, C.; Granese, R.; La Verde, M.; Fagotti, A.; Fanfani, F.; Scambia, G.; Ercoli, A. Robotic Pelvic Exenteration for Gynecologic Malignancies, Anatomic Landmarks, and Surgical Steps: A Systematic Review. Front. Surg. 2021, 8, 790152. [Google Scholar] [CrossRef] [PubMed]
- Peiretti, M.; Zapardiel, I.; Zanagnolo, V.; Landoni, F.; Morrow, C.P.; Maggioni, A. Management of recurrent cervical cancer: A review of the literature. Surg. Oncol. 2012, 21, e59–e66. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, N.; Chiantera, V.; Ercoli, A.; Fagotti, A.; Tortorella, L.; Conte, C.; Cappuccio, S.; Di Donna, M.C.; Gallotta, V.; Scambia, G.; et al. Minimally Invasive Pelvic Exenteration for Gynecologic Malignancies: A Multi-Institutional Case Series and Review of the Literature. J. Minim. Invasive Gynecol. 2019, 26, 1316–1326. [Google Scholar] [CrossRef]
- Stanca, M.; Căpîlna, D.M.; Căpîlna, M.E. Long-Term Survival, Prognostic Factors, and Quality of Life of Patients Undergoing Pelvic Exenteration for Cervical Cancer. Cancers 2022, 14, 2346. [Google Scholar] [CrossRef]
- Graves, S.; Seagle, B.L.; Strohl, A.E.; Shahabi, S.; Nieves-Neira, W. Survival After Pelvic Exenteration for Cervical Cancer: A National Cancer Database Study. Int. J. Gynecol. Cancer 2017, 27, 390–395. [Google Scholar] [CrossRef]
- Stelzner, S.; Heinze, T.; Heimke, M.; Gockel, I.; Kittner, T.; Brown, G.; Mees, S.T.; Wedel, T. Beyond Total Mesorectal Excision: Compartment-based Anatomy of the Pelvis Revisited for Exenterative Pelvic Surgery. Ann. Surg. 2023, 278, e58–e67. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, P.A.; Tekkis, P.P.; Constantinides, V.A.; Patel, U.; Goldin, R.D.; Darzi, A.W.; John Nicholls, R.; Brown, G. Diagnostic accuracy and value of magnetic resonance imaging (MRI) in planning exenterative pelvic surgery for advanced colorectal cancer. Eur. J. Cancer 2013, 49, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Lambaudie, E.; Narducci, F.; Leblanc, E.; Bannier, M.; Houvenaeghel, G. Robotically-assisted laparoscopic anterior pelvic exenteration for recurrent cervical cancer: Report of three first cases. Gynecol. Oncol. 2010, 116, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Adams, S.; Eun, D.; Lee, D.; Randall, T.C. Robotic-assisted laparoscopic exenteration in recurrent cervical cancer Robotics improved the surgical experience for 2 women with recurrent cervical cancer. Am. J. Obstet. Gynecol. 2010, 202, 663.e1. [Google Scholar] [CrossRef] [PubMed]
- Jauffret, C.; Lambaudie, E.; Bannier, M.; Buttarelli, M.; Houvenaeghel, G. Robot-assisted laparoscopy in the management of recurrent pelvic cancer. Gynecol. Obstet. Fertil. 2011, 39, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Lawande, A.; Kenawadekar, R.; Desai, R.; Malireddy, C.; Nallapothula, K.; Puntambekar, S.P. Robotic total pelvic exenteration. J. Robot. Surg. 2014, 8, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, I.T.; Chu, W.; Tozzi, F.; Lau, C.; Wakabayashi, M.; Chan, K.; Lee, B. Robotic Total Pelvic Exenteration: Video-Illustrated Technique. Ann. Surg. Oncol. 2017, 24, 3422–3423. [Google Scholar] [CrossRef]
- Nguyen Xuan, H.T.; Myriam, D.; Charlotte, N.; Richard, D.; Anne-Sophie, B.; Olivier, T.M.; Arnaud, M.; Fabrice, L. Pelvic exenteration by robotically-assisted laparoscopy: A feasibility series of 6 cases. Gynecol. Oncol. Rep. 2018, 25, 56–59, Erratum in Gynecol. Oncol. Rep. 2021, 35, 100703. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, J.; Xiao, L. Disease-free survival after robotic-assisted laparoscopic total pelvic exenteration for recurrent cervical adenocarcinoma: A case report. Medicine 2018, 97, e11611. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Debnath, S.; Rawal, S. Salvage robotic anterior pelvic exenteration for cervical cancer: Technique and feasibility. J. Robot. Surg. 2021, 15, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Dudus, L.; Minciuna, C.; Tudor, S.; Lacatus, M.; Stefan, B.; Vasilescu, C. Robotic or laparoscopic pelvic exenteration for gynecological malignancies: Feasible options to open surgery. J. Gynecol. Oncol. 2024, 35, e12. [Google Scholar] [CrossRef] [PubMed]
- Brunschwig, A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1948, 1, 177–183. [Google Scholar] [CrossRef]
- Berek, J.S.; Howe, C.; Lagasse, L.D.; Hacker, N.F. Pelvic exenteration for recurrent gynecologic malignancy: Survival and morbidity analysis of the 45-year experience at UCLA. Gynecol. Oncol. 2005, 99, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Y.; Tang, J.Y. Application of robot-assisted laparoscopic pelvic exenteration in treating gynecologic malignancies. Chin. Med. J. 2019, 132, 976–979. [Google Scholar] [CrossRef]
- Martínez, A.; Filleron, T.; Vitse, L.; Querleu, D.; Mery, E.; Balague, G.; Delannes, M.; Soulie, M.; Pomel, C.; Ferron, G. Laparoscopic pelvic exenteration for gynaecological malignancy: Is there any advantage? Gynecol. Oncol. 2011, 120, 374–379. [Google Scholar] [CrossRef]
- Pruthi, R.S.; Stefaniak, H.; Hubbard, J.S.; Wallen, E.M. Robotic anterior pelvic exenteration for bladder cancer in the female: Outcomes and comparisons to their male counterparts. J. Laparoendosc. Adv. Surg. Tech. A 2009, 19, 23–27. [Google Scholar] [CrossRef]
- Jäger, L.; Nilsson, P.J.; Rådestad, A.F. Pelvic exenteration for recurrent gynecologic malignancy: A study of 28 consecutive patients at a single institution. Int. J. Gynecol. Cancer 2013, 23, 755–762. [Google Scholar] [CrossRef]
- Baiocchi, G.; Guimaraes, G.C.; Rosa Oliveira, R.A.; Kumagai, L.Y.; Faloppa, C.C.; Aguiar, S.; Begnami, M.D.; Soares, F.A.; Lopes, A. Prognostic factors in pelvic exenteration for gynecological malignancies. Eur. J. Surg. Oncol. 2012, 38, 948–954. [Google Scholar] [CrossRef]
- Diver, E.J.; Rauh-Hain, J.A.; Del Carmen, M.G. Total pelvic exenteration for gynecologic malignancies. Int. J. Surg. Oncol. 2012, 2012, 693535. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Imesch, P.; Fink, D.; Egger, H. Indications and long-term clinical outcomes in 282 patients with pelvic exenteration for advanced or recurrent cervical cancer. Gynecol. Oncol. 2012, 125, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Chiantera, V.; Rossi, M.; De Iaco, P.; Koehler, C.; Marnitz, S.; Ferrandina, G.; Legge, F.; Parazzini, F.; Scambia, G.; Schneider, A.; et al. Survival after curative pelvic exenteration for primary or recurrent cervical cancer: A retrospective multicentric study of 167 patients. Int. J. Gynecol. Cancer 2014, 24, 916–922. [Google Scholar] [CrossRef]
- Höckel, M.; Horn, L.C.; Einenkel, J. (Laterally) extended endopelvic resection: Surgical treatment of locally advanced and recurrent cancer of the uterine cervix and vagina based on ontogenetic anatomy. Gynecol. Oncol. 2012, 127, 297–302. [Google Scholar] [CrossRef]
- Andikyan, V.; Khoury-Collado, F.; Sonoda, Y.; Gerst, S.R.; Alektiar, K.M.; Sandhu, J.S.; Bochner, B.H.; Barakat, R.R.; Boland, P.J.; Chi, D.S. Extended pelvic resections for recurrent or persistent uterine and cervical malignancies: An update on out of the box surgery. Gynecol. Oncol. 2012, 125, 404–408. [Google Scholar] [CrossRef]
- Landoni, F.; Zanagnolo, V.; Rosenberg, P.G.; Lopes, A.; Radice, D.; Bocciolone, L.; Aletti, G.; Parma, G.; Colombo, N.; Maggioni, A. Neoadjuvant chemotherapy prior to pelvic exenteration in patients with recurrent cervical cancer: Single institution experience. Gynecol. Oncol. 2013, 130, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Meads, C.; Davenport, C.; Małysiak, S.; Kowalska, M.; Zapalska, A.; Guest, P.; Martin-Hirsch, P.; Borowiack, E.; Auguste, P.; Barton, P.; et al. Evaluating PET-CT in the detection and management of recurrent cervical cancer: Systematic reviews of diagnostic accuracy and subjective elicitation. BJOG 2014, 121, 398–407. [Google Scholar] [CrossRef]
- Forner, D.M.; Meyer, A.; Lampe, B. Preoperative assessment of complete tumour resection by magnetic resonance imaging in patients undergoing pelvic exenteration. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 148, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zheng, A.; Wang, F.; Lin, W.; Yang, X.; Han, L.; Chen, Y.; Bai, L. Diagnostic value of 18F-FDG-PET or PET-CT in recurrent cervical cancer: A systematic review and meta-analysis. Nucl. Med. Commun. 2014, 35, 144–150. [Google Scholar] [CrossRef]
- De Cuypere, M.; Lovinfosse, P.; Goffin, F.; Gennigens, C.; Rovira, R.; Duch, J.; Fastrez, M.; Gebhart, G.; Squifflet, J.L.; Luyckx, M.; et al. Added value of para-aortic surgical staging compared to 18F-FDG PET/CT on the external beam radiation field for patients with locally advanced cervical cancer: An ONCO-GF study. Eur. J. Surg. Oncol. 2020, 46, 883–887. [Google Scholar] [CrossRef]
- Thelissen, A.A.B.; Jürgenliemk-Schulz, I.M.; van der Leij, F.; Peters, M.; Gerestein, C.G.; Zweemer, R.P.; van Rossum, P.S.N. Upstaging by para-aortic lymph node dissection in patients with locally advanced cervical cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2022, 164, 667–674. [Google Scholar] [CrossRef] [PubMed]



| Author | Year | Number of Patients | Type of Exenteration | Total Operative Time (mins) | EBL (mL) | Early Postop Complications | Late Postop Complications | Hospital Stay (Days-Range) | Median Follow-Up (Months-Range) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Lim [13] | 2009 | 1 | TPE | 375 | 375 | none | NS | 10 | NS | NS |
| Lambaudi et al. [25] | 2010 | 3 | APE | 480 | 400 | none | Fistula, UTI, ureteral stenosis, perineal abscess | 30 | 2–12 | DFS = 9 m |
| Davis et al. [26] | 2010 | 2 | APE | 540 | 550 | NS | NS | 8 | NS | Recurrence after 8 and 23 m |
| Jauffret et al. [27] | 2011 | 2 | APE | 480 | 300 | none | Wound dehiscence, fistula, sepsis, urosepsis, prerenal failure, obstructive renal failure | 6 (3–24) | 23 | DFS = 5 m and 8 m, OS = 22 m and 23 m |
| Lawande et al. [28] | 2014 | 1 | TPE | 240 | 300 | none | none | 11 | 2 | NS |
| Puntambekar et al. [10] | 2014 | 10 | APE | 180 | 110 | none | none | 5 | 11 | Disease free: 8 patients |
| Konstantinidis et al. [29] | 2017 | 1 | TPE | 641 | 400 | NS | NS | NS | NS | NS |
| Nguyen Xuan et al. [30] | 2018 | 5 | APE (n = 2) PPE (n = 2) TPE (n = 1) | 390–480 | NS | 4 UTI, 1 PE, 1 sepsis | Wound infection, stenosis ileal anastomosis, renal failure, pulmonary embolism | 11.5 | NS | 3 recurrences after 7 m, 1 died after 10 m |
| Yang et al. [31] | 2018 | 1 | TPE | 700 | 300 | none | Fistula | 37 | 17 | no recurrence |
| Bizzarri et al. [19] | 2019 | 11 | APE | 500 | 235 | none | 27.3% of cases | 9 | 15 | DFS = 11 m |
| Jain et al. [32] | 2021 | 14 | APE | 305 | 135 | 36% of cases: urosepsis, anastomosis leak, ileus, fistula, intestinal obstruction | 28.6% of cases: colon perforation, UTI, large bowel obstruction, bleeding, ureteral stricture | 6.5 | 17.5 (10–68) | 5 deaths, 12 m DFS = 68.2%, 12 m OS: 77.1% |
| Dudus et al. [33] | 2024 | 12 | APE (n = 6) TPE (n = 6) | 360 440 | 350 | Morbidity: 16.6%, urinoma, wound infection | Pyelonephritis, hydronephrosis, thrombophlebitis, renal failure, bowel obstruction, iliac artery fistula | 18 (6–38) | 24 | 50% alive, DFS = 12 m, OS = 20 m |
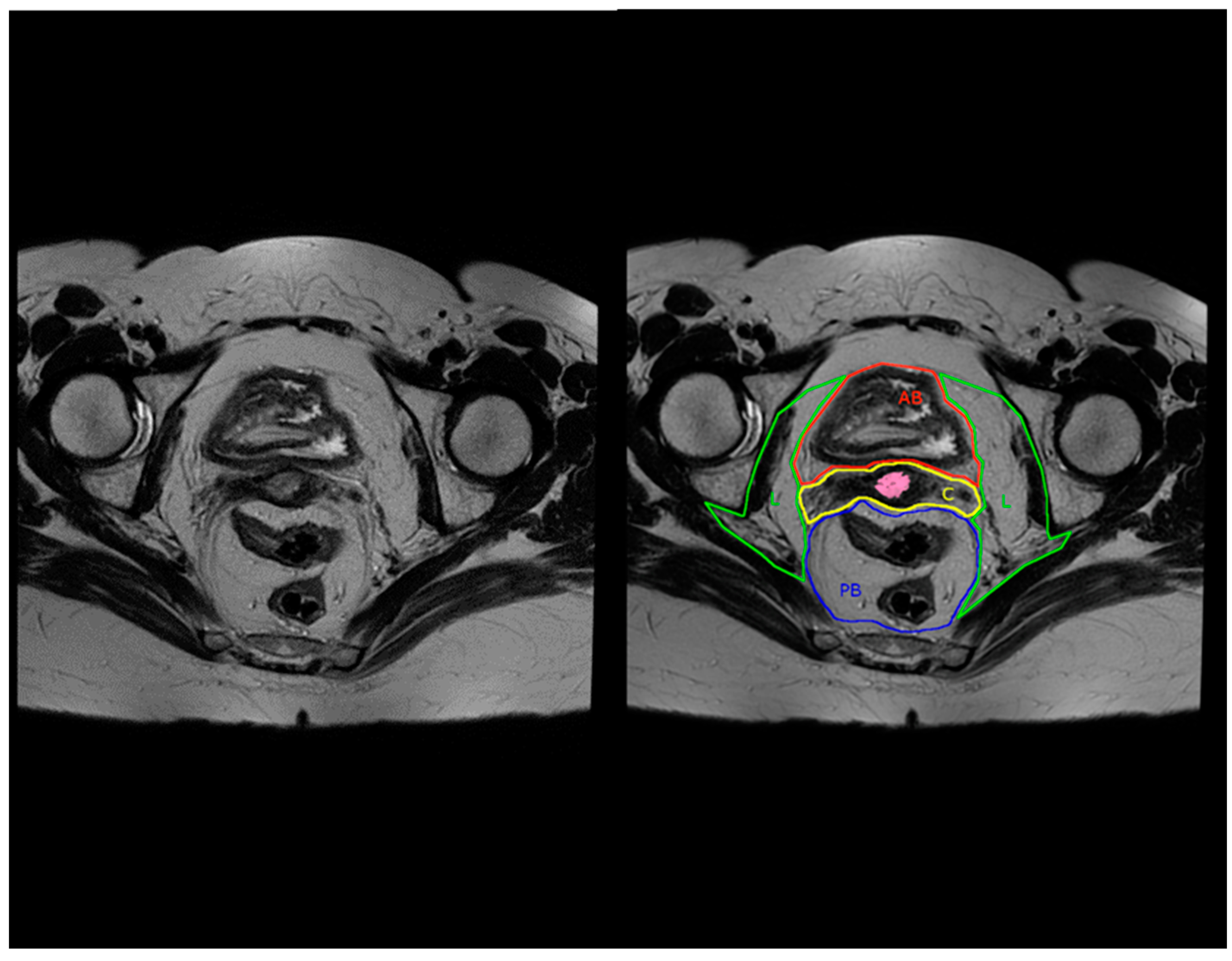
| MRI Compartment Name | Anatomical Structures |
|---|---|
| Peritoneal reflection compartment (PR) | Peritoneum (covering Douglas and vesico-uterine space |
| Anterior above peritoneal reflection compartment (AA) | Abdominal cavity with intestines, mesenteric and omental fat, Retroperitoneal space with proximal two thirds of the ureter, ovarian vessels, genitofemoral nerve, iliopsoas muscle |
| Anterior below peritoneal reflection compartment (AB) | Bladder, urethra, Retzius space, vesico-ureteral junction, superior/inferior vesical vessels, ureter and bladder nerve branches from inferior hypogastric plexus, vesico-uterine ligament |
| Central compartment (C) | Uterine corpus, uterine cervix, proximal round ligaments, parametrium, vagina, paracolpium, uterine and vaginal vessels |
| Posterior below peritoneal reflection compartment (PB) | Rectum, mesorectum, splanchnic branches from the superior/inferior hypogastric plexus and hypogastric nerves, rectosigmoid junction |
| Lateral compartment (L) | Parietal pelvic fascia, inferior hypogastric nerve in meso-ureter, distal third of ureter, iliac vessels, iliac and obturator lymph nodes, sacral nerve plexus, obturator nerve, internal obturator muscle, piriformis muscle, lumbosacral trunk, ovaries, fallopian tubes |
| Inferior compartment (I) | Anorectal junction, pelvic floor muscles, levator ani muscle, transverse perineal muscle (urogenital diaphragm), perineal body, pudendal vessels and nerves |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Trappen, P.; Walgraeve, M.-S.; Roels, S.; Claes, N.; De Cuypere, E.; Baekelandt, F.; Arentsen, H. Robotic-Assisted Pelvic Exenteration for Cervical Cancer: A Systematic Review and Novel Insights into Compartment-Based Imaging. J. Clin. Med. 2024, 13, 3673. https://doi.org/10.3390/jcm13133673
Van Trappen P, Walgraeve M-S, Roels S, Claes N, De Cuypere E, Baekelandt F, Arentsen H. Robotic-Assisted Pelvic Exenteration for Cervical Cancer: A Systematic Review and Novel Insights into Compartment-Based Imaging. Journal of Clinical Medicine. 2024; 13(13):3673. https://doi.org/10.3390/jcm13133673
Chicago/Turabian StyleVan Trappen, Philippe, Marie-Sofie Walgraeve, Sarah Roels, Nele Claes, Eveline De Cuypere, Frederic Baekelandt, and Harm Arentsen. 2024. "Robotic-Assisted Pelvic Exenteration for Cervical Cancer: A Systematic Review and Novel Insights into Compartment-Based Imaging" Journal of Clinical Medicine 13, no. 13: 3673. https://doi.org/10.3390/jcm13133673
APA StyleVan Trappen, P., Walgraeve, M.-S., Roels, S., Claes, N., De Cuypere, E., Baekelandt, F., & Arentsen, H. (2024). Robotic-Assisted Pelvic Exenteration for Cervical Cancer: A Systematic Review and Novel Insights into Compartment-Based Imaging. Journal of Clinical Medicine, 13(13), 3673. https://doi.org/10.3390/jcm13133673






