CPM-Related Mechanisms Could Play a Key Role in the Effects on Pain Sensitivity Induced by Manual Therapy: Three Crossover Trials Investigating the Effects of Manual Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Outcome Measures
2.3.1. Baseline Characteristics
2.3.2. Pressure Pain Thresholds
2.4. Procedure
2.5. Interventions Trial 1
2.6. Interventions Trial 2
2.7. Interventions Trial 3
2.8. Statistical Analysis
2.9. Sample Size Calculation
3. Results
3.1. Trial 1
3.2. Trial 2
3.3. Trial 3
3.4. PPT Washout Period
3.5. Associations between Psychological Factors or Physical Activity Level and the Effect on PPT Produced by PIMP
4. Discussion
4.1. Clinical Implications
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsson, C.; Hansson, E.E.; Sundquist, K.; Jakobsson, U. Chronic Pain in Older Adults: Prevalence, Incidence, and Risk Factors. Scand. J. Rheumatol. 2017, 46, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.E.; Sim, J.; Jordan, J.L.; Jordan, K.P. A Systematic Review and Meta-Analysis of the Prevalence of Chronic Widespread Pain in the General Population. Pain 2016, 157, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Carlesso, L.C.; Macdermid, J.C.; Gross, A.R.; Walton, D.M.; Santaguida, P.L. Treatment Preferences amongst Physical Therapists and Chiropractors for the Management of Neck Pain: Results of an International Survey. Chiropr. Man. Ther. 2014, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Corp, N.; Mansell, G.; Stynes, S.; Wynne-Jones, G.; Morsø, L.; Hill, J.C.; van der Windt, D.A. Evidence-Based Treatment Recommendations for Neck and Low Back Pain across Europe: A Systematic Review of Guidelines. Eur. J. Pain 2021, 25, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Tsokanos, A.; Livieratou, E.; Billis, E.; Tsekoura, M.; Tatsios, P.; Tsepis, E.; Fousekis, K. The Efficacy of Manual Therapy in Patients with Knee Osteoarthritis: A Systematic Review. Medicina 2021, 57, 696. [Google Scholar] [CrossRef] [PubMed]
- George, S.Z.; Fritz, J.M.; Silfies, S.P.; Schneider, M.J.; Beneciuk, J.M.; Lentz, T.A.; Gilliam, J.R.; Hendren, S.; Norman, K.S. Interventions for the Management of Acute and Chronic Low Back Pain: Revision 2021. J. Orthop. Sports Phys. Ther. 2021, 51, CPG1–CPG60. [Google Scholar] [CrossRef] [PubMed]
- Molina-Álvarez, M.; Arribas-Romano, A.; Rodríguez-Rivera, C.; García, M.M.; Fernández-Carnero, J.; Armijo-Olivo, S.; Goicoechea Garcia, C. Manual Therapy Effect in Placebo-Controlled Trials: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14021. [Google Scholar] [CrossRef] [PubMed]
- Coulter, I.D.; Crawford, C.; Vernon, H.; Hurwitz, E.L.; Khorsan, R.; Booth, M.S.; Herman, P.M. Manipulation and Mobilization for Treating Chronic Nonspecific Neck Pain: A Systematic Review and Meta-Analysis for an Appropriateness Panel. Pain Physician 2019, 22, E55–E70. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.E.; Beneciuk, J.M.; Bishop, M.D.; Coronado, R.A.; Penza, C.W.; Simon, C.B.; George, S.Z. Unraveling the Mechanisms of Manual Therapy: Modeling an Approach. J. Orthop. Sports Phys. Ther. 2018, 48, 8–18. [Google Scholar] [CrossRef]
- Shraim, M.A.; Massé-Alarie, H.; Hall, L.M.; Hodges, P.W. Systematic Review and Synthesis of Mechanism-Based Classification Systems for Pain Experienced in the Musculoskeletal System. Clin. J. Pain 2020, 36, 793–812. [Google Scholar] [CrossRef]
- Edwards, R.R.; Dworkin, R.H.; Turk, D.C.; Angst, M.S.; Dionne, R.; Freeman, R.; Hansson, P.; Haroutounian, S.; Arendt-Nielsen, L.; Attal, N.; et al. Patient Phenotyping in Clinical Trials of Chronic Pain Treatments: IMMPACT Recommendations. Pain 2016, 157, 1851–1871. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The Mechanisms of Manual Therapy in the Treatment of Musculoskeletal Pain: A Comprehensive Model. Man. Ther. 2009, 14, 531–538. [Google Scholar] [CrossRef]
- Martínez-Pozas, O.; Sánchez-Romero, E.A.; Beltran-Alacreu, H.; Arribas-Romano, A.; Cuenca-Martínez, F.; Villafañe, J.H.; Fernández-Carnero, J. Effects of Orthopedic Manual Therapy on Pain Sensitization in Patients with Chronic Musculoskeletal Pain: An Umbrella Review with Meta-Meta-Analysis. Am. J. Phys. Med. Rehabil. 2023, 102, 879–885. [Google Scholar] [CrossRef]
- Arribas-Romano, A.; Fernández-Carnero, J.; Molina-Rueda, F.; Angulo-Diaz-Parreño, S.; Navarro-Santana, M.J. Efficacy of Physical Therapy on Nociceptive Pain Processing Alterations in Patients with Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Pain Med. 2020, 21, 2502–2517. [Google Scholar] [CrossRef]
- Gay, C.W.; Alappattu, M.J.; Coronado, R.A.; Horn, M.E.; Bishop, M.D. Effect of a Single Session of Muscle-Biased Therapy on Pain Sensitivity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Pain Res. 2013, 6, 7–22. [Google Scholar] [CrossRef]
- Yarnitsky, D.; Bouhassira, D.; Drewes, A.M.; Fillingim, R.B.; Granot, M.; Hansson, P.; Landau, R.; Marchand, S.; Matre, D.; Nilsen, K.B.; et al. Recommendations on Practice of Conditioned Pain Modulation (CPM) Testing. Eur. J. Pain 2015, 19, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Nahman-Averbuch, H.; Timmers, I. Neural Mechanisms Underlying the Conditioned Pain Modulation Response: A Narrative Review of Neuroimaging Studies. Pain 2023, 164, e25–e46. [Google Scholar] [CrossRef] [PubMed]
- Piché, M. Mechanistic Perspective on Conditioned Pain Modulation. Pain 2023, 164, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.T.; Riley, J.L.; Bishop, M.D.; Beneciuk, J.M.; Godza, M.; Cruz-Almeida, Y.; Bialosky, J.E. A Psychophysical Study Comparing Massage to Conditioned Pain Modulation: A Single Blind Randomized Controlled Trial in Healthy Participants. J. Bodyw. Mov. Ther. 2021, 27, 426–435. [Google Scholar] [CrossRef]
- Nir, R.-R.; Granovsky, Y.; Yarnitsky, D.; Sprecher, E.; Granot, M. A Psychophysical Study of Endogenous Analgesia: The Role of the Conditioning Pain in the Induction and Magnitude of Conditioned Pain Modulation. Eur. J. Pain 2011, 15, 491–497. [Google Scholar] [CrossRef]
- Granot, M.; Weissman-Fogel, I.; Crispel, Y.; Pud, D.; Granovsky, Y.; Sprecher, E.; Yarnitsky, D. Determinants of Endogenous Analgesia Magnitude in a Diffuse Noxious Inhibitory Control (DNIC) Paradigm: Do Conditioning Stimulus Painfulness, Gender and Personality Variables Matter? Pain 2008, 136, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Nahman-Averbuch, H.; Yarnitsky, D.; Granovsky, Y.; Gerber, E.; Dagul, P.; Granot, M. The Role of Stimulation Parameters on the Conditioned Pain Modulation Response. Scand. J. Pain 2013, 4, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Graven-Nielsen, T.; Izumi, M.; Petersen, K.K.; Arendt-Nielsen, L. User-Independent Assessment of Conditioning Pain Modulation by Cuff Pressure Algometry. Eur. J. Pain 2017, 21, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Oono, Y.; Wang, K.; Svensson, P.; Arendt-Nielsen, L. Conditioned Pain Modulation Evoked by Different Intensities of Mechanical Stimuli Applied to the Craniofacial Region in Healthy Men and Women. J. Orofac. Pain 2011, 25, 364–375. [Google Scholar] [PubMed]
- Smith, A.; Pedler, A. Conditioned Pain Modulation Is Affected by Occlusion Cuff Conditioning Stimulus Intensity, but Not Duration. Eur. J. Pain 2018, 22, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Hansson, P.t.; Johansson, B.; Leffler, A.-S. The Influence of Intensity and Duration of a Painful Conditioning Stimulation on Conditioned Pain Modulation in Volunteers. Eur. J. Pain 2014, 18, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Hermans, L.; Van Oosterwijck, J.; Goubert, D.; Goudman, L.; Crombez, G.; Calders, P.; Meeus, M. Inventory of Personal Factors Influencing Conditioned Pain Modulation in Healthy People: A Systematic Literature Review. Pain Pract. 2016, 16, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Nahman-Averbuch, H.; Nir, R.-R.; Sprecher, E.; Yarnitsky, D. Psychological Factors and Conditioned Pain Modulation: A Meta-Analysis. Clin. J. Pain 2016, 32, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Stagg, N.J.; Mata, H.P.; Ibrahim, M.M.; Henriksen, E.J.; Porreca, F.; Vanderah, T.W.; Malan, T.P. Regular Exercise Reverses Sensory Hypersensitivity in a Rat Neuropathic Pain Model: Role of Endogenous Opioids. Anesthesiology 2011, 114, 940–948. [Google Scholar] [CrossRef]
- Hoffmann, P.; Terenius, L.; Thorén, P. Cerebrospinal Fluid Immunoreactive Beta-Endorphin Concentration Is Increased by Voluntary Exercise in the Spontaneously Hypertensive Rat. Regul. Pept. 1990, 28, 233–239. [Google Scholar] [CrossRef]
- Su, C.F.; Chang, Y.Y.; Pai, H.H.; Liu, I.M.; Lo, C.Y.; Cheng, J.T. Mediation of Beta-Endorphin in Exercise-Induced Improvement in Insulin Resistance in Obese Zucker Rats. Diabetes Metab. Res. Rev. 2005, 21, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 Statement: Extension to Randomised Crossover Trials. BMJ 2019, 366, l4378. [Google Scholar] [CrossRef] [PubMed]
- García Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validation of the Spanish version of the Pain Catastrophizing Scale in fibromyalgia. Med. Clin. 2008, 131, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C. Development of the Spanish Edition of the State-Trait Anxiety Inventory. Interam. J. Psychol. 1971, 5, 145–158. [Google Scholar]
- Guillén-Riquelme, A.; Buela-Casal, G. Metaanálisis de Comparación de Grupos y Metaanálisis de Generalización de La Fiabilidad Del Cuestionario State-Trait Anxiety Inventory (STAI). Rev. Española Salud Pública 2014, 88, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kabacoff, R.I.; Segal, D.L.; Hersen, M.; Van Hasselt, V.B. Psychometric Properties and Diagnostic Utility of the Beck Anxiety Inventory and the State-Trait Anxiety Inventory with Older Adult Psychiatric Outpatients. J. Anxiety Disord. 1997, 11, 33–47. [Google Scholar] [CrossRef] [PubMed]
- McCracken, L.M.; Zayfert, C.; Gross, R.T. The Pain Anxiety Symptoms Scale: Development and Validation of a Scale to Measure Fear of Pain. Pain 1992, 50, 67–73. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, A.; Esteve, R.; Ramírez-Maestre, C. The Spanish Version of the Pain Anxiety Symptoms Scale (PASS-20): Preliminary Data on Its Reliability, Validity and Factorial Structure. Eur. J. Pain Suppl. 2011, 5, 265. [Google Scholar] [CrossRef]
- McNeil, D.W.; Rainwater, A.J. Development of the Fear of Pain Questionnaire-III. J. Behav. Med. 1998, 21, 389–410. [Google Scholar] [CrossRef]
- Osman, A.; Breitenstein, J.L.; Barrios, F.X.; Gutierrez, P.M.; Kopper, B.A. The Fear of Pain Questionnaire-III: Further Reliability and Validity with Nonclinical Samples. J. Behav. Med. 2002, 25, 155–173. [Google Scholar] [CrossRef]
- Mantilla Toloza, S.C.; Gómez-Conesa, A. El Cuestionario Internacional de Actividad Física. Un instrumento adecuado en el seguimiento de la actividad física poblacional. Rev. Iberoam. Fisioter. Kinesiol. 2007, 10, 48–52. [Google Scholar] [CrossRef]
- Reezigt, R.R.; Kielstra, S.C.; Coppieters, M.W.; Scholten-Peeters, G.G.M. No Relevant Differences in Conditioned Pain Modulation Effects between Parallel and Sequential Test Design. A Cross-Sectional Observational Study. PeerJ 2021, 9, e12330. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, G.K. Analysis of Cross-over Studies with Missing Data. Stat. Methods Med. Res. 2015, 24, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Alacreu, H.; López-de-Uralde-Villanueva, I.; Calvo-Lobo, C.; Fernández-Carnero, J.; La Touche, R. Clinical Features of Patients with Chronic Non-Specific Neck Pain per Disability Level: A Novel Observational Study. Rev. Assoc. Med. Bras. 2018, 64, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Walton, D.M.; Macdermid, J.C.; Nielson, W.; Teasell, R.W.; Chiasson, M.; Brown, L. Reliability, Standard Error, and Minimum Detectable Change of Clinical Pressure Pain Threshold Testing in People with and without Acute Neck Pain. J. Orthop. Sports Phys. Ther. 2011, 41, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.N.; Rice, D.A.; McNair, P.J. Conditioned Pain Modulation in Populations with Chronic Pain: A Systematic Review and Meta-Analysis. J. Pain 2012, 13, 936–944. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.T.; Deitos, A.; Triñanes Pego, Y.; Fregni, F.; Carrillo-de-la-Peña, M.T. Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. J. Pain 2018, 19, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Romano, A.; Fernández-Carnero, J.; González-Zamorano, Y.; Rodríguez-Lagos, L.; Alguacil-Diego, I.M.; Molina-Álvarez, M.; Tejera, D.M.; Mercado-Romero, F. Manual Therapy Effects on Nonspecific Neck Pain Are Not Mediated by Mechanisms Related to Conditioned Pain Modulation: A Randomized Clinical Trial. J. Clin. Med. 2023, 12, 3894. [Google Scholar] [CrossRef]
- Nijs, J.; George, S.Z.; Clauw, D.J.; Fernández-de-las-Peñas, C.; Kosek, E.; Ickmans, K.; Fernández-Carnero, J.; Polli, A.; Kapreli, E.; Huysmans, E.; et al. Central Sensitisation in Chronic Pain Conditions: Latest Discoveries and Their Potential for Precision Medicine. Lancet Rheumatol. 2021, 3, e383–e392. [Google Scholar] [CrossRef]
- Oono, Y.; Nie, H.; Matos, R.L.; Wang, K.; Arendt-Nielsen, L. The Inter- and Intra-Individual Variance in Descending Pain Modulation Evoked by Different Conditioning Stimuli in Healthy Men. Scand. J. Pain 2011, 2, 162–169. [Google Scholar] [CrossRef]
- Aparecida da Silva, V.; Galhardoni, R.; Teixeira, M.J.; Ciampi de Andrade, D. Not Just a Matter of Pain Intensity: Effects of Three Different Conditioning Stimuli on Conditioned Pain Modulation Effects. Neurophysiol. Clin. 2018, 48, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Ritchie, C.; Pedler, A.; McCamley, K.; Roberts, K.; Sterling, M. Exercise Induced Hypoalgesia Is Elicited by Isometric, but Not Aerobic Exercise in Individuals with Chronic Whiplash Associated Disorders. Scand. J. Pain 2017, 15, 14–21. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Roscher, S.; Strian, F. Inhibitory Effects Do Not Depend on the Subjective Experience of Pain during Heterotopic Noxious Conditioning Stimulation (HNCS): A Contribution to the Psychophysics of Pain Inhibition. Eur. J. Pain 2002, 6, 365–374. [Google Scholar] [CrossRef]
- Graeff, P.; Itter, A.; Wach, K.; Ruscheweyh, R. Inter-Individual Differences Explain More Variance in Conditioned Pain Modulation Than Age, Sex and Conditioning Stimulus Intensity Combined. Brain Sci. 2021, 11, 1186. [Google Scholar] [CrossRef]
- Le Bars, D.; Villanueva, L.; Bouhassira, D.; Willer, J.C. Diffuse Noxious Inhibitory Controls (DNIC) in Animals and in Man. Patol. Fiziol. Eksp. Ter. 1992, 4, 55–65. [Google Scholar] [CrossRef]
- Hoeger Bement, M.K.; Dicapo, J.; Rasiarmos, R.; Hunter, S.K. Dose Response of Isometric Contractions on Pain Perception in Healthy Adults. Med. Sci. Sports Exerc. 2008, 40, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Shepanski, M.A.; Ruble, S.B.; Valic, Z.; Buckwalter, J.B.; Clifford, P.S. Intensity and Duration Threshold for Aerobic Exercise-Induced Analgesia to Pressure Pain. Arch. Phys. Med. Rehabil. 2004, 85, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Pozsgai, M.; Udvarácz, K.; Péter, I.A.; Than, P.; Nusser, N. Effect of Single End-Range and Not End-Range Maitland Mobilization on Pressure Pain Threshold and Functional Measures in Knee Osteoarthritis: Randomised, Controlled Clinical Trial. Eur. J. Phys. Rehabil. Med. 2022, 58, 774–783. [Google Scholar] [CrossRef]
- Snodgrass, S.J.; Rivett, D.A.; Sterling, M.; Vicenzino, B. Dose Optimization for Spinal Treatment Effectiveness: A Randomized Controlled Trial Investigating the Effects of High and Low Mobilization Forces in Patients with Neck Pain. J. Orthop. Sports Phys. Ther. 2014, 44, 141–152. [Google Scholar] [CrossRef]
- Neugebauer, V.; Li, W.; Bird, G.C.; Han, J.S. The Amygdala and Persistent Pain. Neuroscientist 2004, 10, 221–234. [Google Scholar] [CrossRef]
- Hughes, J.W.; Watkins, L.; Blumenthal, J.A.; Kuhn, C.; Sherwood, A. Depression and Anxiety Symptoms Are Related to Increased 24-Hour Urinary Norepinephrine Excretion among Healthy Middle-Aged Women. J. Psychosom. Res. 2004, 57, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Horjales-Araujo, E.; Demontis, D.; Lund, E.K.; Finnerup, N.B.; Børglum, A.D.; Jensen, T.S.; Svensson, P.; Vase, L. Polymorphism in Serotonin Receptor 3B Is Associated with Pain Catastrophizing. PLoS ONE 2013, 8, e78889. [Google Scholar] [CrossRef] [PubMed]
- Naugle, K.M.; Riley, J.L. Self-Reported Physical Activity Predicts Pain Inhibitory and Facilitatory Function. Med. Sci. Sports Exerc. 2014, 46, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Geva, N.; Defrin, R. Enhanced Pain Modulation among Triathletes: A Possible Explanation for Their Exceptional Capabilities. Pain 2013, 154, 2317–2323. [Google Scholar] [CrossRef]
- Naugle, K.M.; Ohlman, T.; Naugle, K.E.; Riley, Z.A.; Keith, N.R. Physical Activity Behavior Predicts Endogenous Pain Modulation in Older Adults. Pain 2017, 158, 383–390. [Google Scholar] [CrossRef]


| TRIAL 1 (n = 23) | TRIAL 2 (n = 24) | TRIAL 3 (n = 24) | Statistic | Between Groups p-Value | ||
|---|---|---|---|---|---|---|
| Sex | Female, No. (%) | 13 (56.5%) | 14 (58.3%) | 8 (33.3%) | Chi2 = 3.71 | 0.156 |
| Male, No. (%) | 10 (43.5%) | 10 (41.7%) | 16 (66.7%) | |||
| Age (y) | Mean (SD) | 24.8 (6.2) | 23.6 (2.8) | 23.9 (5.5) | H = 0.34 | 0.843 |
| Median (IQR) | 23.3 (22.1, 25.5) | 23.6 (21.4, 25.3) | 23.4 (20.3, 25.3) | |||
| Height (m) | Mean (SD) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) | F = 2.72 | 0.073 |
| Median (IQR) | 1.7 (1.6, 1.8) | 1.7 (1.6, 1.8) | 1.7 (1.7, 1.8) | |||
| Weight (kg) | Mean (SD) | 64.1 (11.5) | 66.1 (14) | 67.7 (9.3) | F = 0.54 | 0.586 |
| Median (IQR) | 62 (55, 70) | 62.5 (56.5, 75.5) | 68 (63, 72.8) | |||
| BMI (kg/m2) | Mean (SD) | 22.5 (3) | 22.8 (2.9) | 22.3 (2.2) | F = 0.23 | 0.793 |
| Median (IQR) | 22.6 (19.9, 24.9) | 22.7 (20.1, 24.8) | 21.8 (20.6, 23.9) | |||
| PCS (0–52) | Mean (SD) | 5.7 (6.1) | 7.7 (7.7) | 6.8 (8.1) | H = 0.88 | 0.645 |
| Median (IQR) | 3.5 (0, 11) | 6 (1, 14) | 4.5 (0, 10) | |||
| STAI-S (0–60) | Mean (SD) | 25.7 (3.3) | 25.8 (4.3) | 25.5 (4.2) | F = 0.03 | 0.968 |
| Median (IQR) | 26.0 (22, 29) | 26.5 (22.5, 29) | 25.5 (22, 29) | |||
| PASS-20 (0–100) | Mean (SD) | 17.7 (16.3) | 26 (22.4) | 20.6 (14.3) | H = 1.90 | 0.388 |
| Median (IQR) | 12 (4, 33) | 18.5 (8.5, 35) | 16 (9.5, 32) | |||
| FPQ-III (0–45) | Mean (SD) | 17.9 (5.5) | 20.5 (5.7) | 18.8 (5.4) | F = 1.27 | 0.289 |
| Median (IQR) | 19 (14, 21) | 19.5 (16.5, 24) | 19 (16, 22) | |||
| IPAQ (MET min/week) | Mean (SD) | 2743 (1380) | 3706 (2881) | 3340 (2095) | H = 0.50 | 0.779 |
| Median (IQR) | 2671 (1838, 3680) | 3439 (1386, 4080) | 2853 (1515, 5484) | |||
| PPT (Kg/cm2) | Mean (SD) | 7.97 (2.28) | 7.58 (2.51) | 7.42 (2.28) | F = 0.32 | 0.724 |
| Median (IQR) | 7.98 (6.04, 9.64) | 7.32 (5.47, 9.08) | 6.98 (6.15, 8.93) |
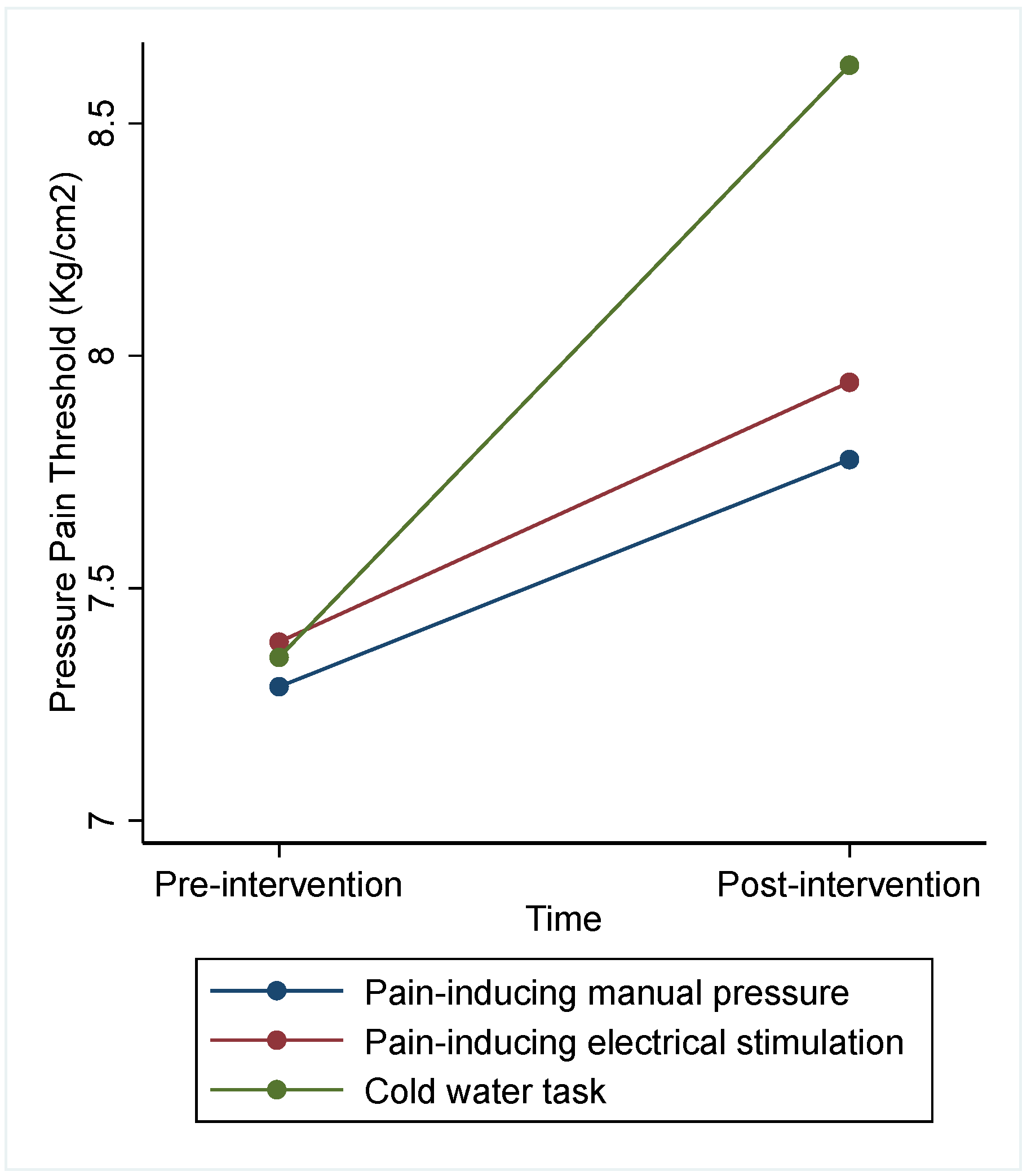
| Intervention | Pain-Inducing Manual Pressure (n = 20) | Pain-Inducing Electrical Stimulation (n = 21) | CPT (n = 22) | Difference between Interventions | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (DS) | Mean (DS) | Chi2 (p Value) | Differences (Bonferroni CI 95%) | |
| PPT pre-intervention | 7.29 (2.61) | 7.38 (2.13) | 7.35 (2.09) | 7.76 (0.021) * | Press vs. elec: −0.06 (0.82; 0.71) Press vs. cold: −0.77 (−1.53; −0.02) * Elec vs. cold: −0.72 (−1.46; 0.02) |
| PPT post-intervention | 7.78 (2.68) | 7.94 (2.46) | 8.63 (2.66) | ||
| Adjusted PPT post-intervention | Marginals (CI 95%) | Marginals (CI 95%) | Marginals (CI 95%) | ||
| 7.86 (7.29; 8.42) | 7.91 (7.36, 8.47) | 8.63 (8.09; 9.17) | |||
| Day | Day 1 (n = 21) | Day 2 (n = 21) | Day 3 (n = 21) | Difference between days | |
| Mean (SD) | Mean (DS) | Mean (DS) | Chi2 (p value) | Differences (Bonferroni CI 95%) | |
| PPT pre-intervention | 7.90 (2.31) | 7.00 (2.26) | 7.13 (2.15) | 2.53 (0.283) | Day 1 vs. Day 2: −0.38 (−1.16; 0.38) Day 1 vs. Day 3: −0.48 (−1.26; 0.38) Day 2 vs. Day 3: −0.09 (−0.84; 0.65) |
| PPT post-intervention | 8.41 (2.45) | 7.86 (2.76) | 8.11 (2.63) | ||
| Adjusted PPT post-intervention | Marginal (CI 95%) | Marginal (CI 95%) | Marginal (CI 95%) | ||
| 7.85 (7.29; 8.41) | 8.24 (7.69; 8.80) | 8.34 (7.79; 8.90) | |||
| Sequence | Seq 1 (n = 7) | Seq 2 (n = 8) | Seq 3 (n = 8) | Difference between sequences | |
| Mean (SD) | Mean (DS) | Mean (DS) | Chi2 (p value) | Differences (Bonferroni CI 95%) | |
| PPT pre-intervention | 8.00 (2.21) | 7.51 (2.32) | 6.58 (2.06) | 0.38 (0.827) | Seq 1 vs. Seq 2: −0.29 (−1.58; 1.00) Seq 1 vs. Seq 3: −0.29 (−1.58; 1.00) Seq 2 vs. 3 Seq: 0.00 (−1.26; 1.26) |
| PPT post-intervention | 8.59 (2.57) | 8.39 (2.73) | 7.47 (2.44) | ||
| Adjusted PPT post-intervention | Marginal (CI 95%) | Marginal (CI 95%) | Marginal (CI 95%) | ||
| 7.95 (7.19; 8.71) | 8.24 (7.50; 8.98) | 8.24 (7.52; 8.96) | |||
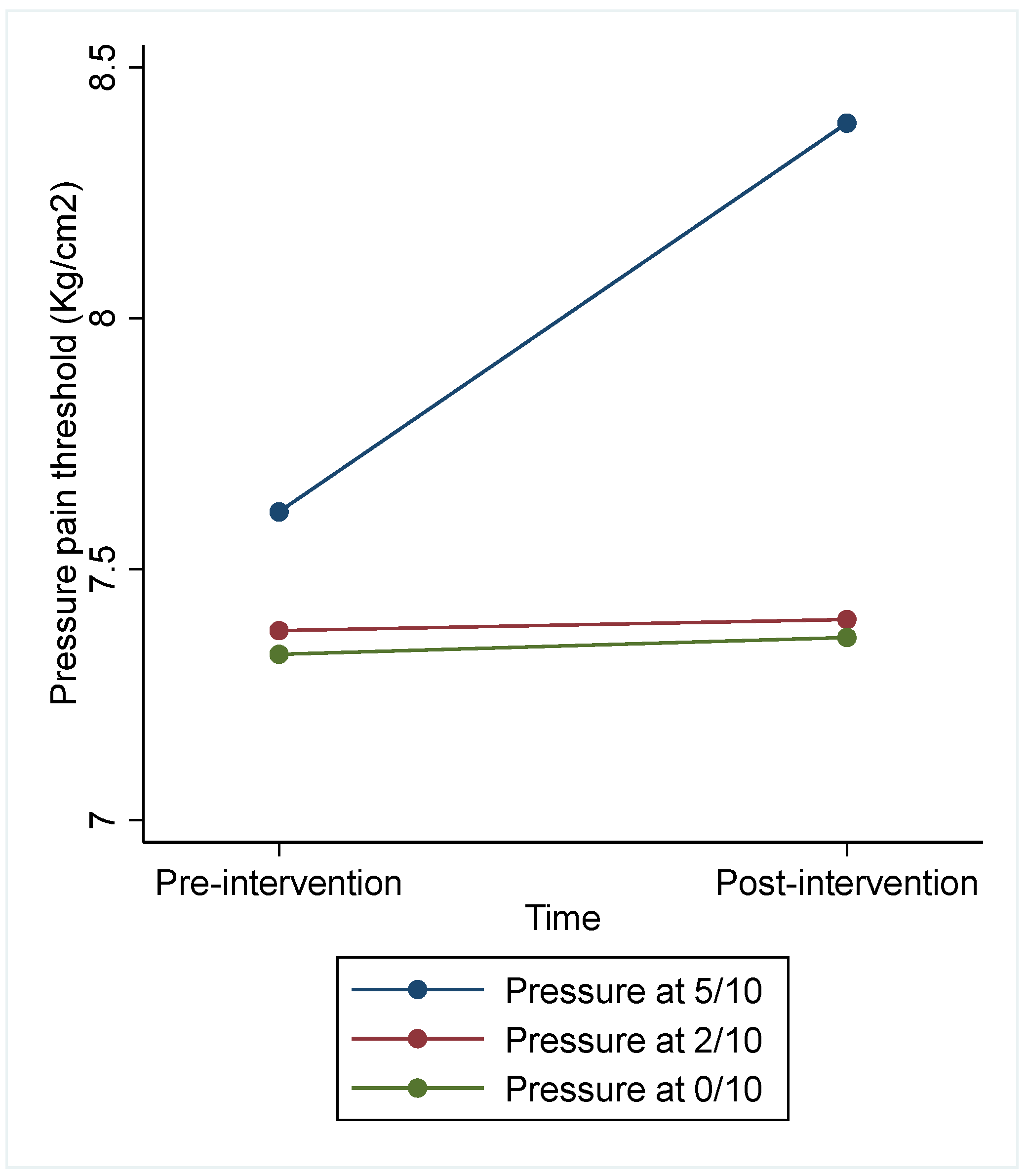
| Intervention | Pressure 5/10 (n = 22) | Pressure 2/10 (n = 23) | Pressure 0/10 (n = 23) | Difference between Interventions | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (DS) | Mean (DS) | Chi2 (p Value) | Differences (Bonferroni CI 95%) | |
| PPT pre-intervention | 7.61 (2.58) | 7.38 (2.57) | 7.33 (2.04) | 9.72 (0.008) * | 5/10 vs. 2/10: 0.79 (0.09; 1.48) * 5/10 vs. 0/10: 0.79 (0.09; 1.49) * 2/10 vs. 0/10: 0.01 (−0.68; 0.69) |
| PPT post-intervention | 8.39 (2.22) | 7.40 (2.39) | 7.36 (2.13) | ||
| Adjusted PPT post-intervention | Marginal (CI 95%) | Marginal (CI 95%) | Marginal (CI 95%) | ||
| 8.24 (7.83; 8.65) | 7.46 (7.06, 7.85) | 7.45 (7.05; 7.85) | |||
| Day | Day 1 (n = 24) | Day 2 (n = 23) | Day 3 (n = 21) | Difference between days | |
| Mean (SD) | Mean (DS) | Mean (DS) | Chi2 (p value) | Differences (Bonferroni CI 95%) | |
| PPT pre-intervention | 7.58 (2.51) | 7.24 (2.43) | 7.49 (2.25) | 2.02 (0.364) | Day 1 vs. Day 2: 0.35 (−0.33; 1.04) Day 1 vs. Day 3: −0.00 (−0.70; 0.69) Day 2 vs. Day 3: −0.36 (−1.06; 0.35) |
| PPT post-intervention | 7.96 (2.49) | 7.29 (2.14) | 7.88 (2.18) | ||
| Adjusted PPT post-intervention | Marginal (CI 95%) | Marginal (CI 95%) | Marginal (CI 95%) | ||
| 7.83 (7.44; 8.22) | 7.47 (7.07; 7.87) | 7.83 (7.41; 8.24) | |||
| Sequence | Seq 1 (n = 8) | Seq 2 (n = 8) | Seq 3 (n = 8) | Difference between sequences | |
| Mean (SD) | Mean (DS) | Mean (DS) | Chi2 (p value) | Differences (Bonferroni CI 95%) | |
| PPT pre-intervention | 7.35 (2.75) | 7.26 (2.50) | 7.71 (1.83) | 1.10 (0.577) | Seq 1 vs. Seq 2: −0.29 (−0.98; 0.39) Seq 1 vs. Seq 3: −0.20 (−0.90; 0.48) Seq 2 vs. Seq 3: 0.08 (−0.61; 0.78) |
| PPT post-intervention | 7.48 (2.54) | 7.67 (2.42) | 7.98 (1.83) | ||
| Adjusted PPT post-intervention | Marginal (CI 95%) | Marginal (CI 95%) | Marginal (CI 95%) | ||
| 7.54 (7.14; 7.94) | 7.83 (7.44; 8.23) | 7.75 (7.34; 8.16) | |||
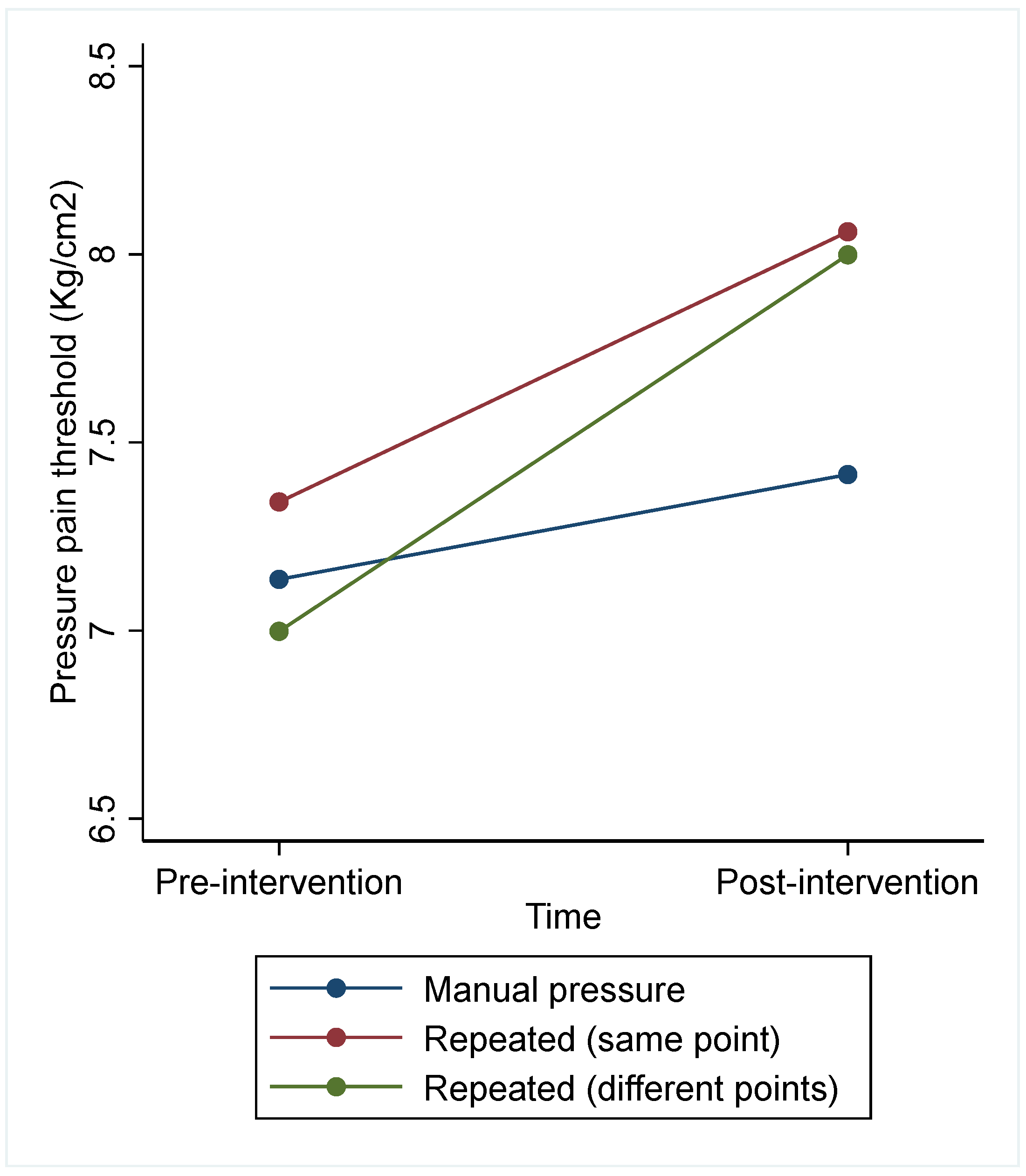
| Intervention | 1. A Manual Pressure Stimulus (n = 22) | 2. Repeated Manual Pressure (Same Point) (n = 23) | 3. Repeated Manual Pressure (Different Points) (n = 22) | Difference between Interventions | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (DS) | Mean (DS) | Chi2 (p Value) | Differences (Bonferroni CI 95%) | |
| PPT pre-intervention | 7.14 (2.08) | 7.34 (2.03) | 7.00 (1.94) | 5.51 (0.064) | 1 vs. 2: −0.45 (−1.19; 0.28) 1 vs. 3: −0.72 (−1.46; 0.02) 2 vs. 3: −0.27 (−1.01; 0.47) |
| PPT post-intervention | 7.41 (1.99) | 8.06 (2.21) | 8.00 (2.60) | ||
| Adjusted PPT post-intervention | Marginal (CI 95%) | Marginal (CI 95%) | Marginal (CI 95%) | ||
| 7.44 (7.01; 7.87) | 7.89 (7.47, 8.31) | 8.16 (7.73; 8.59) | |||
| Day | Day 1 (n = 23) | Day 2 (n = 22) | Day 3 (n = 22) | Difference between days | |
| Mean (SD) | Mean (DS) | Mean (DS) | Chi2 (p value) | Differences (Bonferroni CI 95%) | |
| PPT pre-intervention | 7.24 (2.14) | 6.88 (1.78) | 7.36 (2.09) | 0.48 (0.787) | Day 1 vs. Day 2: 0.20 (−0.54; 0.94) Day 1 vs. Day 3: 0.03 (−0.71; 0.76) Day 2 vs. Day 3: −0.17 (−0.92; 0.58) |
| PPT post-intervention | 7.96 (2.15) | 7.43 (2.05) | 8.09 (2.60) | ||
| Adjusted PPT post-intervention | Marginal (CI 95%) | Marginal (CI 95%) | Marginal (CI 95%) | ||
| 7.90 (7.48; 8.32) | 7.70 (7.27; 8.14) | 7.88 (7.44; 8.31) | |||
| Sequence | Seq 1 (n = 8) | Seq 2 (n = 8) | Seq 3 (n = 8) | Difference between sequences | |
| Mean (SD) | Mean (DS) | Mean (DS) | Chi2 (p value) | Differences (Bonferroni CI 95%) | |
| PPT pre-intervention | 8.01 (1.81) | 7.37 (2.07) | 5.97 (1.55) | 0.97 (0.616) | Seq 1 vs. Seq 2: 0.25 (−0.48; 0.98) Seq 1 vs. Seq 3: 0.29 (−0.51; 1.11) Seq 2 vs. Seq 3: 0.05 (−0.84; 0.74) |
| PPT post-intervention | 8.84 (1.92) | 7.96 (2.51) | 6.53 (1.74) | ||
| Adjusted PPT post-intervention | Marginals (CI 95%) | Marginals (CI 95%) | Marginals (CI 95%) | ||
| 8.00 (7.58; 8.43) | 7.75 (7.32; 8.18) | 7.71 (7.24; 8.18) | |||
| Variables | n | Beta | SE | p | 95% CI |
|---|---|---|---|---|---|
| Anxiety state | 64 | −0.01 | 0.35 | 0.754 | −0.08, 0.06 |
| PPT pre-intervention | 0.86 | 0.06 | 0.000 * | 0.74, 0.98 | |
| Sex | 0.18 | 0.29 | 0.538 | −0.40, 0.76 | |
| Age | 0.02 | 0.03 | 0.500 | −0.04, 0.08 | |
| Pain anxiety | 64 | −0.01 | 0.01 | 0.568 | −0.02, 0.01 |
| PPT pre-intervention | 0.87 | 0.06 | 0.000 * | −0.04, 0.07 | |
| Sex | 0.25 | 0.32 | 0.435 | −0.39, 0.90 | |
| Age | 0.01 | 0.03 | 0.609 | −0.04, 0.72 | |
| Pain Catastrophizing | 64 | −0.02 | 0.02 | 0.385 | −0.06, 0.02 |
| PPT pre-intervention | 0.86 | 0.06 | 0.000 * | 0.75, 0.98 | |
| Sex | 0.21 | 0.29 | 0.467 | −0.37,0.79 | |
| Age | 0.01 | 0.03 | 0.711 | −0.05, 0.07 | |
| Fear of pain | 64 | 0.03 | 0.03 | 0.310 | −0.3, 0.08 |
| PPT pre-intervention | 0.85 | 0.06 | 0.000 * | 0.73, 0.97 | |
| Sex | 0.10 | 0.29 | 0.736 | −0.48, 0.68 | |
| Age | 0.03 | 0.03 | 0.362 | −0.03, 0.08 | |
| Physical activity | 56 | 0.00 | 0.00 | 0.553 | −0.00, 0.00 |
| PPT pre-intervention | 0.88 | 0.06 | 0.000 * | 0.75, 1.00 | |
| Sex | 0.33 | 0.32 | 0.309 | −0.31, 0.96 | |
| Age | 0.02 | 0.03 | 0.534 | −0.04, 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arribas-Romano, A.; Fernández-Carnero, J.; Rodríguez-Lagos, L.; Molina-Álvarez, M.; Zabala-Zambrano, J.; Lezaun-Hernández, L.; Contreras-Padilla, L.; Mercado, F. CPM-Related Mechanisms Could Play a Key Role in the Effects on Pain Sensitivity Induced by Manual Therapy: Three Crossover Trials Investigating the Effects of Manual Pressure. J. Clin. Med. 2024, 13, 3648. https://doi.org/10.3390/jcm13133648
Arribas-Romano A, Fernández-Carnero J, Rodríguez-Lagos L, Molina-Álvarez M, Zabala-Zambrano J, Lezaun-Hernández L, Contreras-Padilla L, Mercado F. CPM-Related Mechanisms Could Play a Key Role in the Effects on Pain Sensitivity Induced by Manual Therapy: Three Crossover Trials Investigating the Effects of Manual Pressure. Journal of Clinical Medicine. 2024; 13(13):3648. https://doi.org/10.3390/jcm13133648
Chicago/Turabian StyleArribas-Romano, Alberto, Josué Fernández-Carnero, Leonardo Rodríguez-Lagos, Miguel Molina-Álvarez, Jesús Zabala-Zambrano, Lucas Lezaun-Hernández, Lucía Contreras-Padilla, and Francisco Mercado. 2024. "CPM-Related Mechanisms Could Play a Key Role in the Effects on Pain Sensitivity Induced by Manual Therapy: Three Crossover Trials Investigating the Effects of Manual Pressure" Journal of Clinical Medicine 13, no. 13: 3648. https://doi.org/10.3390/jcm13133648
APA StyleArribas-Romano, A., Fernández-Carnero, J., Rodríguez-Lagos, L., Molina-Álvarez, M., Zabala-Zambrano, J., Lezaun-Hernández, L., Contreras-Padilla, L., & Mercado, F. (2024). CPM-Related Mechanisms Could Play a Key Role in the Effects on Pain Sensitivity Induced by Manual Therapy: Three Crossover Trials Investigating the Effects of Manual Pressure. Journal of Clinical Medicine, 13(13), 3648. https://doi.org/10.3390/jcm13133648






