Ultrasound Shear Wave Elastography Evaluation of the Liver and Implications for Perioperative Medicine
Abstract
1. Introduction
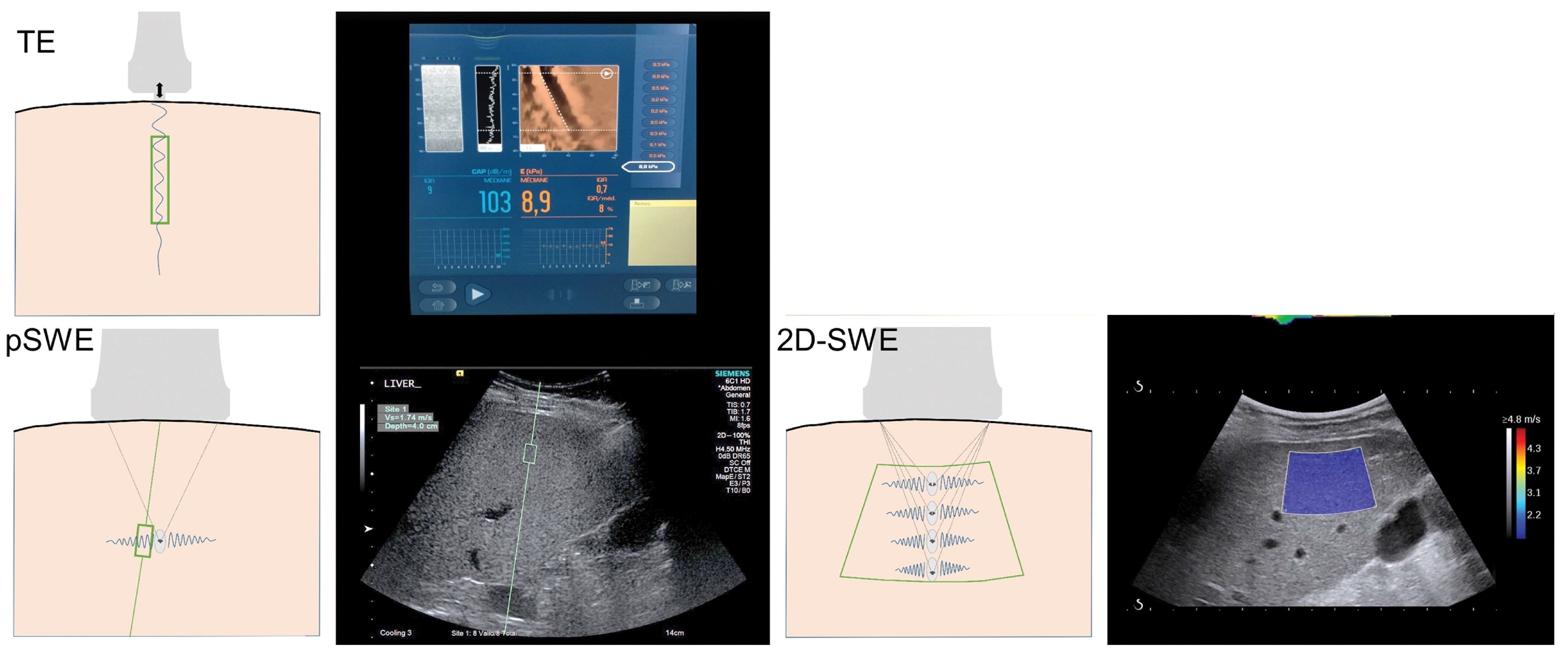
2. Principles of Ultrasound Shear Wave Elastography
3. Clinical Applications of Shear Wave Elastography
3.1. Liver Fibrosis and Inflammation
3.2. MAFLD/MASLD
4. Liver Stiffness and Symptomatic Post Hepatectomy Liver Failure
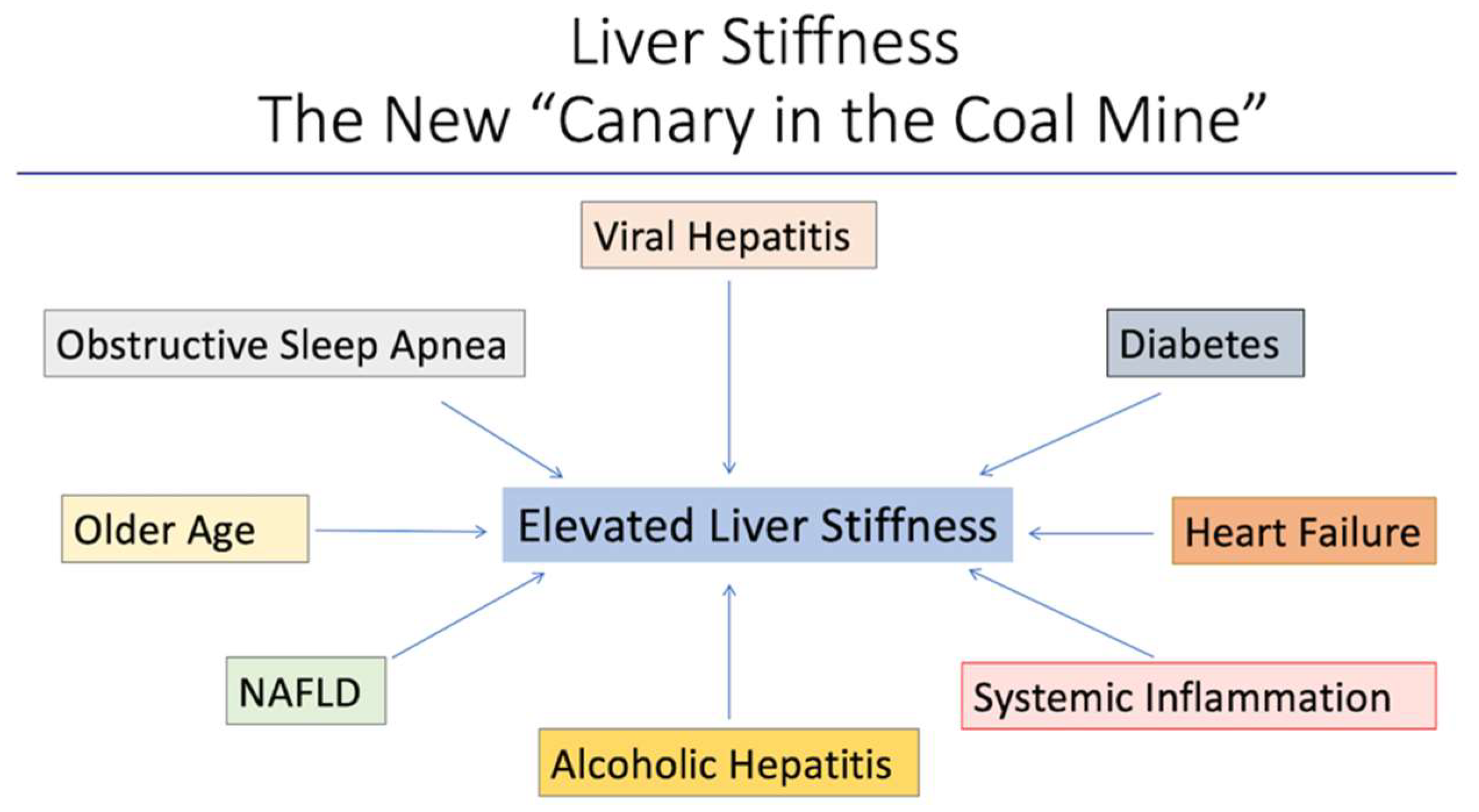
5. The Hepatic Cardiac Axis
6. Congestive Heart Failure
7. Overview of Perioperative Risk for Non-Emergent Cardiac Surgery—Society of Thoracic Surgeons (STS) Risk Calculator
8. Perioperative Risk Stratification by Assessment of Liver Stiffness—How Does It Inform Interventions That Decrease Perioperative Risk
9. Conclusions
Funding
Conflicts of Interest
References
- Bartsch, K.M.; Schleip, R.; Zullo, A.; Hoppe, K.; Klingler, W. The Stiffness Comparison Test: A pilot study to determine inter-individual differences in palpatory skill related to gender, age, and occupation-related experience. J. Bodyw. Mov. Ther. 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Gennisson, J.-L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Ozturk, A.; Olson, M.C.; Samir, A.E.; Venkatesh, S.K. Liver fibrosis assessment: MR and US elastography. Abdom. Radiol. 2022, 47, 3037–3050. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Friedrich-Rust, M.; Poynard, T.; Castera, L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol. Hepatol. 2016, 13, 402–411. [Google Scholar] [CrossRef]
- Kennedy, P.; Wagner, M.; Castéra, L.; Hong, C.W.; Johnson, C.L.; Sirlin, C.B.; Taouli, B. Quantitative Elastography Methods in Liver Disease: Current Evidence and Future Directions. Radiology 2018, 286, 738–763. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Liu, T.; Song, D.; Dong, J.; Zhu, P.; Liu, J.; Liu, W.; Ma, X.; Zhao, L.; Ling, S. Current Understanding of the Pathophysiology of Myocardial Fibrosis and Its Quantitative Assessment in Heart Failure. Front. Physiol. 2017, 8, 238. [Google Scholar] [CrossRef]
- Huang, R.; Fu, P.; Ma, L. Kidney fibrosis: From mechanisms to therapeutic medicines. Signal Transduct. Target. Ther. 2023, 8, 129. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef]
- Santos, A.; Lagares, D. Matrix Stiffness: The Conductor of Organ Fibrosis. Curr. Rheumatol. Rep. 2018, 20, 2. [Google Scholar] [CrossRef]
- Georges, P.C.; Hui, J.; Gombos, Z.; McCormick, M.E.; Wang, A.Y.; Uemura, M.; Mick, R.; Janmey, P.A.; Furth, E.E.; Wells, R.G. Increased stiffness of the rat liver precedes matrix deposition: Implications for fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1147–G1154. [Google Scholar] [CrossRef]
- Mantovani, A.; Turino, T.; Lando, M.G.; Gjini, K.; Byrne, C.D.; Zusi, C.; Ravaioli, F.; Colecchia, A.; Maffeis, C.; Salvagno, G.; et al. Screening for non-alcoholic fatty liver disease using liver stiffness measurement and its association with chronic kidney disease and cardiovascular complications in patients with type 2 diabetes. Diabetes Metab. 2019, 46, 296–303. [Google Scholar] [CrossRef]
- Trzepizur, W.; Boursier, J.; Le Vaillant, M.; Ducluzeau, P.; Dubois, S.; Henni, S.; Abraham, P.; Aubé, C.; Calès, P.; Gagnadoux, F. Increased liver stiffness in patients with severe sleep apnoea and metabolic comorbidities. Eur. Respir. J. 2018, 51, 1800601. [Google Scholar] [CrossRef]
- Taniguchi, T.; Ohtani, T.; Kioka, H.; Tsukamoto, Y.; Onishi, T.; Nakamoto, K.; Katsimichas, T.; Sengoku, K.; Chimura, M.; Hashimoto, H.; et al. Liver Stiffness Reflecting Right-Sided Filling Pressure Can Predict Adverse Outcomes in Patients with Heart Failure. JACC Cardiovasc. Imaging 2019, 12, 955–964. [Google Scholar] [CrossRef]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. Liver fibrosis by FibroScan® independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2019, 40, 347–354. [Google Scholar] [CrossRef]
- Käräjämäki, A.J.; Kettunen, O.; Lepojärvi, S.; Koivurova, O.; Kesäniemi, Y.A.; Huikuri, H.; Ukkola, O. Presence of atrial fibrillation is associated with liver stiffness in an elderly Finnish population. PLoS ONE 2017, 12, e0173855. [Google Scholar] [CrossRef]
- Foucher, J.; Chanteloup, E.; Vergniol, J.; Castéra, L.; Le Bail, B.; Adhoute, X.; Bertet, J. Diagnosis of cirrhosis by transient elastography (FibroScan): A prospective study. Gut 2006, 55, 403–408. [Google Scholar] [CrossRef]
- de Franchis, R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridi, M.; Hiriart, J.B.; Lupsor-Platon, M.; Bronte, F.; Boursier, J.; Elshaarawy, O.; Marra, F.; Thiele, M.; Markakis, G.; Payance, A.; et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J. Hepatol. 2021, 74, 1109–1116. [Google Scholar] [CrossRef]
- Canivet, C.M.; Boursier, J. Screening for Liver Fibrosis in the General Population: Where Do We Stand in 2022? Diagnostics 2022, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Caballería, L.; Pera, G.; Arteaga, I.; Rodríguez, L.; Alumà, A.; Morillas, R.M.; de la Ossa, N.; Díaz, A.; Expósito, C.; Miranda, D.; et al. High Prevalence of Liver Fibrosis Among European Adults with Unknown Liver Disease: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2018, 16, 1138–1145.e5. [Google Scholar] [CrossRef] [PubMed]
- Braude, M.; Roberts, S.S.; Majeed, A.A.; Lubel, J.J.; Prompen, J.; Dev, A.A.; Sievert, W.; Bloom, S.; Gow, P.; Kemp, W.W. Liver stiffness (Fibroscan®) is a predictor of all-cause mortality in people with non-alcoholic fatty liver disease. Liver Int. 2022, 43, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, W.; Dufour, J.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Gofton, C.; Upendran, Y.; Zheng, M.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17. [Google Scholar] [CrossRef] [PubMed]
- Van Kleef, L.A.; Ayada, I.; Alferink, L.J.M.; Pan, Q.; De Knegt, R.J. Metabolic dysfunction–associated fatty liver disease improves detection of high liver stiffness: The Rotterdam Study. Hepatology 2021, 75, 419–429. [Google Scholar] [CrossRef]
- Tsutsumi, T.; Eslam, M.; Kawaguchi, T.; Yamamura, S.; Kawaguchi, A.; Nakano, D.; Koseki, M.; Yoshinaga, S.; Takahashi, H.; Anzai, K.; et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: Generalized estimating equation approach. Hepatol. Res. 2021, 51, 1115–1128. [Google Scholar] [CrossRef]
- Guerreiro, G.T.S.; Longo, L.; Fonseca, M.A.; De Souza, V.E.G.; Álvares-Da-Silva, M.R. Does the risk of cardiovascular events differ between biopsy-proven NAFLD and MAFLD? Hepatol. Int. 2021, 15, 380–391. [Google Scholar] [CrossRef]
- Henry, L.; Paik, J.M.; Younossi, Z.M. The Transition in Terminology: From Non-alcoholic Fatty Liver Disease (NAFLD) to Metabolic Dysfunction-associated Fatty Liver Disease (MAFLD) to Metabolic-associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Paik, J.M.; Stepanova, M.; Ong, J.; Alqahtani, S.; Henry, L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J. Hepatol. 2024, 80, 694. [Google Scholar] [CrossRef]
- Hagström, H.; Vessby, J.; Ekstedt, M.; Shang, Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J. Hepatol. 2023, 80, e76–e77. [Google Scholar] [CrossRef]
- Boeckmans, J.; Sandrin, L.; Knackstedt, C.; Schattenberg, J.M. Liver stiffness as a cornerstone in heart disease risk assessment. Liver Int. 2023, 44, 344–356. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Cescon, M.; Colecchia, A.; Cucchetti, A.; Peri, E.; Montrone, L.; Ercolani, G.; Festi, D.; Pinna, A.D. Value of Transient Elastography Measured with Fibroscan in Predicting the Outcome of Hepatic Resection for Hepatocellular Carcinoma. Ann. Surg. 2008, 256, 706–712. [Google Scholar] [CrossRef]
- Long, H.; Zhong, X.; Su, L.; Huang, T.; Duan, Y.; Ke, W.; Xie, X.; Lin, M. Liver Stiffness Measured by Two-Dimensional Shear Wave Elastography for Predicting Symptomatic Post-hepatectomy Liver Failure in Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2022, 29, 327–336. [Google Scholar] [CrossRef]
- Long, H.; Peng, C.; Ding, H.; Zheng, Y.; Zhou, J.; Chen, W.; Zhong, X.; Shi, Y.; Duan, Y.; Xie, X.; et al. Predicting symptomatic post-hepatectomy liver failure in patients with hepatocellular carcinoma: Development and validation of a preoperative nomogram. Eur. Radiol. 2023, 33, 7665–7674. [Google Scholar] [CrossRef] [PubMed]
- Kleef, L.A.; Sonneveld, M.J.; Zhu, F.; Ikram, M.A.; Kavousi, M.; Knegt, R.J. Liver stiffness is associated with excess mortality in the general population driven by heart failure: The Rotterdam Study. Liver Int. 2023, 43, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- van Kleef, L.A.; Lu, Z.; Ikram, M.A.; de Groot, N.M.S.; Kavousi, M.; de Knegt, R.J. Liver stiffness not fatty liver disease is associated with atrial fibrillation: The Rotterdam study. J. Hepatol. 2022, 77, 931–938. [Google Scholar] [CrossRef]
- Ciardullo, S.; Cannistraci, R.; Mazzetti, S.; Mortara, A.; Perseghin, G. Nonalcoholic Fatty Liver Disease, Liver Fibrosis and Cardiovascular Disease in the Adult US Population. Front. Endocrinol. 2021, 12, 711484. [Google Scholar] [CrossRef]
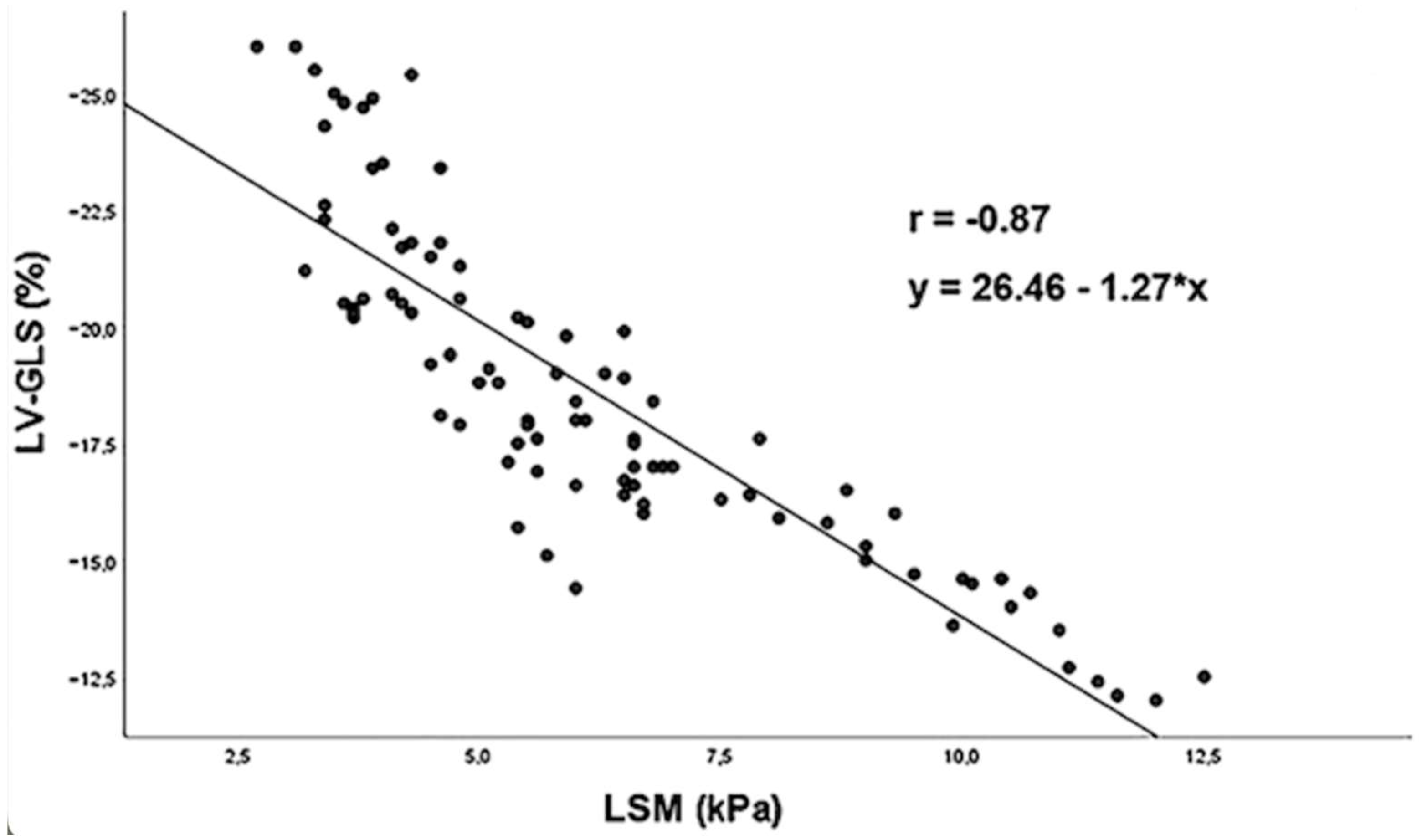
- Sonaglioni, A.; Cerini, F.; Cerrone, A.; Argiento, L.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; Rumi, M.G.; Viganò, M. Liver stiffness measurement identifies subclinical myocardial dysfunction in non-advanced non-alcoholic fatty liver disease patients without overt heart disease. Intern. Emerg. Med. 2022, 17, 1425–1438. [Google Scholar] [CrossRef]
- Millonig, G.; Friedrich, S.; Adolf, S.; Fonouni, H.; Golriz, M.; Mehrabi, A.; Stiefel, P.; Pöschl, G.; Büchler, M.W.; Seitz, H.K.; et al. Liver stiffness is directly influenced by central venous pressure. J. Hepatol. 2010, 52, 206–210. [Google Scholar] [CrossRef]
- Villemain, O.; Sitefane, F.; Pernot, M.; Malekzadeh-Milani, S.; Tanter, M.; Bonnet, D.; Boudjemline, Y. Toward Noninvasive Assessment of CVP Variations Using Real-Time and Quantitative Liver Stiffness Estimation. JACC Cardiovasc. Imaging 2017, 10, 1285–1286. [Google Scholar] [CrossRef]
- Taniguchi, T.; Sakata, Y.; Ohtani, T.; Mizote, I.; Takeda, Y.; Asano, Y.; Masuda, M.; Minamiguchi, H.; Kanzaki, M.; Ichibori, Y.; et al. Usefulness of Transient Elastography for Noninvasive and Reliable Estimation of Right-Sided Filling Pressure in Heart Failure. Am. J. Cardiol. 2014, 113, 552–558. [Google Scholar] [CrossRef]
- Soloveva, A.; Kobalava, Z.; Fudim, M.; Ambrosy, A.P.; Villevalde, S.; Bayarsaikhan, M.; Garmash, I.; Naumenko, M. Relationship of Liver Stiffness with Congestion in Patients Presenting with Acute Decompensated Heart Failure. J. Card. Fail. 2019, 25, 176–187. [Google Scholar] [CrossRef]
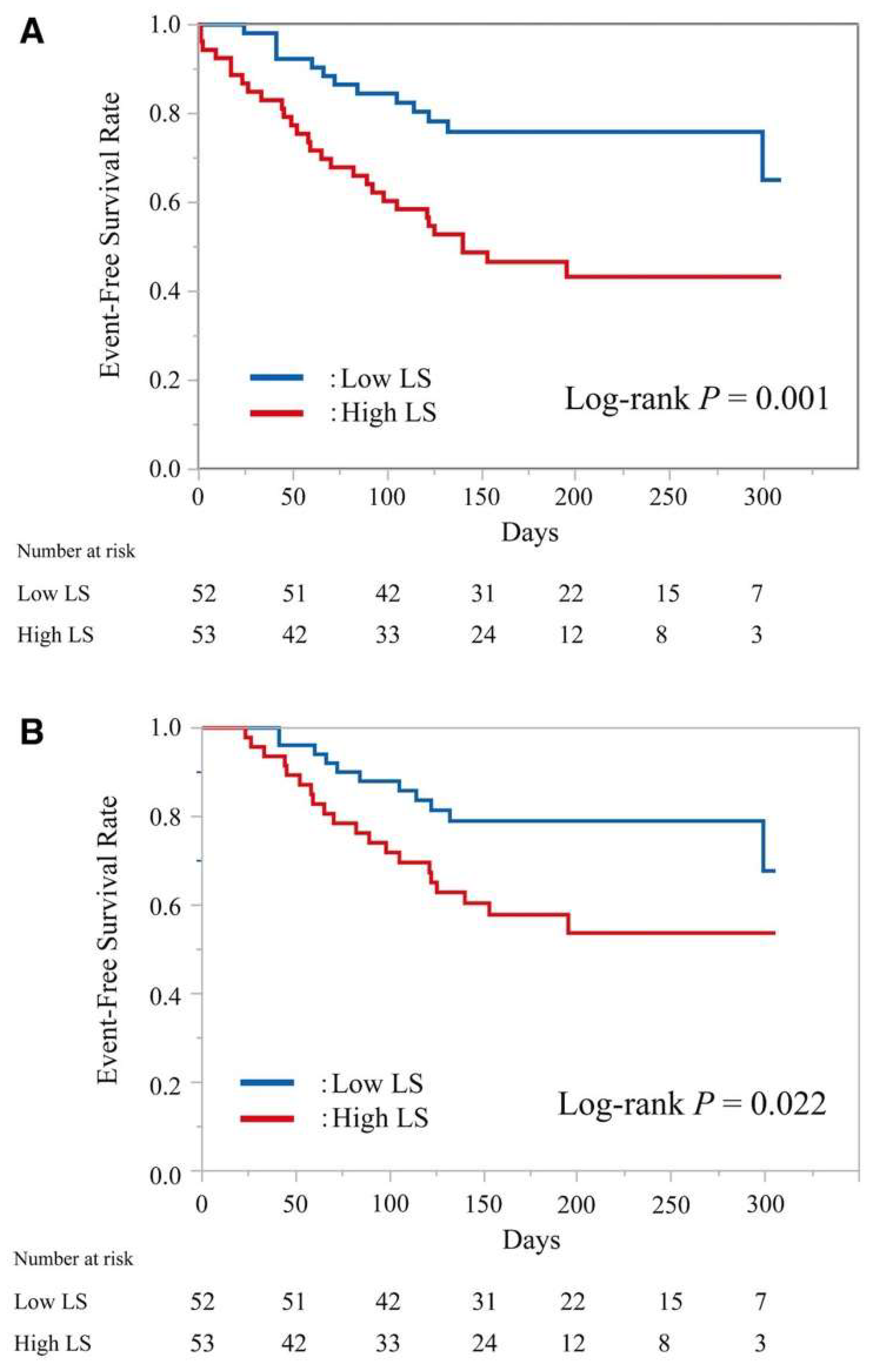
- Saito, Y.; Matsumoto, N.; Aizawa, Y.; Fukamachi, D.; Kitano, D.; Toyama, K.; Fujito, H.; Sezai, A.; Okumura, Y. Prognostic Value of Liver Stiffness Measured by Two-Dimensional Elastography in Acute Decompensated Heart Failure with Preserved Ejection Fraction. Int. Heart J. 2021, 62, 821–828. [Google Scholar] [CrossRef]
- Saito, Y.; Kato, M.; Nagashima, K.; Monno, K.; Aizawa, Y.; Okumura, Y.; Matsumoto, N.; Moriyama, M.; Hirayama, A. Prognostic Relevance of Liver Stiffness Assessed by Transient Elastography in Patients with Acute Decompensated Heart Failure. Circ. J. 2018, 82, 1822–1829. [Google Scholar] [CrossRef]
- STS Short-Term/Operative Risk Calculator Adult Cardiac Surgery Database—All Procedures. Available online: https://acsdriskcalc.research.sts.org (accessed on 2 December 2023).
- Garatti, A.; Daprati, A.; Cottini, M.; Russo, C.F.; Dalla Tomba, M.; Troise, G.; Salsano, A.; Santini, F.; Scrofani, R.; Nicolò, F.; et al. Cardiac Surgery in Patients with Liver Cirrhosis (CASTER) Study: Early and Long-Term Outcomes. Ann. Thorac. Surg. 2021, 111, 1242–1251. [Google Scholar] [CrossRef]
- Macaron, C.; Hanouneh, I.A.; Suman, A.; Lopez, R.; Johnston, D.; Carey, W.W. Safety of Cardiac Surgery for Patients with Cirrhosis and Child–Pugh Scores Less Than 8. Clin. Gastroenterol. Hepatol. 2012, 10, 535–539. [Google Scholar] [CrossRef]
- Ostovar, R.; Schroeter, F.; Erb, M.; Hartrumpf, M.; Chopsonidou, S.; Albes, J.M. Liver cirrhosis: Still an elusive risk factor in the current EuroSCORE system. Eur. J. Cardio-Thorac. Surg. 2022, 62, ezac128. [Google Scholar] [CrossRef]
- Suffredini, G.; Slowey, C.; Sun, J.; Gao, W.D.; Choi, C.W.; Aziz, H.; Kilic, A.; Schena, S.; Lawton, J.; Hamilton, J.P.; et al. Preoperative Liver Stiffness is Associated with Hospital Length of Stay After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2022, 36, 4093–4099. [Google Scholar] [CrossRef]
- Nguyen, T.; Wardell, R.; Chitturi, S.; Teoh, N.; Farrell, G. When the liver gets stiff, the tough get moving. J. Gastroenterol. Hepatol. 2020, 35, 953–959. [Google Scholar] [CrossRef]
- Niezen, S.; Mehta, M.; Jiang, Z.G.; Tapper, E.B. Coffee Consumption Is Associated with Lower Liver Stiffness: A Nationally Representative Study. Clin. Gastroenterol. Hepatol. 2022, 20, 2032–2040.e6. [Google Scholar] [CrossRef]
- Leung, C.W.; Tapper, E.B. Sugar-sweetened Beverages Are Associated with Increased Liver Stiffness and Steatosis Among Apparently Healthy Adults in the United States. Clin. Gastroenterol. Hepatol. 2022, 20, 959–961.e1. [Google Scholar] [CrossRef]
- Newsome, P.N.; Ambery, P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J. Hepatol. 2023, 79, 1557–1565. [Google Scholar] [CrossRef]
- Gracen, L.; Muthukumara, W.; Aikebuse, M.; Russell, A.; O’Beirne, J.; Irvine, K.M.; Williams, S.; Puri, G.; Valery, P.C.; Hayward, K.L.; et al. Lower prevalence of elevated liver stiffness measurements in people with type 2 diabetes taking sodium-glucose co-transporter 2 inhibitors or glucagon-like peptide-1 receptor agonists. Ann. Hepatol. 2023, 28, 101142. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Facciorusso, A.; Loomba, R.; Falck-Ytter, Y.T. Magnitude and Kinetics of Decrease in Liver Stiffness After Antiviral Therapy in Patients with Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 27–38.e4. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Kaplan, L.M.; Frías, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman, M.L. Triple–Hormone-Receptor Agonist Retatrutide for Obesity—A Phase 2 Trial. N. Engl. J. Med. 2023, 389, 514–526. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suffredini, G.; Gao, W.D.; Dodd-o, J.M. Ultrasound Shear Wave Elastography Evaluation of the Liver and Implications for Perioperative Medicine. J. Clin. Med. 2024, 13, 3633. https://doi.org/10.3390/jcm13133633
Suffredini G, Gao WD, Dodd-o JM. Ultrasound Shear Wave Elastography Evaluation of the Liver and Implications for Perioperative Medicine. Journal of Clinical Medicine. 2024; 13(13):3633. https://doi.org/10.3390/jcm13133633
Chicago/Turabian StyleSuffredini, Giancarlo, Wei Dong Gao, and Jeffrey M. Dodd-o. 2024. "Ultrasound Shear Wave Elastography Evaluation of the Liver and Implications for Perioperative Medicine" Journal of Clinical Medicine 13, no. 13: 3633. https://doi.org/10.3390/jcm13133633
APA StyleSuffredini, G., Gao, W. D., & Dodd-o, J. M. (2024). Ultrasound Shear Wave Elastography Evaluation of the Liver and Implications for Perioperative Medicine. Journal of Clinical Medicine, 13(13), 3633. https://doi.org/10.3390/jcm13133633




