Clinical Utility of Disease Activity Indices in Predicting Short-Term Response to Biologics in Patients with Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Collection of Blood Samples and Blood Analysis
2.3. Statistical Analysis
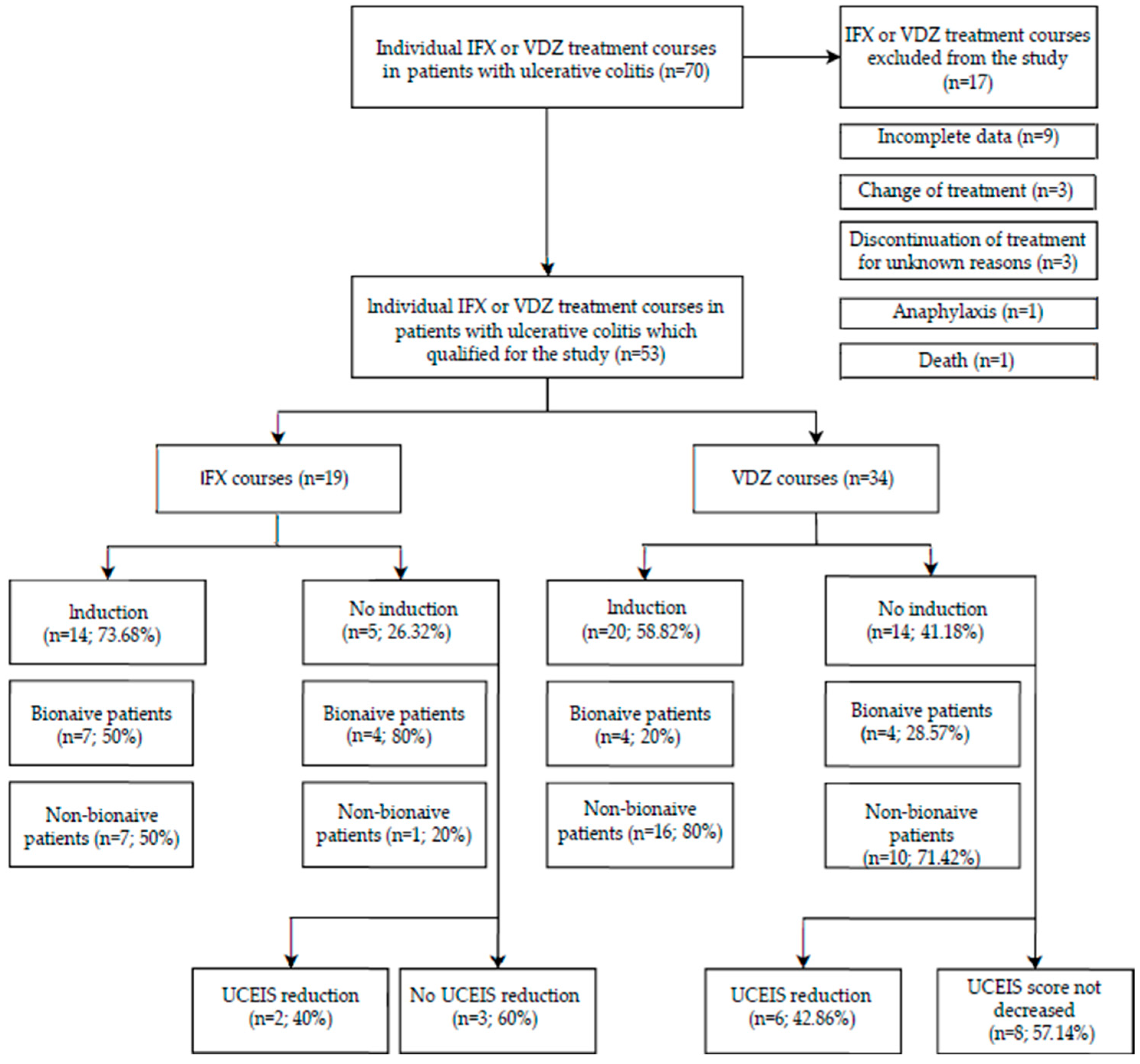
3. Results
3.1. Short-Term Response
3.2. Correlations
3.2.1. IFX
3.2.2. VDZ
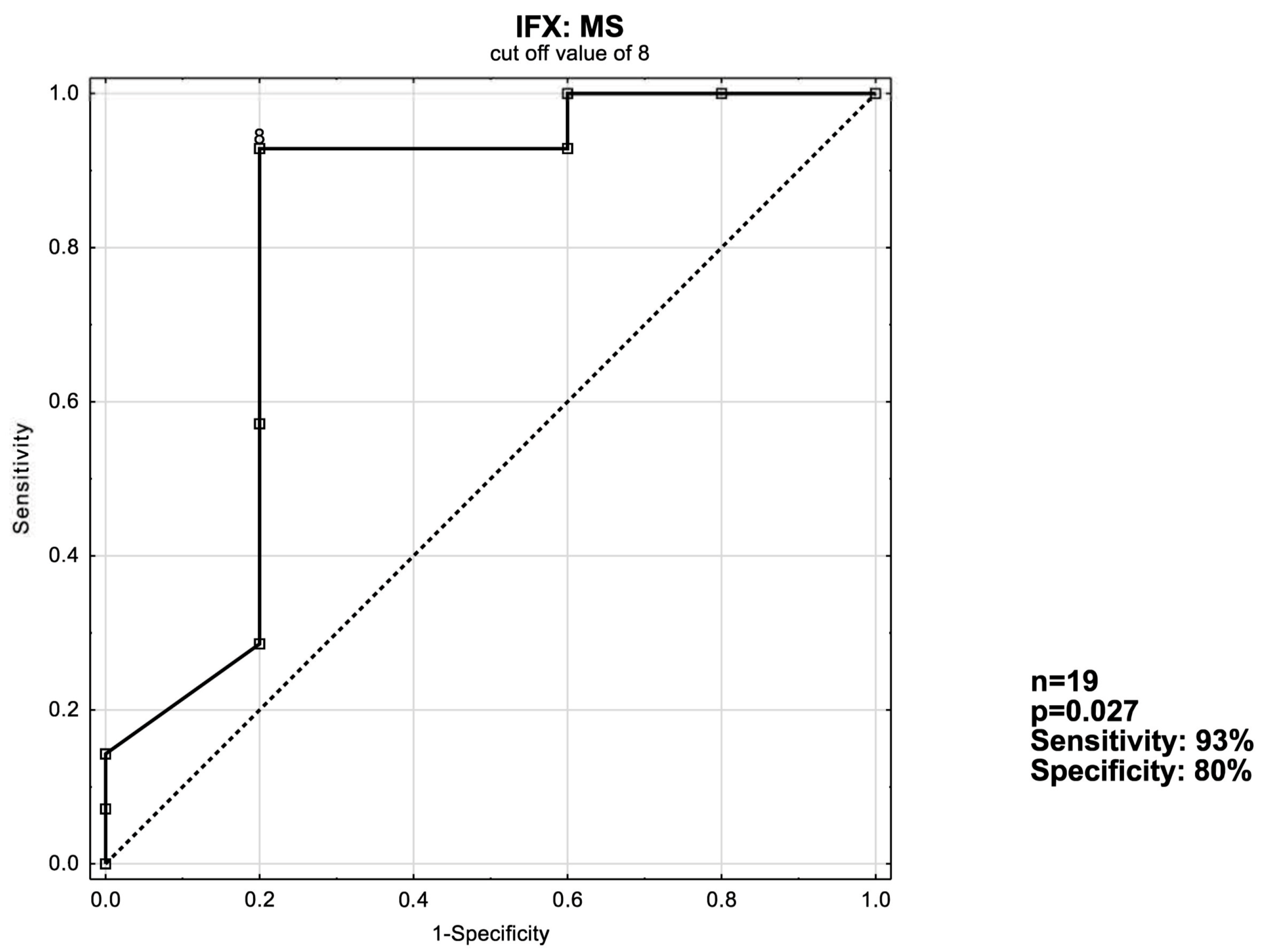
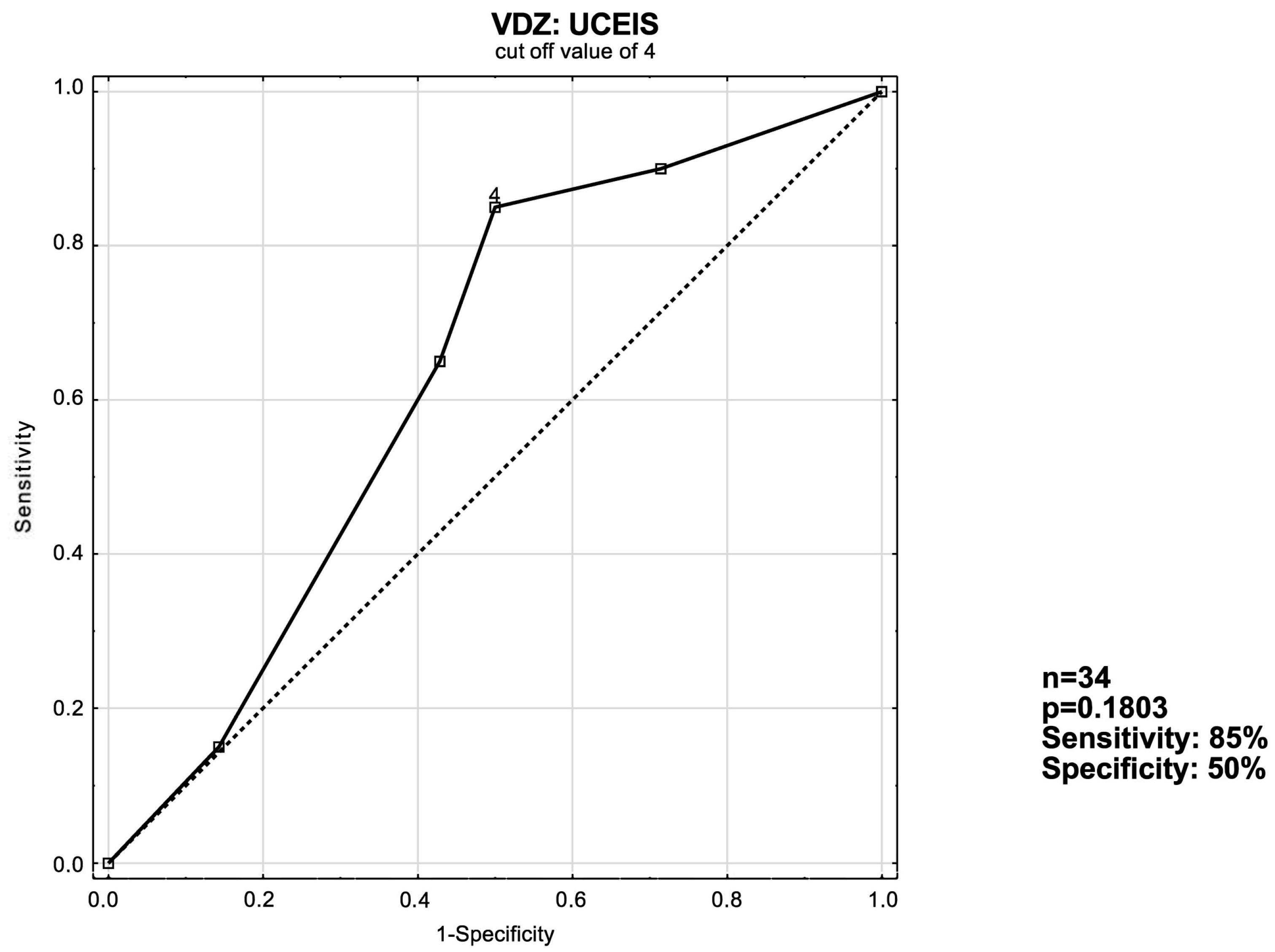
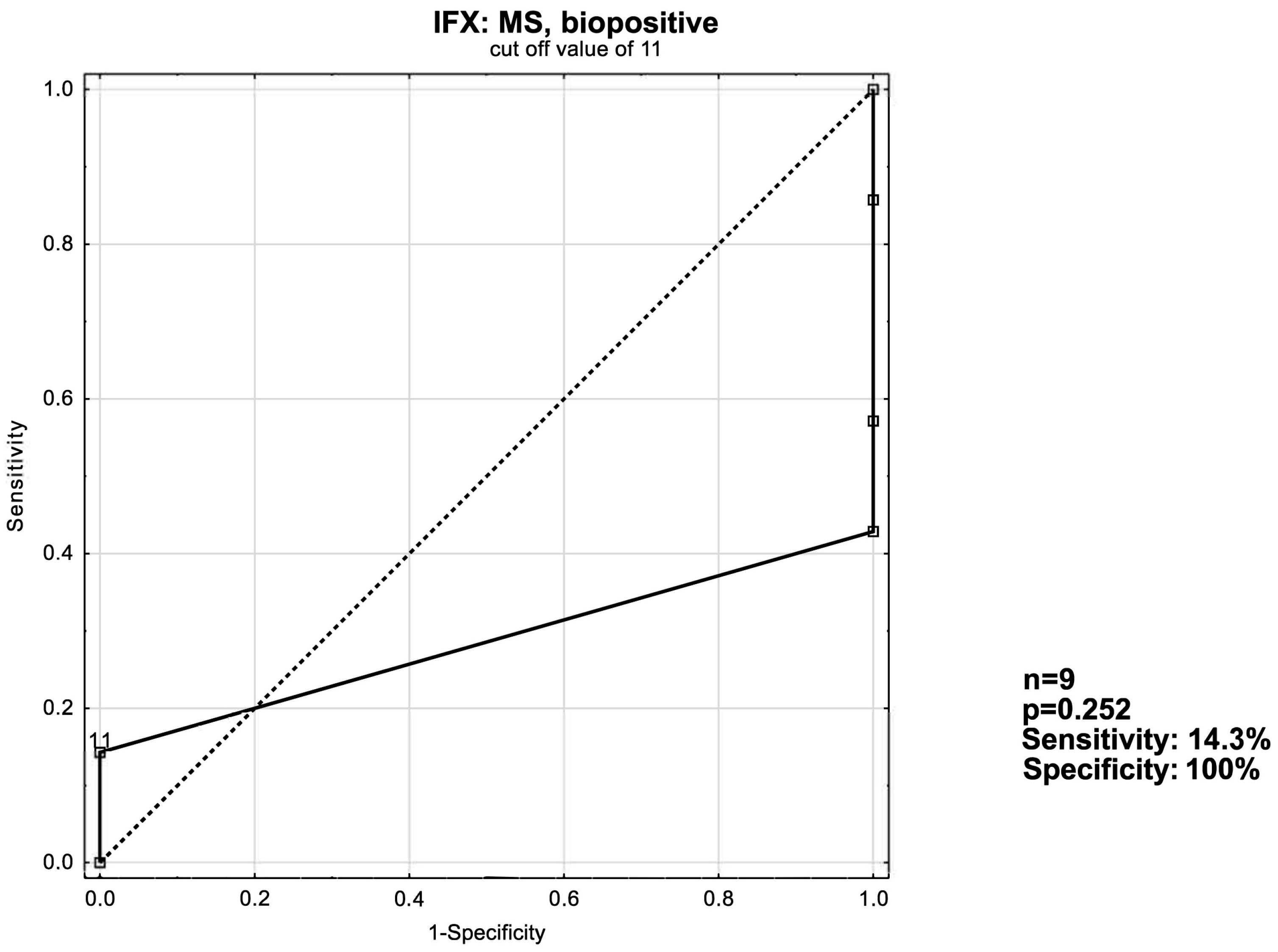
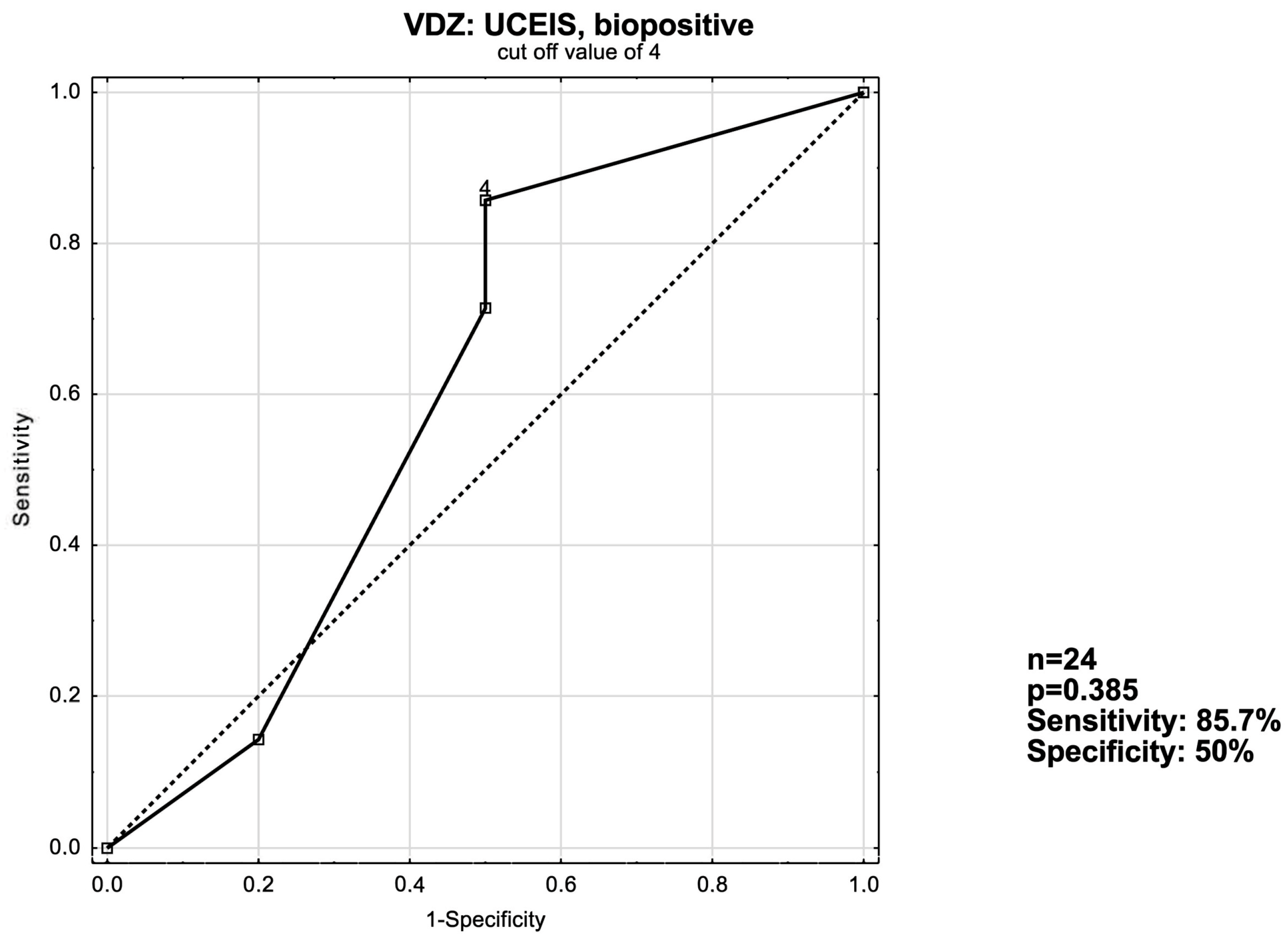
3.3. ROC Curve Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis.-A-Mon. 2019, 65, 100851. [Google Scholar] [CrossRef]
- Arvikar, S.L.; Fisher, M.C. Inflammatory bowel disease associated arthropathy. Curr. Rev. Musculoskelet. Med. 2011, 4, 123–131. [Google Scholar] [CrossRef]
- Rossi, R.E.; Conte, D.; Massironi, S. Primary sclerosing cholangitis associated with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2016, 28, 123–131. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Moss, A.C.; Farraye, F.A. Ulcerative colitis. Mayo Clin. Proc. 2019, 94, 1357–1373. [Google Scholar] [CrossRef]
- Conrad, K.; Roggenbuck, D.; Laass, M.W. Diagnosis and classification of ulcerative colitis. Autoimmun. Rev. 2014, 13, 463–466. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting therapeutic targets in inflammatory bowel disease (stride): Determining therapeutic goals for treat-to-target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef]
- Eder, P.; Łodyga, M.; Gawron-Kiszka, M.; Dobrowolska, A.; Gonciarz, M.; Hartleb, M.; Kłopocka, M.; Małecka-Wojciesko, E.; Radwan, P.; Reguła, J.; et al. Guidelines for the management of ulcerative colitis. Recommendations of the Polish Society of Gastroenterology and the Polish national consultant in gastroenterology. Gastroenterol. Rev. 2023, 18, 1–42. [Google Scholar] [CrossRef]
- Ford, A.C.; Sandborn, W.J.; Khan, K.J.; Hanauer, S.B.; Talley, N.J.; Moayyedi, P. Efficacy of biological therapies in inflammatory bowel disease: Systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 644–659. [Google Scholar] [CrossRef]
- Bhandari, R.; Ogeyingbo, O.D.; Kareem, R.; Gyawali, M.; Venkatesan, N.; Ahmed, R.; Botleroo, R.A.; Elshaikh, A.O. Efficacy and safety of vedolizumab in management of moderate to severe ulcerative colitis: A systematic review. Cureus 2021, 13, e17729. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Osterman, M.T. Biologic therapy for ulcerative colitis. Gastroenterol. Clin. N. Am. 2020, 49, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Berlin, C.; Berg, E.L.; Briskin, M.J.; Andrew, D.P.; Kilshaw, P.J.; Holzmann, B.; Weissman, I.L.; Hamann, A.; Butcher, E.C. A4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin madcam-1. Cell 1993, 74, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Luzentales-Simpson, M.; Pang, Y.C.; Zhang, A.; Sousa, J.A.; Sly, L.M. Vedolizumab: Potential mechanisms of action for reducing pathological inflammation in inflammatory bowel diseases. Front. Cell Dev. Biol. 2021, 9, 612830. [Google Scholar] [CrossRef]
- Stark, R.; König, H.-H.; Leidl, R. Costs of inflammatory bowel disease in Germany. PharmacoEconomics 2006, 24, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, P.; Stawowczyk, E.; Mossakowska, M.; Pilc, A. Disease activity, quality of life, and indirect costs of ulcerative colitis in Poland. Gastroenterol. Rev. 2017, 1, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Everhov, Å.H.; Halfvarson, J.; Ludvigsson, J.F.; Askling, J.; Myrelid, P.; Söderling, J.; Olen, O.; Neovius, M.; SWI-BREG Group. Healthcare use, work loss and total costs in incident and prevalent crohn’s disease and ulcerative colitis: Results from a nationwide study in Sweden. Aliment. Pharmacol. Ther. 2020, 52, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Pakdin, M.; Zarei, L.; Bagheri Lankarani, K.; Ghahramani, S. The cost of illness analysis of inflammatory bowel disease. BMC Gastroenterol. 2023, 23, 21. [Google Scholar] [CrossRef] [PubMed]
- Haga, K.; Shibuya, T.; Osada, T.; Sato, S.; Fukuo, Y.; Kobayashi, O.; Yamada, T.; Asaoka, D.; Ito, K.; Nomura, K.; et al. Early clinical remission is a predictor of long-term remission with the use of vedolizumab for ulcerative colitis. Biomedicines 2022, 10, 2526. [Google Scholar] [CrossRef]
- Murthy, S.K.; Greenberg, G.R.; Croitoru, K.; Nguyen, G.C.; Silverberg, M.S.; Steinhart, A.H. Extent of early clinical response to infliximab predicts long-term treatment success in active ulcerative colitis. Inflamm. Bowel Dis. 2015, 21, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- García-Bosch, O.; Aceituno, M.; Ordás, I.; Etchevers, J.; Sans, M.; Feu, F.; Panés, J.; Ricart, E. Long-term follow-up of patients treated with infliximab for ulcerative colitis: Predictive factors of response—An observational study. Dig. Dis. Sci. 2016, 61, 2051–2059. [Google Scholar] [CrossRef]
- Papamichael, K.; Rivals-Lerebours, O.; Billiet, T.; Vande Casteele, N.; Gils, A.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.J.; Mantzaris, G.J.; Peyrin-Biroulet, L. Long-term outcome of patients with ulcerative colitis and primary non-re-sponse to infliximab. J. Crohn’s Colitis 2016, 10, 1015–1023. [Google Scholar] [CrossRef]
- Dinesen, L.C.; Walsh, A.J.; Protic, M.N.; Heap, G.; Cummings, F.; Warren, B.F.; George, B.; Mortensen, N.J.M.; Travis, S.P.L. The pattern and outcome of acute severe colitis. J. Crohn’s Colitis 2010, 4, 431–437. [Google Scholar] [CrossRef]
- Festa, S.; Scribano, M.L.; Pugliese, D.; Bezzio, C.; Principi, M.; Ribaldone, D.G.; Allocca, M.; Mocci, G.; Bodini, G.; Spagnuolo, R.; et al. Long-term outcomes of acute severe ulcerative colitis in the rescue therapy era: A multicentre cohort study. United Eur. Gastroenterol. J. 2021, 9, 507–516. [Google Scholar] [CrossRef]
- Chen, K.; Shang, S.; Yu, S.; Cui, L.; Li, S.; He, N. Identification and exploration of pharmacological pyroptosis-related biomarkers of ulcerative colitis. Front. Immunol. 2022, 13, 998470. [Google Scholar] [CrossRef]
- Lian, Z.; Hu, J.; Cheng, C.; Liu, Y.; Zhu, L.; Shen, H. Association with controlling nutritional status score and disease activity of ulcerative colitis. J. Int. Med. Res. 2023, 51, 03000605231184046. [Google Scholar] [CrossRef]
- Rocha, R.; Sousa, U.H.; Reis, T.L.; Santana, G.O. Nutritional status as a predictor of hospitalization in inflammatory bowel disease: A Review. World J. Gastrointest. Pharmacol. Ther. 2019, 10, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.M.; Cross, R.K. Association of age at diagnosis and ulcerative colitis phenotype. Dig. Dis. Sci. 2012, 57, 2402–2407. [Google Scholar] [CrossRef]
- Fries, W.; Demarzo, M.G.; Navarra, G.; Viola, A. Ulcerative colitis in adulthood and in older patients: Same disease, same outcome, same risks? Drugs Aging 2022, 39, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Gupta, V.; Mohsen, W.; Chapman, T.P.; Subhaharan, D.; Kakkadasam Ramaswamy, P.; Kumar, S.; Kedia, S.; McGregor, C.G.; Ambrose, T.; et al. Early manage-ment of acute severe UC in the biologics era: Development and international validation of a prognostic clinical index to predict steroid response. Gut 2022, 72, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Laredo, V.; Gargallo-Puyuelo, C.J.; Gomollón, F. How to choose the biologic therapy in a bio-naïve patient with inflammatory bowel disease. J. Clin. Med. 2022, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Bokemeyer, B.; Lügering, A.; Bettenworth, D.; Teich, N.; Fischer, I.; Hammer, L.; Kolterer, S.; Rath, S.; Stallmach, A.; et al. Clinical predictors for a complicated course of disease in an inception cohort of patients with ulcerative colitis: Results from the prospective, observational EPICOL study. Int. J. Color. Dis. 2022, 37, 485–493. [Google Scholar] [CrossRef]
- Xu, W.; Ou, W.; Fu, J.; Gu, Y.; Cui, L.; Zhong, J.; Du, P. Cut-off value of ulcerative colitis endoscopic index of severity (UCEIS) score for predicting the need for pouch construction in ulcerative colitis: Results of a multicenter study with long-term follow-up. Gastroenterol. Rep. 2021, 9, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Ikeya, K.; Hanai, H.; Sugimoto, K.; Osawa, S.; Kawasaki, S.; Iida, T.; Maruyama, Y.; Watanabe, F. The ulcerative colitis endo-scopic index of severity more accurately reflects clinical outcomes and long-term prognosis than the Mayo endoscopic score. J. Crohn’s Colitis 2015, 10, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Zhang, T.; Ding, C.; Dai, X.; Li, Y.; Guo, Z.; Wei, Y.; Gong, J.; Zhu, W.; Li, J. Ulcerative colitis endoscopic index of severity (UCEIS) versus Mayo endoscopic score (MES) in guiding the need for colectomy in patients with acute severe colitis. Gastroenterol. Rep. 2017, 6, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Corte, C.; Fernandopulle, N.; Catuneanu, A.M.; Burger, D.; Cesarini, M.; White, L.; Keshav, S.; Travis, S. Association between the ulcerative colitis endoscopic index of severity (UCEIS) and outcomes in acute severe ulcerative colitis. J. Crohn’s Colitis 2015, 9, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Di Ruscio, M.; Variola, A.; Vernia, F.; Lunardi, G.; Castelli, P.; Bocus, P.; Geccherle, A. Role of ulcerative colitis endoscopic index of severity (UCEIS) versus Mayo endoscopic Subscore (MES) in predicting patients′ response to biological therapy and the need for colectomy. Digestion 2020, 102, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, L.; Wang, M.; Shao, B.; Ye, L.; Zhang, Y.; Cao, Q. Use of the ulcerative colitis endoscopic index of severity and Mayo endoscopic score for predicting the therapeutic effect of mesalazine in patients with ulcerative colitis. Laparosc. Endo-Scopic Robot. Surg. 2021, 4, 33–39. [Google Scholar] [CrossRef]
- Yan, J.; Liu, A.; Fang, L.; Wu, J.; Ding, X.; Xu, Y. The ulcerative colitis endoscopic index of severity score is superior to reflecting long-term prognosis in ulcerative colitis patients treated with vedolizumab. Medicine 2023, 102, e35799. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.C.; Dulai, P.S.; Marshall, J.K.; Jairath, V.; Reinisch, W.; Narula, N. Predicting endoscopic improvement in ulcerative colitis using the ulcerative colitis severity index. Inflamm. Bowel Dis. 2023, 30, 370–381. [Google Scholar] [CrossRef]
- Vasudevan, A.; Gibson, P.R.; Langenberg, D.R. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: What should the clinician expect, what should patients be told? World J. Gastroenterol. 2017, 23, 6385–6402. [Google Scholar] [CrossRef]


| Clinical Remission after 14 Weeks of IFX Therapy | ||||
|---|---|---|---|---|
| Remission | No Remission | p-Value | ||
| Subjects, n | 14 | 5 | NA | |
| Sex | women, n (%) | 6 (42.86%) | 2 (40.00%) | 0.6641 |
| men, n (%) | 8 (57.14%) | 3 (60.00%) | ||
| Age, years | 36.57 ± 12.35 | 42.8 ± 15.59 | 0.3771 | |
| Pancolitis, n (%) | 9 | 4 | ||
| Extent of lesions | Left colon, n (%) | 4 | 0 | 0.4097 |
| Rectum, n (%) | 1 | 1 | ||
| Bionaïve | Yes, n (%) | 7 (50.00%) | 4 (80.00%) | 0.2668 |
| No, n (%) | 7 (50.00%) | 1 (20.00%) | ||
| Glucocorticoid use | Yes, n (%) | 13 (92.86%) | 5 (100%) | 0.7368 |
| No, n (%) | 1 (7.14%) | 0 (0%) | ||
| Disease duration, years | 7.57 ± 5.61 | 8.6 ± 5.77 | 0.7311 | |
| Hemoglobin, g/dL | 12.331 ± 2.03 | 12.53 ± 5.77 | 0.8664 | |
| White blood cell count, ×103/µL | 9.76 ± 3.31 | 10.26 ± 2.51 | 0.7864 | |
| Lymphocyte count, ×103/µL | 2.02 ± 1.02 | 1.69 ± 1.10 | 0.5875 | |
| CRP, mg/L | 24.25 ± 39.93 | 17.10 ± 18.03 | 0.5335 | |
| Calprotectin, µg/L | 1213 ± 783.20 | 814.7 ± 1114.00 | 0.6098 | |
| Clinical Remission after 14 Weeks of VDZ Therapy | ||||
|---|---|---|---|---|
| Remission | No Remission | p-Value | ||
| Subjects, n | 20 | 14 | NA | |
| Sex | women, n (%) | 11 (55.00%) | 4 (28.57%) | 0.1194 |
| men, n (%) | 9 (45.00%) | 10 (71.43%) | ||
| Age, years | 36.12 ± 12.10 | 38.29 ± 16.11 | 0.9860 | |
| Pancolitis, n (%) | 11 (55.00%) | 8 (57.14%) | ||
| Extent of lesions | Left colon, n (%) | 8 (40.00%) | 5 (35.71%) | 1.000 |
| Rectum, n (%) | 1 (5.00%) | 1 (7.14%) | ||
| Bionaïve | Yes, n (%) | 14 (70.00%) | 4 (28.57%) | 0.0204 |
| No, n (%) | 6 (30.00%) | 10 (71.43%) | ||
| Glucocorticoid use | Yes, n (%) | 19 (95.00%) | 12 (85.71%) | 0.3650 |
| No, n (%) | 1 (5.00%) | 2 (14.29%) | ||
| Disease duration, years | 7.95 ± 5.72 | 7.714 ± 6.64 | 0.9125 | |
| Hemoglobin, g/dL | 13.57 ± 1.36 | 13.86 ± 1.59 | 0.5785 | |
| White blood cell count, ×103/µL | 8.54 ± 3.06 | 8.86 ± 3.07 | 1.0000 | |
| Lymphocyte count, ×103/µL | 1.96 ± 0.91 | 2.24 ± 1.38 | 1.0000 | |
| CRP, mg/L | 10.11 ± 9.87 | 12.67 ± 25.85 | 0.6358 | |
| Calprotectin, µg/L | 1385.1 ± 702.37 | 752.00 ± 527.6 | 0.0758 | |
| Before Treatment | After 14 Weeks of Treatment | n | p-Value | ||
|---|---|---|---|---|---|
| UCEIS | Induction | 4.786 ± 1.528 | 2.500 ± 1.160 | 14 | <0.001 |
| No induction | 4.800 ± 1.483 | 4.400 ± 1.342 | 5 | 1.0 | |
| Total | 4.790 ± 1.475 | 3.000 ± 1.453 | 19 | 0.0012 | |
| eMS | Induction | 2.571 ± 0.646 | 1.500 ± 0.519 | 14 | <0.001 |
| No induction | 2.800 ± 0.447 | 2.600 ± 0.548 | 5 | N/A | |
| Total | 2.632 ± 0.597 | 1.790 ± 0.713 | 19 | <0.001 | |
| MS | Induction | 8.929 ± 1.492 | 3.2857 ± 1.326 | 14 | <0.001 |
| No induction | 6.600 ± 2.302 | 6.600 ± 2.510 | 5 | 0.371 | |
| Total | 8.316 ± 1.976 | 4.158 ± 2.218 | 19 | <0.001 |
| Before Treatment | After 14 Weeks of Treatment | n | p-Value | ||
|---|---|---|---|---|---|
| UCEIS | Induction | 4.550 ± 1.146 | 2.200 ± 1.7652 | 20 | <0.001 |
| No induction | 3.786 ± 1.528 | 3.500 ± 1.829 | 14 | 0.75183 | |
| Total | 4.235 ± 1.350 | 2.735 ± 1.879 | 34 | 0.00208 | |
| eMS | Induction | 2.800 ± 0.410 | 1.500 ± 1.051 | 20 | <0.001 |
| No induction | 2.571 ± 0.646 | 1.928 ± 1.071 | 14 | 0.0771 | |
| Total | 2.706 ± 0.524 | 1.677 ± 1.0652 | 34 | <0.001 | |
| MS | Induction | 7.55 ± 2.013 | 2.600 ± 2.036 | 20 | <0.001 |
| No induction | 6.428 ± 2.441 | 5.071 ± 2.200 | 14 | 0.005546 | |
| Total | 7.062 ± 2.299 | 3.618 ± 2.412 | 34 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romaniuk, F.; Franus, A.; Sobolewska-Włodarczyk, A.; Gąsiorowska, A. Clinical Utility of Disease Activity Indices in Predicting Short-Term Response to Biologics in Patients with Ulcerative Colitis. J. Clin. Med. 2024, 13, 3455. https://doi.org/10.3390/jcm13123455
Romaniuk F, Franus A, Sobolewska-Włodarczyk A, Gąsiorowska A. Clinical Utility of Disease Activity Indices in Predicting Short-Term Response to Biologics in Patients with Ulcerative Colitis. Journal of Clinical Medicine. 2024; 13(12):3455. https://doi.org/10.3390/jcm13123455
Chicago/Turabian StyleRomaniuk, Filip, Anna Franus, Aleksandra Sobolewska-Włodarczyk, and Anita Gąsiorowska. 2024. "Clinical Utility of Disease Activity Indices in Predicting Short-Term Response to Biologics in Patients with Ulcerative Colitis" Journal of Clinical Medicine 13, no. 12: 3455. https://doi.org/10.3390/jcm13123455
APA StyleRomaniuk, F., Franus, A., Sobolewska-Włodarczyk, A., & Gąsiorowska, A. (2024). Clinical Utility of Disease Activity Indices in Predicting Short-Term Response to Biologics in Patients with Ulcerative Colitis. Journal of Clinical Medicine, 13(12), 3455. https://doi.org/10.3390/jcm13123455






