A Retrospective Cohort Analysis of Transarterial Chemoembolization for Hepatocellular Cancer at a Tertiary Center in Switzerland
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M. Global Cancer Statistics in the Year 2000. Lancet Oncol. 2001, 2, 533–543. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International Trends in Hepatocellular Carcinoma Incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Valery, P.C.; Laversanne, M.; Clark, P.J.; Petrick, J.L.; McGlynn, K.A.; Bray, F. Projections of Primary Liver Cancer to 2030 in 30 Countries Worldwide. Hepatology 2018, 67, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ducreux, M.; Lencioni, R.; Di Bisceglie, A.M.; Galle, P.R.; Dufour, J.F.; Greten, T.F.; Raymond, E.; Roskams, T.; De Baere, T.; et al. EASL-EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. Eur. J. Cancer 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Llovet, J.M.; Fuster, J.; Bruix, J. Barcelona-Clínic Liver Cancer Group The Barcelona Approach: Diagnosis, Staging, and Treatment of Hepatocellular Carcinoma. Liver Transpl. 2004, 10, S115–S120. [Google Scholar] [CrossRef]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M. American Association for the Study of Liver Diseases Management of Hepatocellular Carcinoma: An Update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J. A Concise Review of Updated Guidelines Regarding the Management of Hepatocellular Carcinoma around the World: 2010-2016. Clin. Mol. Hepatol. 2016, 22, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bruix, J. Systematic Review of Randomized Trials for Unresectable Hepatocellular Carcinoma: Chemoembolization Improves Survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Lladó, L.; Virgili, J.; Figueras, J.; Valls, C.; Dominguez, J.; Rafecas, A.; Torras, J.; Fabregat, J.; Guardiola, J.; Jaurrieta, E. A Prognostic Index of the Survival of Patients with Unresectable Hepatocellular Carcinoma after Transcatheter Arterial Chemoembolization. Cancer 2000, 88, 50–57. [Google Scholar] [CrossRef]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Diagnostik und Therapie des Hepatozellulären Karzinoms und biliärer Karzi-nome, Langversion 4.0, 2023, AWMF-Registernummer: 032-053OL. Available online: https://www.leitli-nienprogramm-onkologie.de/leitlinien/hcc-und-biliaere-karzinome/ (accessed on 1 December 2023).
- Kikuchi, L.; Chagas, A.L.; Alencar, R.S.S.M.; Tani, C.; Diniz, M.A.; D’Albuquerque, L.A.C.; Carrilho, F.J. Adherence to BCLC Recommendations for the Treatment of Hepatocellular Carcinoma: Impact on Survival According to Stage. Clinics 2017, 72, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A Simple Prognostic Scoring System for Patients Receiving Transarterial Embolisation for Hepatocellular Cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef]
- Park, Y.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.-H.; Yeon, J.E.; Byun, K.S.; Kim, H.S.; Kim, J.H.; et al. Feasibility of Dynamic Risk Assessment for Patients with Repeated Trans-Arterial Chemoembolization for Hepatocellular Carcinoma. BMC Cancer 2019, 19, 363. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial Embolisation or Chemoembolisation versus Symptomatic Treatment in Patients with Unresectable Hepatocellular Carcinoma: A Randomised Controlled Trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-H.; Hsu, C.-Y.; Hsia, C.-Y.; Lee, Y.-H.; Huang, Y.-H.; Chiou, Y.-Y.; Lin, H.-C.; Huo, T.-I. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma ≤ 2 Cm in a Propensity Score Model. Ann. Surg. 2016, 263, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-M.; Yeh, M.-L.; Chuang, S.-C.; Wang, L.-Y.; Lin, Z.-Y.; Chen, S.-C.; Tsai, J.-F.; Wang, S.-N.; Kuo, K.-K.; Dai, C.-Y.; et al. Survival Comparison between Surgical Resection and Percutaneous Radiofrequency Ablation for Patients in Barcelona Clinic Liver Cancer Early Stage Hepatocellular Carcinoma. Indian J. Gastroenterol. 2013, 32, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Gory, I.; Fink, M.; Bell, S.; Gow, P.; Nicoll, A.; Knight, V.; Dev, A.; Rode, A.; Bailey, M.; Cheung, W.; et al. Radiofrequency Ablation versus Resection for the Treatment of Early Stage Hepatocellular Carcinoma: A Multicenter Australian Study. Scand. J. Gastroenterol. 2015, 50, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in Combination with Ablation in Patients with Advanced Hepatocellular Carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef]
- Swan, R.Z.; Sindram, D.; Martinie, J.B.; Iannitti, D.A. Operative Microwave Ablation for Hepatocellular Carcinoma: Complications, Recurrence, and Long-Term Outcomes. J. Gastrointest. Surg. 2013, 17, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Ohmoto, K.; Yoshioka, N.; Tomiyama, Y.; Shibata, N.; Kawase, T.; Yoshida, K.; Kuboki, M.; Yamamoto, S. Comparison of Therapeutic Effects between Radiofrequency Ablation and Percutaneous Microwave Coagulation Therapy for Small Hepatocellular Carcinomas. J. Gastroenterol. Hepatol. 2009, 24, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, J.S.; Choi, I.J.; Chung, J.W.; Park, J.H.; Kim, C.Y. The Safety and Efficacy of Transcatheter Arterial Chemoembolization in the Treatment of Patients with Hepatocellular Carcinoma and Main Portal Vein Obstruction. A Prospective Controlled Study. Cancer 1997, 79, 2087–2094. [Google Scholar] [CrossRef]
- Georgiades, C.S.; Hong, K.; D’Angelo, M.; Geschwind, J.-F.H. Safety and Efficacy of Transarterial Chemoembolization in Patients with Unresectable Hepatocellular Carcinoma and Portal Vein Thrombosis. J. Vasc. Interv. Radiol. 2005, 16, 1653–1659. [Google Scholar] [CrossRef]
- Luo, J.; Guo, R.-P.; Lai, E.C.H.; Zhang, Y.-J.; Lau, W.Y.; Chen, M.-S.; Shi, M. Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis: A Prospective Comparative Study. Ann. Surg. Oncol. 2011, 18, 413–420. [Google Scholar] [CrossRef]
- Okusaka, T.; Okada, S.; Ishii, H.; Nose, H.; Nagahama, H.; Nakasuka, H.; Ikeda, K.; Yoshimori, M. Prognosis of Hepatocellular Carcinoma Patients with Extrahepatic Metastases. Hepatogastroenterology 1997, 44, 251–257. [Google Scholar] [PubMed]
- Uka, K.; Aikata, H.; Takaki, S.; Shirakawa, H.; Jeong, S.-C.; Yamashina, K.; Hiramatsu, A.; Kodama, H.; Takahashi, S.; Chayama, K. Clinical Features and Prognosis of Patients with Extrahepatic Metastases from Hepatocellular Carcinoma. World J. Gastroenterol. 2007, 13, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Kim, J.H.; Park, I.S.; Ko, G.-Y.; Yoon, H.-K.; Sung, K.-B.; Lim, Y.-S.; Lee, H.C.; Chung, Y.H.; Lee, Y.S.; et al. Reappraisal of Repeated Transarterial Chemoembolization in the Treatment of Hepatocellular Carcinoma with Portal Vein Invasion. J. Gastroenterol. Hepatol. 2009, 24, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.-J.; Kim, K.M.; Jin, Y.-J.; Shim, J.H.; Ko, G.-Y.; Yoon, H.-K.; Sung, K.-B.; Lee, J.-L.; Kang, Y.-K.; Lim, Y.-S.; et al. Clinical Outcome of 251 Patients with Extrahepatic Metastasis at Initial Diagnosis of Hepatocellular Carcinoma: Does Transarterial Chemoembolization Improve Survival in These Patients? J. Gastroenterol. Hepatol. 2011, 26, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.E.; Lee, J.-H.; Kim, H.Y.; Hwang, S.Y.; Kim, J.S.; Chung, J.W.; Yoon, J.-H.; Lee, H.-S.; Kim, Y.J. Transarterial Chemoembolization Can Be Safely Performed in Patients with Hepatocellular Carcinoma Invading the Main Portal Vein and May Improve the Overall Survival. Radiology 2011, 258, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Kedarisetty, C.; Venkataraman, J.; Srinivasan, V.; Deepashree, T.; Uthappa, M.; Ilankumaran, K.; Govil, S.; Reddy, M.; Rela, M. Combination of TACE and Sorafenib Improves Outcomes in BCLC Stages B/C of Hepatocellular Carcinoma: A Single Centre Experience. Ann. Hepatol. 2017, 16, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.H.; Shim, J.H.; Kim, M.-J.; Ryu, M.-H.; Ryoo, B.-Y.; Kang, Y.-K.; Shin, Y.M.; Kim, K.M.; Lim, Y.-S.; Lee, H.C. Sorafenib Alone versus Sorafenib Combined with Transarterial Chemoembolization for Advanced-Stage Hepatocellular Carcinoma: Results of Propensity Score Analyses. Radiology 2013, 269, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.D.; Mills, C.; Sawhney, R.; Sood, S.; Bird, V.; Mishra, G.; Dev, A.; Kemp, W.; Lubel, J.; Roberts, S.K.; et al. SIRT Compared with DEB-TACE for Hepatocellular Carcinoma: A Real-World Study (the SITAR Study). J. Gastrointest. Cancer 2021, 52, 907–914. [Google Scholar] [CrossRef]
- Luz, J.H.M.; Luz, P.M.; Martin, H.S.; Gouveia, H.R.; Levigard, R.B.; Nogueira, F.D.; Rodrigues, B.C.; de Miranda, T.N.; Mamede, M.H. DEB TACE for Intermediate and Advanced HCC—Initial Experience in a Brazilian Cancer Center. Cancer Imaging 2017, 17, 5. [Google Scholar] [CrossRef]
- Takayasu, K.; Arii, S.; Ikai, I.; Omata, M.; Okita, K.; Ichida, T.; Matsuyama, Y.; Nakanuma, Y.; Kojiro, M.; Makuuchi, M.; et al. Prospective Cohort Study of Transarterial Chemoembolization for Unresectable Hepatocellular Carcinoma in 8510 Patients. Gastroenterology 2006, 131, 461–469. [Google Scholar] [CrossRef]
- Adhoute, X.; Penaranda, G.; Naude, S.; Raoul, J.L.; Perrier, H.; Bayle, O.; Monnet, O.; Beaurain, P.; Bazin, C.; Pol, B.; et al. Retreatment with TACE: The ABCR SCORE, an Aid to the Decision-Making Process. J. Hepatol. 2015, 62, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Jia, Z.; Ying, X.; Zhang, D.; Li, S.; Tian, F.; Jiang, G. The Incidence and Outcome of Major Complication Following Conventional TAE/TACE for Hepatocellular Carcinoma. Medicine 2016, 95, e5606. [Google Scholar] [CrossRef]
- Marcacuzco Quinto, A.; Nutu, O.-A.; San Román Manso, R.; Justo Alonso, I.; Calvo Pulido, J.; Manrique Municio, A.; García-Sesma, Á.; Loinaz Segurola, C.; Martínez Caballero, J.; Jiménez Romero, L.C. Complications of Transarterial Chemoembolization (TACE) in the Treatment of Liver Tumors. Cir. Esp. 2018, 96, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Toprak, N.U.; Sayin, E.; Akilli, F.M.; Gundogdu, A. Sepsis Caused by Anaerococcus Nagyae after Transarterial-Chemoembolization for Hepatocellular Carcinoma: Case Report and Literature Review. Anaerobe 2021, 72, 102464. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, A.A.; Abbas, Z.; Luck, N.H.; Hassan, S.M.; Faiq, S.M. Adverse Events Following Transarterial Chemoembolization for Hepatocellular Carcinoma and Factors Predicting Such Events. J. Pak. Med. Assoc. 2013, 63, 239–244. [Google Scholar] [PubMed]
- Paul, S.B.; Gamanagatti, S.; Sreenivas, V.; Chandrashekhara, S.H.; Mukund, A.; Gulati, M.S.; Gupta, A.K.; Acharya, S.K. Trans-Arterial Chemoembolization (TACE) in Patients with Unresectable Hepatocellular Carcinoma: Experience from a Tertiary Care Centre in India. Indian. J. Radiol. Imaging 2011, 21, 113–120. [Google Scholar] [CrossRef]
- Mukund, A.; Bhardwaj, K.; Choudhury, A.; Sarin, S.K. Survival and Outcome in Patients Receiving Drug-Eluting Beads Transarterial Chemoembolization for Large Hepatocellular Carcinoma (>5 Cm). J. Clin. Exp. Hepatol. 2021, 11, 674–681. [Google Scholar] [CrossRef]
| Patient Characteristic | Overall Patients (N = 101) |
|---|---|
| Age in years [mean, SD] | 67.5 ± 10.1 |
| Gender | |
| Female [Amount, Percent] | 25 (24.75) |
| Male [Amount, Percent] | 76 (75.25) |
| BMI [mean, SD] | 27.7 ± 5.9 |
| Charlson Comorbidity Index [mean, SD] | 8.5 ± 3 |
| Cirrhosis confirmed by histology | |
| Yes | 85 (84.2) |
| No | 16 (15.8) |
| Meld Score [mean, SD] | 10.4 ± 4.4 |
| APRI score [mean, SD] | 2.4 ± 4.7 |
| Child-Pugh Score | |
| A [Amount, Percent] | 50 (49.5) |
| B [Amount, Percent] | 45 (44.6) |
| C [Amount, Percent] | 6 (5.9) |
| BCLC stage [Amount, Percent] | |
| Stage 0 | 4 (4) |
| Stage A | 31 (30.7) |
| Stage B | 50 (49.5) |
| Stage C | 10 (9.9) |
| Stage D | 6 (5.9) |
| Hepatoma arterial-embolization prognostic (HAP) score [Amount, Percent] | |
| Risk group A (Score 0) | 0 (0) |
| Risk group B (Score 1) | 16 (15.8) |
| Risk group C (Score 2) | 49 (49) |
| Risk group D (Score >2) | 10 (9.9) |
| N/A | 26 (25.5) |
| Amount of lesions [Amount, SD] | 2.8 ± 1.5 |
| Diameter of the greatest leison [Mean in mm, SD] | 41.4 ± 31.5 |
| Bilobular lesions [Amount, Percent] | 42 (41.6) |
| Metastasis [Amount, Percent] | |
| M0 | 90 (89.1) |
| M1 | 11 (10.9) |
| Characteristic | Single TACE (n = 23) | 2 × TACE (n = 16) | Surgery Followed by TACE (n = 8) | 3 × TACE (n = 8) | 4 × TACE (n = 5) | >4 × TACE (n = 4) |
|---|---|---|---|---|---|---|
| Age in years [mean, SD] | 72 ± 9.3 | 71.8 ± 9.3 | 67.9 ± 4.6 | 66.9 ± 9.5 | 64.6 ± 9.3 | 66.8 ± 4.3 |
| CCI [mean, SD] | 10.3 ± 3.8 | 7.3 ± 2.1 | 8.1 ± 1.9 | 8.5 ± 1.7 | 7.8 ± 0.8 | 8.2 ± 2.2 |
| Amount of lesions [mean, SD] | 2.4 ± 1.2 | 2.9 ± 1.7 | 2.4 ± 1.7 | 3.6 ± 2.1 | 3.2 ± 0.8 | 4.9 ± 2.3 |
| Diameter biggest lesion [mean, SD] | 59.3 ± 40.6 | 52.3 ± 41.1 | 35.5 ± 33.8 | 32.4 ± 18 | 51.8 ± 30.4 | 31 ± 6.7 |
| Bilobular lesoions [Amount, Percent] | 9 (39.1) | 7 (43.8) | 4 (50) | 3 (37.5) | 3 (60) | 3 (75) |
| BCLC Stage [Amount, Percent] | ||||||
| Stage 0 | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) |
| Stage A | 5 (21.73) | 5 (31.25) | 2 (25) | 2 (25) | 0 (0) | 0 (0) |
| Stage B | 9 (39.13) | 11 (68.75) | 3 (37.5) | 6 (75) | 5 (100) | 3 (75) |
| Stage C | 6 (26.08) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) |
| Stage D | 3 (13.04) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 1 (25) |
| Progress [Amount, Percent] | ||||||
| Yes | 13 (56.5) | 14 (87.5) | 6 (75) | 8 (100) | 5 (100) | 3 (75) |
| Growth target lesion | 8 (61.5) | 9 (64.3) | 4 (66.7) | 4 (50) | 2 (40) | 3 (100) |
| New lesion | 1 (7.7) | 3 (21.4) | 2 (33.3) | 3 (37.5) | 2 (40) | 0 (0) |
| Mixed reponse (Growth + new lesion) | 3 (23.1) | 2 (14.3) | 0 (0) | 0 (0) | 1 (20) | 0 (0) |
| Metastasis | 1 (7.7) | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) |
| Time to progress [mean days, SD] | 164.8 ± 188.3 | 194.1 ± 94.8 | 164.5 ± 46.5 | 327.9 ± 325.9 | 384.2 ± 184.6 | 160.3 ± 79.6 |
| Survival [median months, IQR] | 8.2 (2–20.3) | 19.2 (13.8–23) | 15.5 (7.5–37.2) | 20.8 (14.4–35.4) | 26.7 (15.7–40.2) | 18.5 (13.7–23.1) |
| Outcome | Interventions (N = 186) |
|---|---|
| Progress occured [Amount, Percent] | 140 (75.3) |
| Growth of target lesion | 84 (60) |
| New lesion | 29 (20.7) |
| Mixed, Growth + New Lesion | 21 (15) |
| Metastasis | 6 (4.3) |
| Time to progress [Mean in days, SD] | 287.4 ± 354 |
| Complications occured [Amount, Percent] | 32 (17.2) |
| Clavien-Dindo I | 12 (6.5) |
| Clavien-Dindo II | 18 (9.7) |
| Clavien-Dindo III | 0 (0) |
| Clavien-Dindo IV | 0 (0) |
| Clavien-Dindo V | 2 (1.1) |
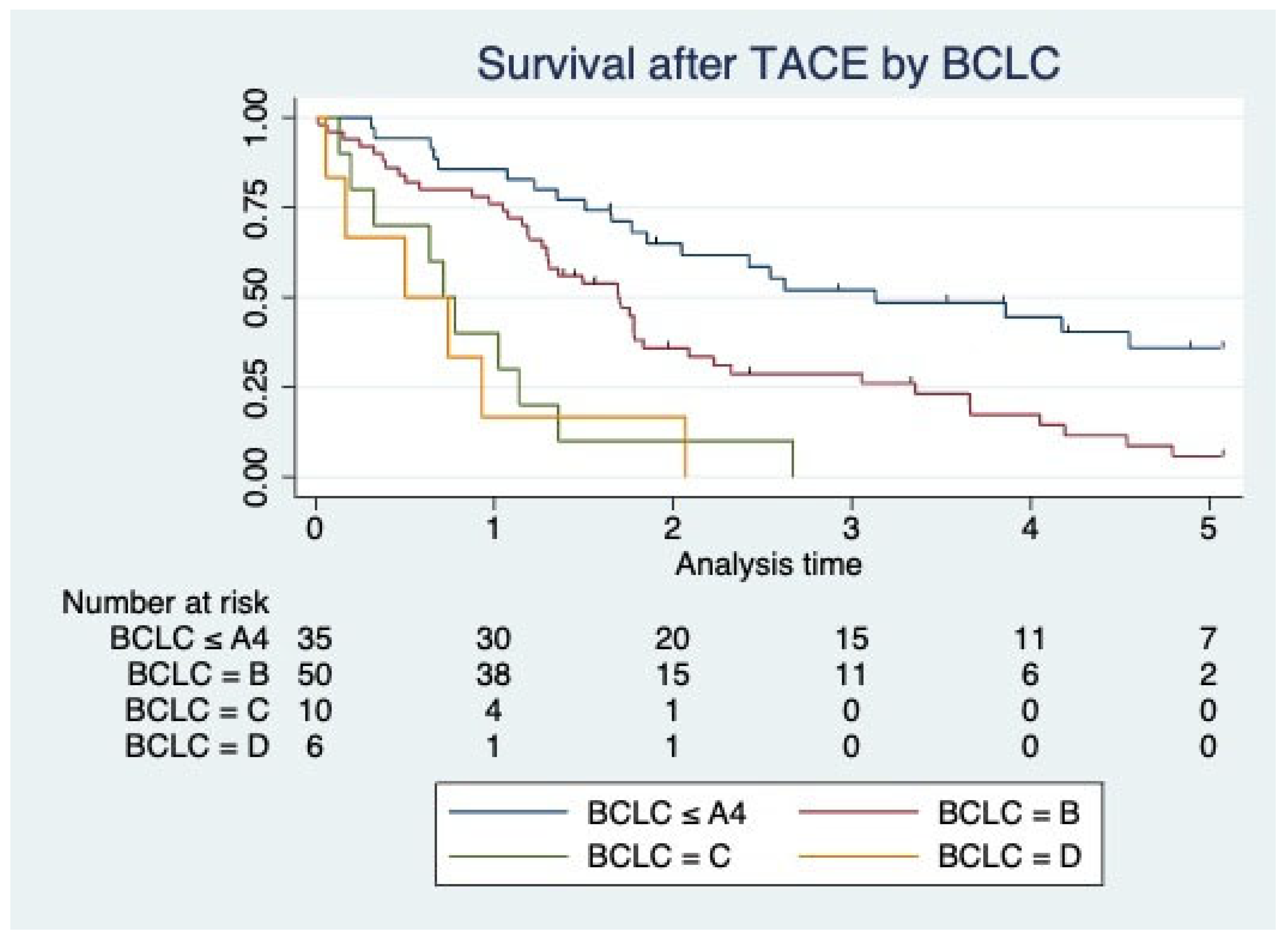
| Survival Characteristic | Overall Patients (N = 101) |
|---|---|
| Survival, median in months (IQR) | |
| Entire cohort | 21.4 (11.7–37.6) |
| BCLC ≤ A4 | 30.5 (19.9–56.5) |
| BCLC B | 20.5 (12.6–29.4) |
| BCLC C | 10.8 (3.9–16.3) |
| BCLC D | 7.5 (2–11.2) |
| Survival, mean in months (SD) | |
| Entire cohort | 22.79 (15.29) |
| BCLC ≤ A4 | 34.15 (17.53) |
| BCLC B | 25.03 (23.48) |
| BCLC C | 11.87 (9.32) |
| BCLC D | 8.77 (8.58) |
| Survival influenced by Lesion size, HR (95% CI) | |
| 12–25 mm | 1.09 (0.45–2.61) |
| 25–35 mm | 1.66 (0.67–4.09) |
| 35–45 mm | 2.35 (0.87–6.38) |
| 45–55 mm | 1.2 (0.46–3.15) |
| >55 mm | 2.8 (1.15–6.78) |
| Univariate Analysis—Correlation with survival, HR (95% CI) | |
| Bilobular Disease | 2.11 (1.31–3.39) |
| Charlson Comorbidity Index | 1.08 (1.01–1.17) |
| Child Score | 1.37 (1.18–1.60) |
| Meld Score | 1.06 (1.01–1.12) |
| APRI Score | 1.03 (0.996–1.08) |
| M1 vs M0 | 3.14 (1.58–6.23) |
| Size of lesion | 1.01 (1.00–1.02) |
| Amount of lesion | 1.19 (1.02–1.40) |
| BMI | 1.00 (0.99–1.00) |
| Age | 1.02 (0.99–1.05) |
| Sex (Male vs. Female) | 0.98 (0.57–1.66) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haak, F.; Karli, T.; Takes, M.; Zech, C.J.; Kollmar, O.; Soysal, S.D. A Retrospective Cohort Analysis of Transarterial Chemoembolization for Hepatocellular Cancer at a Tertiary Center in Switzerland. J. Clin. Med. 2024, 13, 3279. https://doi.org/10.3390/jcm13113279
Haak F, Karli T, Takes M, Zech CJ, Kollmar O, Soysal SD. A Retrospective Cohort Analysis of Transarterial Chemoembolization for Hepatocellular Cancer at a Tertiary Center in Switzerland. Journal of Clinical Medicine. 2024; 13(11):3279. https://doi.org/10.3390/jcm13113279
Chicago/Turabian StyleHaak, Fabian, Tobias Karli, Martin Takes, Christoph J. Zech, Otto Kollmar, and Savas D. Soysal. 2024. "A Retrospective Cohort Analysis of Transarterial Chemoembolization for Hepatocellular Cancer at a Tertiary Center in Switzerland" Journal of Clinical Medicine 13, no. 11: 3279. https://doi.org/10.3390/jcm13113279
APA StyleHaak, F., Karli, T., Takes, M., Zech, C. J., Kollmar, O., & Soysal, S. D. (2024). A Retrospective Cohort Analysis of Transarterial Chemoembolization for Hepatocellular Cancer at a Tertiary Center in Switzerland. Journal of Clinical Medicine, 13(11), 3279. https://doi.org/10.3390/jcm13113279






