Dexmedetomidine as an Adjuvant to Nerve Block for Cancer Surgery: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Selection of Studies
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Clinical Outcomes
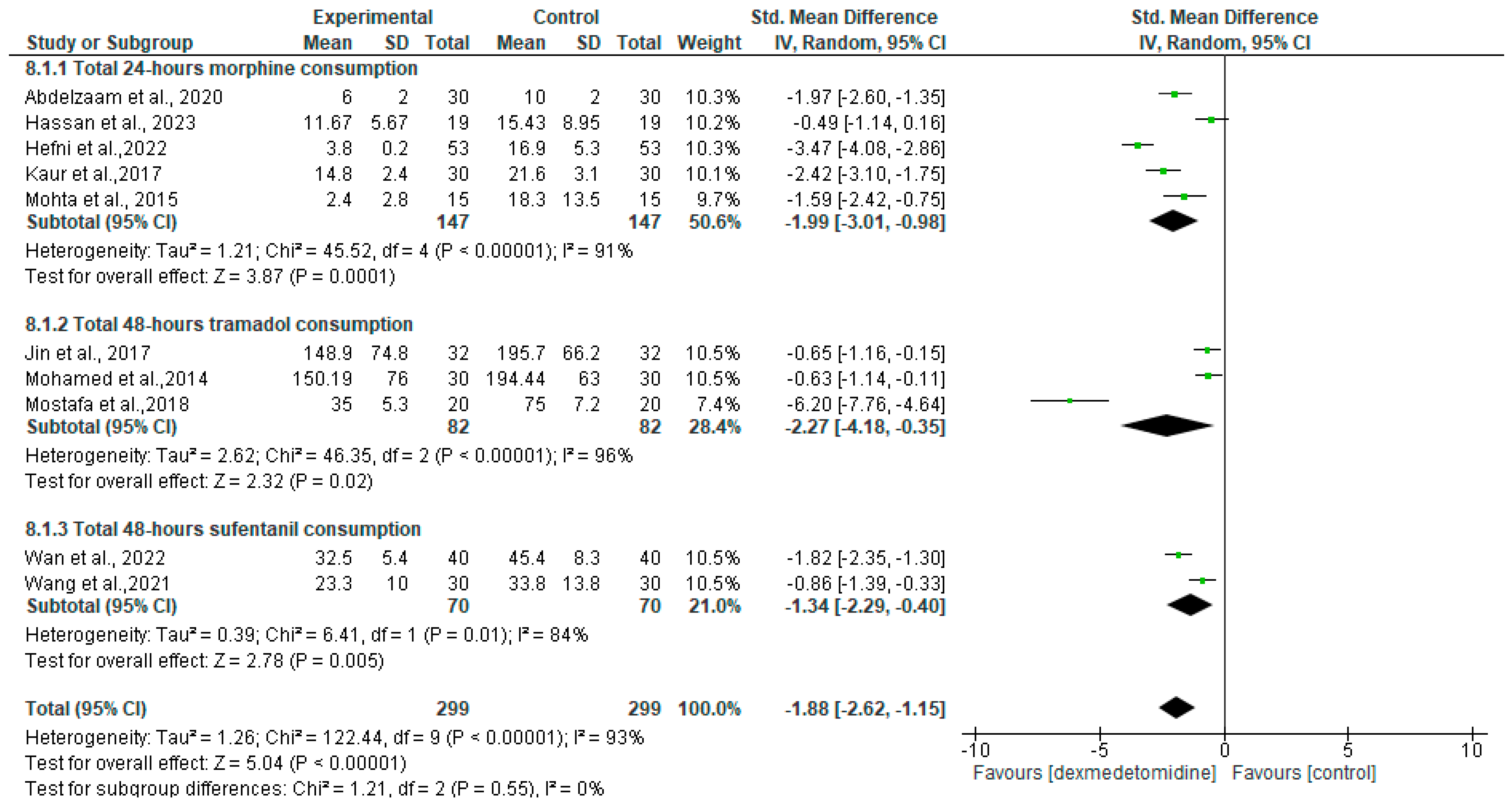
3.4. Intraoperative Outcome
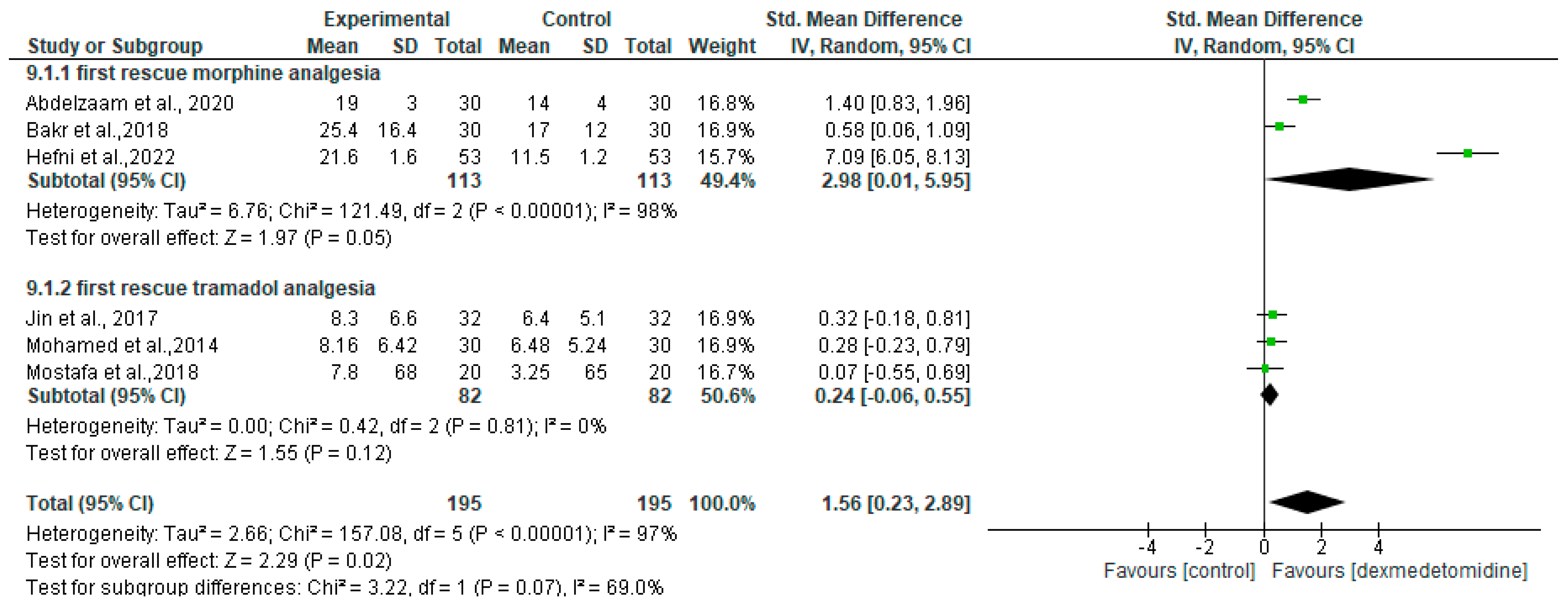
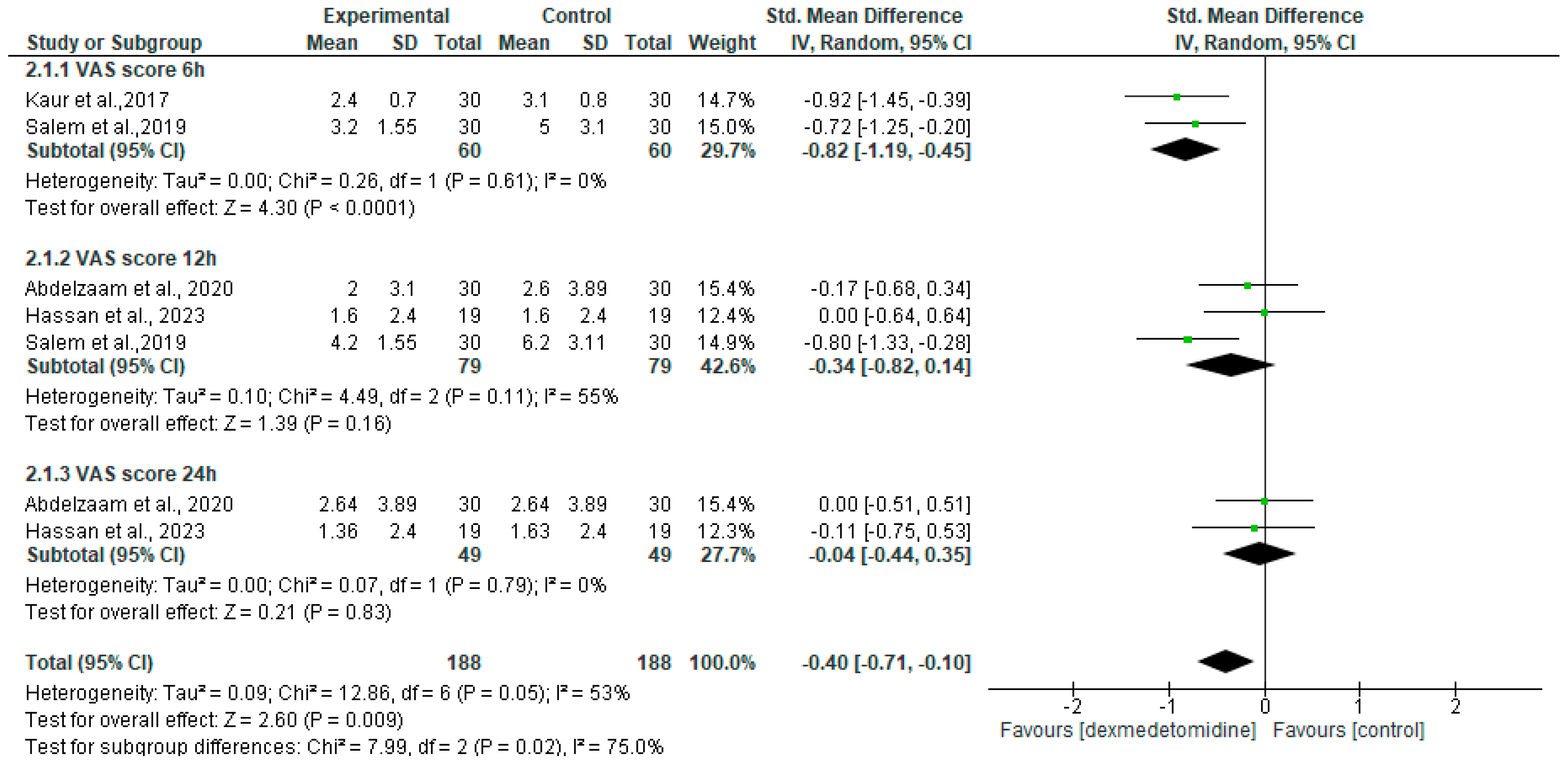
3.5. Postoperative Mean Pain Score
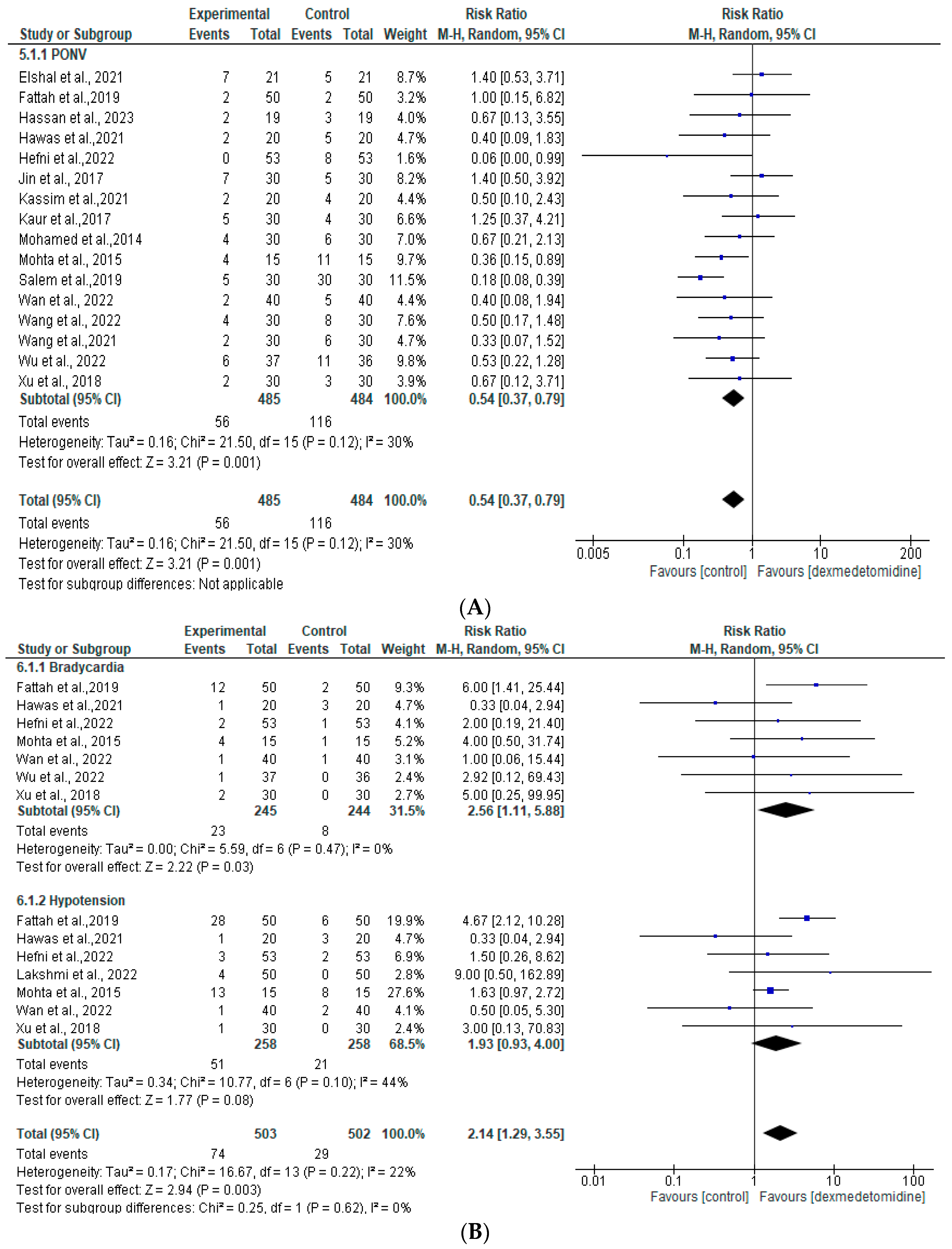
3.6. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, F.M.; Beckman, M. Updates on Management of Gastric Cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef]
- Coffey, J.; Wang, J.; Smith, M.; Bouchier-Hayes, D.; Cotter, T.; Redmond, H. Excisional Surgery for Cancer Cure: Therapy at a Cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Brogi, E.; Forfori, F. Anesthesia and Cancer Recurrence: An Overview. J. Anesth. Analg. Crit. Care 2022, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Montejano, J.; Jevtovic-Todorovic, V. Anesthesia and Cancer, Friend or Foe? A Narrative Review. Front. Oncol. 2021, 11, 803266. [Google Scholar] [CrossRef]
- Xie, S.; Li, L.; Meng, F.; Wang, H. Regional Anesthesia Might Reduce Recurrence and Metastasis Rates in Adult Patients with Cancers after Surgery: A Meta-Analysis. BMC Anesthesiol. 2024, 24, 19. [Google Scholar] [CrossRef]
- Tedore, T. Regional Anaesthesia and Analgesia: Relationship to Cancer Recurrence and Survival. Br. J. Anaesth. 2015, 115, ii34–ii45. [Google Scholar] [CrossRef]
- Naccache, N.; Jabbour, H.; Nasser-Ayoub, E.; Abou Zeid, H.; Naja, Z. Regional Analgesia and Breast Cancer Surgery. J. Med. Liban. 2019, 57, 110–114. [Google Scholar]
- Lee, S. Dexmedetomidine: Present and Future Directions. Korean J. Anesthesiol. 2019, 72, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Hetta, D.F.; Fares, K.M.; Abedalmohsen, A.M.; Abdel-Wahab, A.H.; Elfadl, G.M.A.; Ali, W.N. Epidural Dexmedetomidine Infusion for Perioperative Analgesia in Patients Undergoing Abdominal Cancer Surgery: Randomized Trial. J. Pain Res. 2018, 11, 2675–2685. [Google Scholar] [CrossRef]
- Kwak, H.J.; Chang, Y.J.; Lee, K.C.; Jung, W.S.; Kwon, S.; Jo, Y.Y. Antiemetic Efficacy of Dexmedetomidine versus Dexmedetomidine-Dexamethasone Combination in Patients Undergoing Breast Surgery. J. Int. Med. Res. 2019, 47, 5060–5069. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R.; Bonner, A.; Alexander, P.E.; Siemieniuk, R.A.; Furukawa, T.A.; Rochwerg, B.; Hazlewood, G.S.; Alhazzani, W.; Mustafa, R.A.; Murad, M.H.; et al. Advances in the GRADE Approach to Rate the Certainty in Estimates from a Network Meta-Analysis. J. Clin. Epidemiol. 2018, 93, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Abdelzaam, E.m.; Abd Alazeem, E.S. Efficacy of Dexmedetomidine as an Adjuvant to Bupivacaine in the Ultrasound-Guided Serratus Anterior Plane Block for Postmastectomy Analgesia. Egypt. J. Anaesth. 2020, 36, 319–323. [Google Scholar] [CrossRef]
- Bakr, M.A.; Mohamed, S.A.; Mohamad, M.F.; Mohamed, M.A.; El Sherif, F.A.; Mosad, E.; Abel-Hamed, M.F. Effect of Dexmedetomidine Added to Modified Pectoral Block on Postoperative Pain and Stress Response in Patient Undergoing Modified Radical Mastectomy. Pain Physician 2018, 21, 87–96. [Google Scholar]
- Hawas, A.M.; Ebrahim Elhossary, Z.; El Desouky, I.; Abd, M.M.; Zakzouk, A. Analgesia in Unilateral Breast Surgeries Using Ultra Sound-Guided Serratus Plane Block with Dexmedetomidine versus Fentanyl with Bupivacaine. J. Cardiovasc. Dis. Res. 2021, 12, 819–826. [Google Scholar]
- Hefni, A.F.; Eldeek, A.M.; Ismael, S.A.; Shaban, A.R. Comparing Effect of Adding Ketamine Versus Dexmedetomidine to Bupivacaine in Pecs- Block on Postoperative Pain Control in Patients Undergoing Breast Surgery. Clin. J. Pain 2022, 38, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.J.; Wen, L.Y.; Zhang, Y.L.; Li, G.; Sun, P.; Zhou, X. Thoracic Paravertebral Regional Anesthesia for Pain Relief in Patients with Breast Cancer Surgery. Medicine 2017, 96, e8107. [Google Scholar] [CrossRef]
- Kaur, H.; Arora, P.; Singh, G.; Singh, A.; Aggarwal, S.; Kumar, M. Dexmedetomidine as an Adjunctive Analgesic to Ropivacaine in Pectoral Nerve Block in Oncological Breast Surgery: A Randomized Double-Blind Prospective Study. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, K.B.; Gollapalli, V.K.; Pativada, V.R. Effects of Adding Dexmedetomidine to Ropivacaine for Paravertebral Block in Breast Cancer Surgery. Eur. J. Mol. Clin. Med. 2022, 9, 210–218. [Google Scholar]
- Mohamed, S.A.; Fares, K.M.; Mohamed, A.A.; Aliedin, N.H. Dexmedetomidine as an Adjunctive Analgesic with Bupivacaine in Paravertebral Analgesia for Breast Cancer Surgery. Pain Physician 2014, 17, 589–598. [Google Scholar] [CrossRef]
- Mohta, M.; Kalra, B.; Sethi, A.K.; Kaur, N. Efficacy of Dexmedetomidine as an Adjuvant in Paravertebral Block in Breast Cancer Surgery. J. Anesth. 2016, 30, 252–260. [Google Scholar] [CrossRef]
- Mostafa, M.O.; Botros, J.M.; Khaleel, A.M.S. Effect of Dexmedetomidine versus Nalbuphine as an Adjuvant on Paravertebral Block to Manage Postoperative Pain after Mastectomies. Anesth. Pain Med. 2018, 8, e13308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ran, G.; Chen, X.; Xie, C.; Wang, J.; Liu, X.; Lu, Y.; Fang, W. The Effect of Ultrasound-Guided Erector Spinae Plane Block Combined with Dexmedetomidine on Postoperative Analgesia in Patients Undergoing Modified Radical Mastectomy: A Randomized Controlled Trial. Pain Ther. 2021, 10, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kang, Y.; Li, Y.; Fu, B. Impact of Ultrasound-Guided Deep Serratus Anterior Plane Block Combined with Dexmedetomidine as an Adjuvant to Ropivacaine Inpatient Quality of Recovery Scores Undergoing Modified Radical Mastectomy: A Randomized Controlled Trial. Front. Oncol. 2022, 12, 858030. [Google Scholar] [CrossRef] [PubMed]
- Salem, W.T.; Alsamahy, K.A.; Ibrahim, W.A.; Alsaed, A.S.; Salaheldin, M.M. Effect of Adding Dexmedetomidine to Bupivacaine in Ultrasound Guided Rectus Sheath Block: A Randomized Controlled Double-Blinded Study. Open Anesth. J. 2019, 13, 25–30. [Google Scholar] [CrossRef]
- Wan, W.; Hou, Z.; Qiu, Q. Postoperative Analgesic Effect of Dexmedetomidine Combined with TPVB Applied to Open Gastrectomy for Gastric Cancer. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Wei, S.; Zhang, G.; Ni, C.; Sun, L.; Zheng, H. Dexmedetomidine Added to Ropivacaine for Ultrasoundguided Erector Spinae Plane Block Prolongs Analgesia Duration and Reduces Perioperative Opioid Consumption after Thoracotomy: A Randomized, Controlled Clinical Study. Clin. J. Pain 2022, 38, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Elshal, M.M.; Gamal, R.M.; Ahmed, A.M.; Gouda, N.M.; Abdelhaq, M.M. Efficacy of Adding Dexmedetomidine as Adjuvant with Bupivacaine in Ultrasound-Guided Erector Spinae Plane Block for Post Thoracotomy Pain: Randomized Controlled Study: Dexmedetomidine as Adjuvant in ESPB for PTP. Egypt. J. Anaesth. 2021, 37, 425–431. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Hu, X.; Chen, X.; Zhang, J.; Wang, Y. Multilevel Thoracic Paravertebral Block Using Ropivacaine with/without Dexmedetomidine in Video-Assisted Thoracoscopic Surgery. J. Cardiothorac. Vasc. Anesth. 2018, 32, 318–324. [Google Scholar] [CrossRef]
- Fattah, M.E.A.; Taha, S.N.; Gaber, S.M.H.; Elramley, M.A.; Fathy, M. Comparative Study to Show the Efficacy of Throacic Paravertebral Block, with and without Dexmedetomidine, versus General Anesthesia in Modified Radical Mastectomies. Res. J. Pharm. Biol. Chem. Sci. 2019, 10, 1066–1076. [Google Scholar]
- Hassan, M.E.; Abdelgalil, A.S. Efficacy of Dexmedetomidine as an Adjuvant in Erector Spinae Plane Block in Breast Cancer Surgery: A Randomized Controlled Trial. Anaesth. Pain Intensive Care 2023, 27, 16–22. [Google Scholar] [CrossRef]
- Kassim, D.Y.; Mahmoud, H.E.M.; Fakhry, D.M.; Mansour, M.A.E.S. Comparative Study of Dexmedetomidine versus Fentanyl as Adjuvants to Bupivacaine in Ultrasound-Guided Transversus Abdominis Plane Block in Patients Undergoing Radical Cystectomy: A Prospective Randomised Study. BMC Anesthesiol. 2022, 22, 340. [Google Scholar] [CrossRef]
- Ke, H.H.; Liou, J.Y.; Teng, W.N.; Hsu, P.K.; Tsou, M.Y.; Chang, W.K.; Ting, C.K. Opioid-Sparing Anesthesia with Dexmedetomidine Provides Stable Hemodynamic and Short Hospital Stay in Non-Intubated Video-Assisted Thoracoscopic Surgery: A Propensity Score Matching Cohort Study. BMC Anesthesiol. 2023, 23, 110. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Feng, C.; Jin, Y.; Zhao, X. Dexmedetomidine as an Adjuvant in Peripheral Nerve Block. Drug Des. Dev. Ther. 2023, 17, 1463–1484. [Google Scholar] [CrossRef]
- Bao, N.; Shi, K.; Wu, Y.Q.; He, Y.; Chen, Z.; Gao, Y.; Xia, Y.; Papadimos, T.J.; Wang, Q.; Zhou, R. Dexmedetomidine Prolongs the Duration of Local Anesthetics When Used as an Adjuvant through Both Perineural and Systemic Mechanisms: A Prospective Randomized Double-Blinded Trial. BMC Anesthesiol. 2022, 22, 176. [Google Scholar] [CrossRef]
- Donatiello, V.; Alfieri, A.; Napolitano, A.; Maffei, V.; Coppolino, F.; Pota, V.; Passavanti, M.B.; Pace, M.C.; Sansone, P. Opioid Sparing Effect of Intravenous Dexmedetomidine in Orthopaedic Surgery: A Retrospective Analysis. J. Anesth. Analg. Crit. Care 2022, 2, 49. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Z.; Li, Z.; Dai, W.; Lyu, J.; Bai, X. Perineural Dexmedetomidine in Femoral Nerve Blocks Increases the Duration of Postoperative Analgesia for Anterolateral Thigh Flap Donor Sites in Patients with Oral Cancer. Neurosci. Lett. 2023, 812, 137369. [Google Scholar] [CrossRef]
- El Sherif, F.A.; Abdel-Ghaffar, H.; Othman, A.; Mohamed, S.; Omran, M.; Shouman, S.; Hassan, N.; Allam, A.; Hassan, S. Pharmacokinetics and Pharmacodynamics of Dexmedetomidine Administered as an Adjunct to Bupivacaine for Transversus Abdominis Plane Block in Patients Undergoing Lower Abdominal Cancer Surgery. J. Pain Res. 2022, 15, 1–12. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X. Dexmedetomidine Contributes to Reduced Anesthesia Dosages and Improves Anesthetic Effectiveness in the Radical Resection of Gastric Cancer. Int. J. Clin. Exp. Med. 2020, 13, 6533–6541. [Google Scholar]
- Lee, C.; Kim, Y.D.; Kim, J.N. Antihyperalgesic Effects of Dexmedetomidine on High-Dose Remifentanil-Induced Hyperalgesia. Korean J. Anesthesiol. 2013, 64, 301–307. [Google Scholar] [CrossRef]
- Jain, G.; Bansal, P.; Ahmad, B.; Singh, D.; Yadav, G. Effect of the Perioperative Infusion of Dexmedetomidine on Chronic Pain after Breast Surgery. Indian J. Palliat. Care 2012, 18, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Fares, K.M.; Othman, A.H.; Alieldin, N.H. Efficacy and Safety of Dexmedetomidine Added to Caudal Bupivacaine in Pediatric Major Abdominal Cancer Surgery. Pain Physician 2014, 17, 393–400. [Google Scholar]
- Mazy, A.; El-Domiaty, A.; Mageed, N.A.; Motawi, A.A.; Messeha, M. Comparison between Thoracic Paravertebral Block and Segmental Thoracic Spinal Anesthesia in Breast Cancer Surgery. Ain-Shams J. Anesthesiol. 2022, 14, 88. [Google Scholar] [CrossRef]
- Abdallah, F.W.; Dwyer, T.; Chan, V.W.S.; Niazi, A.U.; Ogilvie-Harris, D.J.; Oldfield, S.; Patel, R.; Oh, J.; Brull, R. IV and Perineural Dexmedetomidine Similarly Prolong the Duration of Analgesia after Interscalene Brachial Plexus Block: A Randomized, Three-Arm, Triple-Masked, Placebo-Controlled Trial. Anesthesiology 2016, 124, 683–695. [Google Scholar] [CrossRef]
- Marhofer, D.; Kettner, S.C.; Marhofer, P.; Pils, S.; Weber, M.; Zeitlinger, M. Dexmedetomidine as an Adjuvant to Ropivacaine Prolongs Peripheral Nerve Block: A Volunteer Study. Br. J. Anaesth. 2013, 110, 438–442. [Google Scholar] [CrossRef]
- Gao, Z.; Xiao, Y.; Wang, Q.; Li, Y. Comparison of Dexmedetomidine and Dexamethasone as Adjuvant for Ropivacaine in Ultrasound-Guided Erector Spinae Plane Block for Video-Assisted Thoracoscopic Lobectomy Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Transl. Med. 2019, 7, 668. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; He, Z.; Sun, Z.; Wu, X.; Zhong, J. Protective Effect of Dexmedetomidine Infusion Combined with Epidural Blockade on Postoperative Complications after Surgery: A Prospective Randomized Controlled Clinical Trial. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Das, R.; Das, R.K.; Sahoo, S.; Nanda, S. Role of Dexmedetomidine as an Anaesthetic Adjuvant in Breast Cancer Surgery as a Day-Care Procedure: A Randomised Controlled Study. Indian J. Anaesth. 2018, 62, 182–187. [Google Scholar] [CrossRef]
- Bielka, K.; Kuchyn, I.; Babych, V.; Martycshenko, K.; Inozemtsev, O. Dexmedetomidine Infusion as an Analgesic Adjuvant during Laparoscopic Cholecystectomy: A Randomized Controlled Study. BMC Anesthesiol. 2018, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Fu, R.; Lei, W. Beneficial Effects of Dexmedetomidine on Early Postoperative Cognitive Dysfunction in Pediatric Patients with Tonsillectomy. Exp. Ther. Med. 2018, 16, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kong, L.S.; Zhu, X.X.; Wang, R.X.; Liu, Y.; Chen, L.R. Effect of Dexmedetomidine on Postoperative Cognitive Dysfunction and Inflammation in Patients after General Anaesthesia: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 2019, 98, e15383. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the Clinical Importance of Treatment Outcomes in Chronic Pain Clinical Trials: IMMPACT Recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Orhon Ergun, M.; Guclu Ozturk, E.; Zengin, S.U. Effects of Erector Spinae Plane Block on Postoperative Pain and Quality of Recovery Questionnaire Scores in Video-Assisted Thoracoscopic Surgery: A Randomized Controlled Study. Cureus 2023, 15, e36089. [Google Scholar] [CrossRef]
- Dyck, J.B.; Maze, M.; Haack, C.; Vuorilehto, L.; Shafer, S.L. The Pharmacokinetics and Hemodynamic Effects of Intravenous and Intramuscular Dexmedetomidine Hydrochloride in Adult Human Volunteers. Anesthesiology 1993, 78, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Vilo, S.; Rautiainen, P.; Kaisti, K.; Aantaa, R.; Scheinin, M.; Manner, T.; Olkkola, K.T. Pharmacokinetics of Intravenous Dexmedetomidine in Children under 11 Yr of Age. Br. J. Anaesth. 2008, 100, 697–700. [Google Scholar] [CrossRef]
- Nair, A.S.; Saifuddin, M.S.; Naik, V.; Rayani, B.K. Dexmedetomidine in Cancer Surgeries: Present Status and Consequences with Its Use. Indian J. Cancer 2020, 57, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhou, M.; Feng, J.-J.; Wu, L.; Fang, S.-P.; Ge, X.-Y.; Sun, H.-J.; Ren, P.-C.; Lv, X. Efficacy of Dexmedetomidine on Postoperative Nausea and Vomiting: A Meta-Analysis of Randomized Controlled Trials. Int. J. Clin. Exp. Med. 2015, 8, 8450–8471. [Google Scholar] [PubMed]
- Long, Y.; Wang, D.; Chen, S.; Xu, Y.; Feng, C.; Ji, F.-H.; Cheng, H.; Peng, K. Effect of Balanced Opioid-Free Anaesthesia on Postoperative Nausea and Vomiting after Video-Assisted Thoracoscopic Lung Resection: Protocol for a Randomised Controlled Trial. BMJ Open 2022, 12, e066202. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Pelli, M.; Loffredo, C.; La Regina, R.; Policastro, F.; Fiorelli, S.; De Blasi, R.A.; Coluzzi, F.; Rocco, M. Opioid-Free Anesthesia versus Opioid-Inclusive Anesthesia for Breast Cancer Surgery: A Retrospective Study. J. Anesth. Analg. Crit. Care 2021, 1, 6. [Google Scholar] [CrossRef]

| Reference | Study Design | Country | Surgery | Sample Size | Age (y) | ASA Physical Status | Dosage and Administration | Comparison | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD or Median (IQR) | |||||||||||
| Intervention | Control | Intervention | Control | Intervention | Control | ||||||
| N | N | ||||||||||
| Abdelzaam et al., 2020 [13] | RCT | Egypt | Breast cancer surgery | 30 | 30 | 46 ± 6 | 43 ± 7 | I–II | I–II | GA + serratus plane block: 0.25% bupivacaine 0.5 mL/kg + dexmedetomidine 0.5 μg/kg | GA + serratus plane block: 0.25% bupivacaine 0.5 mL/kg |
| Bakr et al., 2018 [14] | RCT | Egypt | Breast cancer surgery | 30 | 30 | 47.3 ± 9.7 | 48.5 ± 13.7 | I–II | I–II | GA + pecs block: 30 mL of 0.25% bupivacaine + 1 μg/kg dexmedetomidine | GA + pecs block: 30 mL of 0.25% bupivacaine |
| Fattah et al., 2019 [30] | RCT | Egypt | Breast cancer surgery | 50 | 50 | 20–55 | 20–55 | II–III | II–III | GA + TPVB: 20 mL of bupivacaine 0.5% + 0.5 μg/kg dexmedetomidine | GA + TPVB: 20 mL of 0.5% bupivacaine |
| Hassan et al., 2023 [31] | RCT | Egypt | Breast cancer surgery | 19 | 19 | 45.4 ± 12.7 | 49.5 ± 11.9 | II | II | GA + ESPB: 19 mL of bupivacaine 0.5% + 1 mL of normal saline containing 1 μg/kg dexmedetomidine | GA + ESPB: 20 mL of bupivacaine 0.5% |
| Hawas et al., 2021 [15] | RCT | Egypt | Breast cancer surgery | 20 | 20 | 41.8 ± 2.6 | 45.3 ± 1.7 | II | II | GA + serratus plane block: 30 mL of bupivacaine 0.25% + 1 μg/kg dexmedetomidine | GA + serratus plane block: 30 mL of bupivacaine 0.25% + 25 μg fentanyl |
| Hefni et al., 2022 [16] | RCT | Egypt | Breast cancer surgery | 53 | 53 | 47.2 ± 5.2 | 46.1 ± 4.2 | I–III | I–III | GA + pecs block: 30 mL of bupivacaine 0.25% + 1 μg/kg dexmedetomidine in 2 mL volume | GA + pecs block: 30 mL of bupivacaine 0.25% |
| Jin et al., 2017 [17] | RCT | China | Breast cancer surgery | 32 | 32 | 57.6 ± 10.3 | 58.8 ± 11.0 | I–III | I–III | GA + TPVB: 20 mL of 0.25% bupivacaine + 1 mg/kg dexmedetomidine | GA + TPVB: 20 mL of 0.25% bupivacaine |
| Kaur et al., 2017 [18] | RCT | India | Breast cancer surgery | 30 | 30 | 51.6 ± 10 | 46.2 ± 10 | I–II | I–II | GA + pecs block: 30 mL of 0.25% ropivacaine + dexmedetomidine 1 μg/kg | GA + pecs block: 30 mL of 0.25% ropivacaine |
| Lakshmi et al., 2022 [19] | RCT | India | Breast cancer surgery | 50 | 50 | 50.84 ± 6.36 | 47.8 ± 4.94 | I–III | I–III | GA + TPVB: 0.3 mL/kg of ropivacaine 0.5% + 1 μg/kg Dexmedetomidine | GA + TPVB: 0.3 mL/kg Ropivacaine 0.5% + 1 mL normal saline |
| Mohamed et al., 2014 [20] | RCT | Egypt | Breast cancer surgery | 30 | 30 | 50.50 ± 7.7 | 50.36 ± 60 | I–III | I–III | GA + TVPB: 20 mL of bupivacaine 0.25% + 1 μg/kg dexmedetomidine | GA + TVPB: 20 mL of bupivacaine 0.25% |
| Mohta et al., 2015 [21] | RCT | India | Breast cancer surgery | 15 | 15 | 46.6 ± 10.5 | 49.9 ± 10.6 | I–III | I–III | GA + PVB: 0.3 mL/kg of 0.5% bupivacaine + dexmedetomidine 1 μg/kg in a volume of 1 mL | GA + PVB: 0.3 mL/kg of 0.5% bupivacaine + 1 mL normal saline |
| Mostafa et al., 2018 [22] | RCT | Egypt | Breast cancer surgery | 20 | 20 | 55.9 ± 6 | 55.8 ± 5.8 | I–III | I–III | GA + PVB: 0.3 mL/kg of 0.5% bupivacaine + dexmedetomidine 1 µg/kg | GA + PVB: 0.3 mL/kg of 0.5% bupivacaine + normal saline 1 mL |
| Wang et al., 2021 [23] | RCT | China | Breast cancer surgery | 30 | 30 | 51.93 ± 9.18 | 52.83 ± 8.76 | I–II | I–II | GA + ESPB: 30 mL of 0.33 ropivacaine + 30 mL of dexmedetomidine 1 μg/kg | GA + ESPB: 30 mL of 0.33% ropivacaine |
| Wu et al., 2022 [24] | RCT | China | Breast cancer surgery | 37 | 36 | 54.62 ± 7.44 | 54.08 ± 6.28 | I–II | I–II | GA + dSAPB: 30 mL of 0.375% ropivacaine + dexmedetomidine 1 μg/kg | GA + dSAPB: 30 mL of 0.375% ropivacaine |
| Salem et al., 2019 [25] | RCT | Egypt | Abdominal cancer surgery | 30 | 30 | NR | NR | I–II | I–II | GA + BRSB: 20 mL of 0.25% bupivacaine + dexmedetomidine 2 μg/kg | GA + BRSB: 20 mL of 0.25% bupivacaine |
| Wan et al., 2022 [26] | RCT | China | Gastric cancer | 40 | 40 | 58.6 ± 10.77 | 57.2 ± 11.34 | I–III | I–III | GA + TVPB: 15 mL 0.5% of ropivacaine + 2 mL dexmedetomidine (1 μg/kg) | GA + TVPB: 15 mL of ropivacaine (0.5%) + 2 mL normal saline |
| Wang et al., 2022 [27] | RCT | China | Abdominal cancer surgery | 30 | 30 | 57.9 ± 6.0 | 55.8 ± 7.0 | I–III | I–III | GA + ESPB: 28 mL of 0.5% ropivacaine + interfascial dexmedetomidine 0.5 μg/kg in 2 mL | GA + ESPB: 28 mL of 0.5% ropivacaine + 2 mL of normal saline |
| Elshal et al., 2021 [28] | RCT | Egypt | Thoracic cancer surgey | 21 | 21 | 46.76 ± 9.89 | 44.62 ± 10.77 | II | II | GA + ESPB: 28 mL of bupivacaine 0.25% + 2 mL of dexmedetomidine 0.5 μg/kg | GA + ESPB: 28 mL of bupivacaine 0.25% + 2 mL saline |
| Xu et al., 2018 [29] | RCT | China | Lung cancer | 30 | 30 | 59.2 ± 7 9.7 | 59.5 ± 7 9.7 | I–II | I–II | GA + TVPB: 75 mg/20 mL of ropivacaine 0.375% + dexmedetomidine 1 μg/kg | GA + TVPB: 75 mg/20 mL of ropivacaine 0.375% |
| Kassim et al., 2021 [32] | RCT | Egypt | Radikal cystectomy | 20 | 20 | 61.5 ± 6.8 | 60.4 ± 6.8 | I–II | I–II | GA + TAP block: 20 mL of 0.25% bupivacaine + dexmedetomidine 1 μg/kg | GA + TAP block: 20 mL of 0.25% bupivacaine + 2 mL normal saline |
| Reference | Clinical Outcome | Intraoperative Outcome | Mean Pain Score | Laboratory Outcome | Any Adverse Events | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | (Mean ± SD) | N (%) | ||||||
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Abdelzaam et al., 2020 [13] | Time of first rescue dose (h): 19 ± 3 Total morphine consumption 24 h postoperatively (mg): 6 ± 2 | Time of first rescue dose (h): 14 ± 4 Total morphine consumption 24 h postoperatively (mg): 10 ± 2 | NR | NR | VAS score at rest: PACU: 1 (0–4) 4 h: 1 (0–3) 8 h: 2 (0–4) 12 h: 2 (0–4) 16 h: 2 (0–5) 20 h: 3 (0–5) 24 h: 3 (0–5) VAS score at movement: PACU: 1 (0–4) 4 h: 1 (0–4) 8 h: 2 (0–4) 12 h: 2 (0–4) 16 h: 2 (0–4) 20 h: 3(0–6) 24 h: 3(0–6) | VAS score at rest: PACU: 0 (0–4) 4 h: 1 (0–4) 8 h: 2 (0–3) 12 h: 3 (0–5) 16 h: 3 (0–5) 20 h: 3 (0–5) 24 h: 3 (0–5) VAS score at movement: PACU: 0 (0–4) 4 h: 1 (0–4) 8 h: 2 (1–3) 12 h: 3(1–6) 16 h: 3(0–5) 20 h: 4(0–6) 24 h: 4(0–6) | NR | NR | NR | NR |
| Bakr et al., 2018 [14] | Time to first request of analgesia (h): 25.4 ± 16.4 Total PCA morphine 48 h postoperatively (mg): 9 ± 3.6 | Time to first request of analgesia (h): 17 ± 12 Total PCA morphine 48 h postoperatively (mg): 12 ± 3.6 | NR | NR | VAS score 12 h: 2.1 ± 1 | VAS score 12 h: 2.7 ± 1.1 | Cortisol level (μg/dL): 205.9 ± 142.6 prolactin level (ng/mL): 28.3 ± 22.1 | Cortisol level (μg/dL): 257.3 ± 163.2 prolactin level (ng/mL): 41.7 ± 21.2 | NR | NR |
| Fattah et al., 2019 [30] | Total opioid consumption 24 h postoperatively (mg): 5 Need for extra sedation: 27 (61.4) | Total opioid consumption 24 h postoperatively (mg): 5.15 Need for extra sedation: 28 (63.6) | NR | NR | VAS score 30 min: 1.5 ± 1 | VAS score 30 min: 1 ± 1.75 | NR | NR | Nausea and vomiting: 2 (4.5%) Bradycardia: 12 (27.3%) Hypotension: 28 (63.6%) | Nausea and vomiting: 2 (4.5%) Bradycardia: 2 (4.5%) Hypotension: 6 (13.6%) |
| Hassan et al., 2023 [31] | Total postoperative morphine consumption 24 h postoperatively (mg): 13.0 (6.0–32.0) | Total postoperative morphine consumption 24 h postoperatively (mg): 11.0 (4.0–22.0) | Intraoperative fentanyl consumption (μg): 110 (60.0−210.0) | Intraoperative fentanyl consumption (μg): 120.0 (60.0−200.0) | Postoperative numeric rating score at rest: After 30 min: 2 (1–3) After 2 h: 2 (0–3) After 4 h: 2 (0–3) After 8 h: 2 (0–3) After 12 h: 2 (0–3) After 24 h: 1 (0–3) Postoperative numeric rating score on movement: After 30 min: 3 (2–6) After 2 h: 3 (1–5) After 4 h: 3 (1–5) After 8 h: 3 (1–6) After 12 h: 3 (1–6) After 24 h: 2 (1–4) | Postoperative numeric rating score at rest After 30 min: 2 (1–3) After 2 h: 2 (0–3) After 4 h: 2 (0–3) After 8 h: 2 (0–3) After 12 h: 2 (0–3) After 24 h: 2 (0–3) Postoperative numeric rating score on movement: After 30 min: 3 (1–4) After 2 h: 4 (2–6) After 4 h: 3 (2–4) After 8 h: 3 (2–6) After 12 h: 3 (2–6) After 24 h: 2 (1–4) | NR | NR | Nausea and vomiting (PONV): 2 (10.5%) Pruritis: 0 (0) Respiratory depression: 0 (0) Block-related complication: 0 (0) | Nausea and vomiting (PONV): 3 (15.8%) Pruritis: 0 (0) Respiratory depression: 0 (0) Block-related complication: 0 (0) |
| Hawas et al., 2021 [15] | Total pethidine 24 h postoperatively (mg): 61 ± 12.7 | Total pethidine 24 h postoperatively (mg): 86.2 ± 16.7 | NR | NR | NR | NR | NR | NR | Nausea and vomiting: 2 (10%) Bradycardia and hypotension: 1 (5%) | Nausea and vomiting: 5 (25%) Bradycardia and hypotension: 3 (15%) |
| Hefni et al., 2022 [16] | Time to first rescue analgesic (min): 21.6 ± 1.6 Total 24 h morphine consumption (mg): 3.8 ± 0.2 | Time to first rescue analgesic (min): 11.5 ± 1.2 Total 24 h morphine consumption (mg): 16.9 ± 5.3 | Fentanyl supplementation (μg): 57 ± 7 | Fentanyl supplementation (μg): 58 ± 6 | NR | NR | NR | NR | Nausea and/or vomiting: 0 (0) Bradycardia: 2 (3.7%) Hypotension: 3 (5.6%) | Nausea and/or vomiting: 8 (15.1) Bradycardia: 1 (1.89%) Hypotension: 2 (3.77%) |
| Jin et al., 2017 [17] | Time to first request pain medicine (h): 8.3 ± 6.6 Total tramadol consumption 48 h postoperatively (mg): 148.9 ± 74.8 | Time to first request pain medicine (h): 6.4 ± 5.1 Total tramadol consumption (mg): 195.7 ± 66.2 | Heart rate 0 min: 84.1 ± 6.9 30 min: 68.4 ± 8.3 60 min: 74.6 ± 10.7 120 min: 77.8 ± 9.8 Systolic blood pressure 0 min: 129.1 ± 11.6 30 min: 102.4 ± 11.8 60 min: 124.8 ± 12.2 120 min: 127.0 ± 12.5 Diastolic blood pressure 0 min: 81.4 ± 7.5 30 min: 64.9 ± 7.8 60 min: 75.1 ± 5.9 120 min: 78.0 ± 7.0 | Heart rate 0 min: 83.5 ± 6.8 30 min: 79.1 ± 6.6 60 min: 80.1 ± 8.4 120 min: 80.3 ± 8.5 Systolic blood pressure 0 min: 127.8 ± 12.3 30 min: 120.1 ± 13.3 60 min: 131.9 ± 12.9 120 min: 133.2 ± 8.7 Diastolic blood pressure 0 min: 81.2 ± 7.1 30 min: 76.4 ± 7.4 60 min: 77.7 ± 7.6 120 min: 77.9 ± 6.7 | VAS score 0 h: 2.5 ± 0.4 3 h: 2.2 ± 0.5 6 h: 2.4 ± 0.5 12 h: 2.5 ± 0.6 24 h: 2.4 ± 0.5 36 h: 2.3 ± 0.4 48 h: 2.3 ± 0.4 | VAS score 0 h: 2.6 ± 0.4 3 h: 2.3 ± 0.6 6 h: 2.5 ± 0.6 12 h: 2.7 ± 0.7 24 h: 2.7 ± 0.6 36 h: 2.6 ± 0.5 48 h: 2.5 ± 0.6 | NR | NR | Nausea: 4 (11.1%) Vomiting: 3 (8.3%) Pneumothorax: 1 (2.8%) | Nausea: 3 (8.3%) Vomiting: 2 (5.6%) Pneumothorax: 0 |
| Kaur et al., 2017 [18] | Duration of analgesia (min): 469.6 ± 81.5 Total morphine consumption 24 h postoperatively (mg): 14.8 ± 2.4 | Duration of analgesia (min): 298.2 ± 42.3 Total morphine consumption 24 h postoperatively (mg): 21.6 ± 3.1 | NR | NR | VAS score 1 h: 2.3 ± 0.6 5 h: 2.4 ± 0.7 | VAS score 1 h: 2.4 ± 0.7 5 h: 3.1 ± 0.8 | NR | NR | Nausea and/or vomiting: 5 (16.6%) | Nausea and/or vomiting: 4 (13.3%) |
| Lakshmi et al., 2022 [19] | Total tramadol consumption 24 h postoperatively (mg/kg): 0.88 ± 0.707 Patient satisfaction score: Good (84%), Average (12%), Poor (4%) | Total tramadol consumption 24 h postoperatively (mg/kg): 2.84 ± 0 Patient satisfaction score: Good (28%), Average (56%), Poor (8%) | Heart rate: 79.90 ± 5.89 Onset of sensory blocks (min): 3.4 ± 0.70 | Heart rate: 80.73 ± 6.79 Onset of sensory blocks (min): 4.76 ± 0.707 | NR | NR | NR | NR | Hypotension: 4 (8.0%) | Hypotension: 0 |
| Mohamed et al., 2014 [20] | Time to first request of analgesia (h): 8.16 ± 6.42 Total tramadol consumption 48 h postoperatively (mg): 150.19 ±76.98 | Time to first request of analgesia (h): 6.48 ± 5.24 Total tramadol consumption 48 h postoperatively (mg): 194.44 ± 63.91 | Heart rate 30 min: 69.8 ± 8.29 60 min: 74.43 ± 11.03 Systolic blood pressure 0 min: 130.00 ± 11.49 30 min: 101.33 ± 11.67 120 min: 127.20 ± 12.79 | Heart rate 30 min: 79.33 ± 6.62 60 min: 80.40 ± 8.45 Systolic blood pressure 0 min: 126.57 ± 13.39 30 min: 118.60 ± 13.16 120 min: 134.0 ± 8.46 | VAS score 1 h: 2.46 ± 0.6 | VAS score 1 h: 2.51 ± 0.7 | NR | NR | Nausea and/or vomiting: 4 (13.3%) | Nausea and/or vomiting: 6 (20.0%) |
| Mohta et al., 2015 [21] | Total morphine consumption 24 h postoperatively (mg): 2.4 ± 2.8 PCA morphine requirement 24 h postoperatively (mg): 1.5 ± 2.3 Fentanyl requirement (mcg): 54.6 ± 11.4 Time to mobilize (hour): 23.2 ± 4.0 Time to discharge (days): 5.2 ± 0.4 | Total morphine consumption 24 h postoperatively (mg): 18.3 ± 13.5 PCA morphine requirement 24 h postoperatively (mg): 15.3 ± 13.1 Fentanyl requirement (mcg): 58.0 ± 10.3 Time to mobilize (hour): 43.4 ± 6.1 Time to discharge (days): 5.7 ± 0.5 | NR | NR | NR | NR | NR | NR | Nausea and vomiting (PONV): 4 (26.7%) Hypotension: 13 (86.7%) Bradycardia: 4 (26.7%) | Nausea and vomiting (PONV): 11 (73.3%) Hypotension: 8 (53.3%) Bradycardia: 1 (6.67%) |
| Mostafa et al., 2018 [22] | Time to first request of analgesia (h): 7.8 ± 68 Total tramadol consumptions 48 h postoperatively (mg): 35 ± 5.3 | Time to first request of analgesia (h): 3.25 ± 65 Total tramadol consumptions 48 h postoperatively (mg): 75 ± 7.2 | HR 30 min: 66 ± 7 90 min: 75 ± 6 | HR 30 min: 74 ± 3 60 min: 77 ± 5 | NR | NR | NR | NR | NR | NR |
| Wang et al., 2021 [23] | PACU stay (min): 34.5 (29–48) Length of stay (days): 8 (6–18) Total flurbiprofen consumption 48 h postoperatively (mg): 100 (52–115) | PACU stay (min): 30 (24–45) Length of stay (days): 8 (6–15) Total flurbiprofen consumption 48 h postoperatively (mg): 150 (94–160) | Intraoperative propofol (mg): 461.7 ± 108.6 Intraoperative sufentanil (μg): 25 (20–30) | Intraoperative propofol (mg): 462.6 ± 112.1 Intraoperative sufentanil (μg): 30 (20–35) | VAS score at rest: 1 h: 0 (0–0) 6 h: 1 (1–1) 12 h: 1 (1–1) 24 h: 1 (1–1) 48 h: 1 (1–1) VAS score in movement: 1 h: 1 (1–1) 6 h 1 (1–1) 12 h: 2 (1–2) 24 h: 3 (2–3) 48 h: 3 (2–3) | VAS score at rest: 1 h: 0 (0–1) 6 h: 1 (1–1) 12 h: 1.5 (1–2) 24 h: 2 (1–2) 48 h: 1 (1–2) VAS score in movement: 1 h: 1 (1–1) 6 h: 1 (1–2) 12 h: 2.5 (2–3) 24 h: 3 (3–3) 48 h: 3 (2–3) | NR | NR | Nausea: 2 (6.65) Vomit: 0 Bradycardia: 0 Hypotension: 0 | Nausea: 4 (13.3%) Vomit: 2 (6.6%) Bradycardia: 0 Hypotension: 0 |
| Wu et al., 2022 [24] | PACU stay: 22.43 ± 3.98 Postoperative QoR-15 score: 109.5 (107–114) Patient satisfaction score:8.62 ± 0.59 | PACU stay: 22.06 ± 3.76 Postoperative QoR-15 score: 107 (103–112) Patient satisfaction score: 8.28 ± 0.70 | Total intraoperative propofol consumption (mg): 464.23 ± 28.21 Total intraoperative sufentanil consumption (μg): 21.22 ± 2.98 Total intraoperative remifentanil consumption (μg): 146.74 ± 14.99 | Total intraoperative propofol consumption (mg): 470.27 ± 30.41 Total intraoperative sufentanil consumption (μg): 21.39 ± 3.07 Total intraoperative remifentanil consumption (μg): 151.54 ± 14.58 | VAS score at rest: After 1 h: 0 (0–0.5) After 6 h: 0 (0–1) After 12 h: 1 (1–2) After 24 h: 2 (2–3) After 48 h: 2 (1–2) VAS score during movement: After 1 h: 1 (0–1) After 6 h: 1 (0–1) After 12 h: 2 (1–3) After 24 h: 3 (2–3) After 48 h: 2 (2–3) | VAS score at rest: After 1 h: 0 (0–0.5) After 6 h: 0 (0–1) After 12 h: 1 (1–2) After 24 h: 2 (2–3) After 48 h: 2 (1–2) VAS score during movement: After 1 h: 1 (0–1) After 6 h: 1 (0–1) After 12 h: 2 (1–3) After 24 h: 3 (2–3) After 48 h: 2 (2–3) | NR | NR | Nausea and vomiting (PONV): After 24 h: 6 (16.2%) After 48 h: 5 (13.5%) Bradycardia: 1 (2.7%) Dizziness: 3 (8.1%) Delirium: 0 | Nausea and vomiting (PONV): After 24 h: 11 (30.6%) After 48 h: 8 (22.25%) Bradycardia: 0 Dizziness: 4 (11.1%) Delirium: 0 |
| Salem et al., 2019 [25] | Time to first request of analgesia (h): 15 (4–20) Total Morphine consumption 24 h postoperatively (mg): 1.06± 2.33 | Time to first request of analgesia (h): 6 (4–14) Total Morphine consumption 24 h postoperatively (mg): 3.28 ± 4.67 | NR | NR | VAS score 2 h: 1 (0–2) 6 h: 4 (2–4) 12 h: 5 (3–5) | VAS score 2 h: 2 (0–3) 6 h: 5 (3–7) 12 h: 7 (4–8) | Serum cortisol (μg/dL): baseline: 16.1 ± 1.4 postop: 8.6 ± 1.1 | Serum cortisol (μg/dL): baseline: 16.3 ± 1.4 postop: 14.4 ± 1.8 | Nausea and/or vomiting: 5 (16.6%) | Nausea and/or vomiting: 30 (100%) |
| Wan et al., 2022 [26] | Duration of PCA (h): 4.7 ± 1.5 Total sufentanil consumption via PCA in 48 h after operation (ml): 32.5 ± 5.4 PCA pressing times in 48 h after operation (n): 11.4 ± 1.7 | Duration of PCA (h): 6.3 ± 2.4 Total sufentanil consumption via PCA in 48 h after operation (ml): 45.4 ± 8.3 PCA pressing times in 48 h after operation (n): 14.3 ± 2.5 | NR | NR | NR | NR | NR | NR | Nausea and vomiting (PONV): 2 (5.0%) Cardiac arrhythmia: 0 Respiratory depression: 0 Bradycardia: 1 (2.5%) Hypotension: 1 (2.5%) | Nausea and vomiting (PONV): 5 (12.5%) Cardiac arrhythmia: 0 Respiratory depression: 1 (2.5%) Bradycardia: 1 (2.5%) Hypotension: 2 (5.0%) |
| Wang et al., 2022 [27] | Total sufentanil consumption 48 h postoperatively (μg): 23.3 ± 10.0 | Total sufentanil consumption 48 h postoperatively (μg): 33.8 ± 13.8 | NR | NR | NR | NR | NR | NR | Nausea and/or vomiting: 4 (13.3%) | Nausea and/or vomiting: 8 (26.7%) |
| Elshal et al., 2021 [28] | Patients required rescue analgesia in the 1st 24 h postoperative: 11 (54%) Total morphine consumption in 24 h postoperative (mg): 3 (0–3) Time to first request of rescue analgesia (h): 6 (6–12) | Patients required rescue analgesia in the 1st 24 h postoperative: 15 (71.4%) Total morphine consumption in 24 h postoperative (mg): 3 (0–6) Time to first request of rescue analgesia (h): 4 (3–6) | Total intraoperative fentanyl consumption (μg): 153.33 ± 23.09 | Total intraoperative fentanyl consumption (μg): 169.05 ± 31.88 | NR | NR | NR | NR | Intraoperative hypothermia: 6 (28.6%) Nausea and vomiting (PONV): 7 (33.3%) Block-related complication: 0 | Intraoperative hypothermia: 7 (33.3%) Nausea and vomiting (PONV): 5 (23.8%) Block-related complication: 0 |
| Xu et al., 2018 [29] | Patient satisfaction (5-point Likert scale): 1: 0 2: 0 3: 1 (3.3%) 4: 9 (30%) 5: 20 (66.7%) | Patient satisfaction (5-point Likert scale): 1: 0 2: 0 3: 7 (23.3%) 4: 17 (56.7%) 5: 6 (20.0%) | Total intraoperative fentanyl consumption (μg): 561.7 ± 145.4 | Total intraoperative fentanyl consumption (μg): 583.3 ± 124.1 | NR | NR | NR | NR | Nausea and vomiting (PONV): 2 (6.7%) Bradycardia: 2 (6.7%) Hypotension: 1 (3.3%) Respiratory depression: 0 | Nausea and vomiting (PONV): 3 (10.0%) Bradycardia: 0 Hypotension: 0 Respiratory depression: 0 |
| Kassim et al., 2021 [32] | Time to first request for analgesic (h): 8.90 ± 2.47 Total nalbuphine consumption 24 h postoperatively (mg/kg): 0.15 ± 0.0 | Time to first request for analgesic (h): 4.4 ± 1.05 Total nalbuphine consumption 24 h postoperatively (mg/kg): 0.24 ± 0.08 | NR | NR | VAS score 0 h: 0.45 ± 0.51 2 h: 0.75 ± 0.4 6 h: 2.45 ± 0.51 | VAS score 0 h: 0.90 ± 0.31 2 h: 1.75 ± 0.4 6 h: 3.20 ± 0.6 | NR | NR | Nausea and/or vomiting: 2 (10.0%) | Nausea and/or vomiting: 4 (20%) |
| Outcomes | Effect in Intervention Group | Participants | Studies (n) | Quality of Evidence (GRADE) | |
|---|---|---|---|---|---|
| Dexmedetomidine | Control | ||||
| Total 24 h morphine consumption | SMD = −1.99 [95% CI −3.01 to −0.98] | 147 | 147 | 5 | Low 1,2 |
| Total 48 h postoperative tramadol consumption | SMD = −2.27 [95% CI −4.18 to −0.35] | 82 | 82 | 3 | Low 2,3 |
| Total 48 h postoperative sulfentanil consumption | SMD = −1.34 [95% CI −2.29 to −0.40] | 70 | 70 | 2 | Moderate 2 |
| First rescue morphine analgesia | SMD = 2.98 [95% CI 0.01 to 5.95] | 113 | 113 | 3 | Low 2,3 |
| First rescue tramadol analgesia | SMD = 0.24 [95% CI −0.06 to 0.55] | 82 | 82 | 3 | Moderate 3 |
| Postoperative mean VAS score | SMD = −0.40 [95% CI −0.71 to −0.10] | 188 | 188 | 4 | Low 1,2 |
| Adverse events: postoperative nausea and vomiting | RR 0.54 [95% CI 0.37 to 0.79] | 485 | 484 | 16 | Moderate 1 |
| Adverse events: bradycardia | RR 2.56 [95% CI 1.11 to 5.88] | 245 | 244 | 7 | Moderate 1 |
| Adverse events: hypotension | RR 1.93 [95% CI 0.0.93 to 4.00] | 258 | 258 | 7 | Low 1,2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waloejo, C.S.; Musalim, D.A.P.; Budi, D.S.; Pratama, N.R.; Sulistiawan, S.S.; Wungu, C.D.K. Dexmedetomidine as an Adjuvant to Nerve Block for Cancer Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3166. https://doi.org/10.3390/jcm13113166
Waloejo CS, Musalim DAP, Budi DS, Pratama NR, Sulistiawan SS, Wungu CDK. Dexmedetomidine as an Adjuvant to Nerve Block for Cancer Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(11):3166. https://doi.org/10.3390/jcm13113166
Chicago/Turabian StyleWaloejo, Christrijogo Soemartono, Dian Anggraini Permatasari Musalim, David Setyo Budi, Nando Reza Pratama, Soni Sunarso Sulistiawan, and Citrawati Dyah Kencono Wungu. 2024. "Dexmedetomidine as an Adjuvant to Nerve Block for Cancer Surgery: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 11: 3166. https://doi.org/10.3390/jcm13113166
APA StyleWaloejo, C. S., Musalim, D. A. P., Budi, D. S., Pratama, N. R., Sulistiawan, S. S., & Wungu, C. D. K. (2024). Dexmedetomidine as an Adjuvant to Nerve Block for Cancer Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(11), 3166. https://doi.org/10.3390/jcm13113166






