Sitting Postural Management to Prevent Migration Percentage Progression in Non-Ambulatory Children with Cerebral Palsy: Randomized Controlled Trial Preliminary Data
Abstract
1. Introduction
2. Materials and Methods
- -
- Slow passive ROM (pROM) in the supine position: abduction with the hip and knee flexed at 90° and the hip and knee extended (gracilis), knee extension, and hip extension according to the Thomas test;
- -
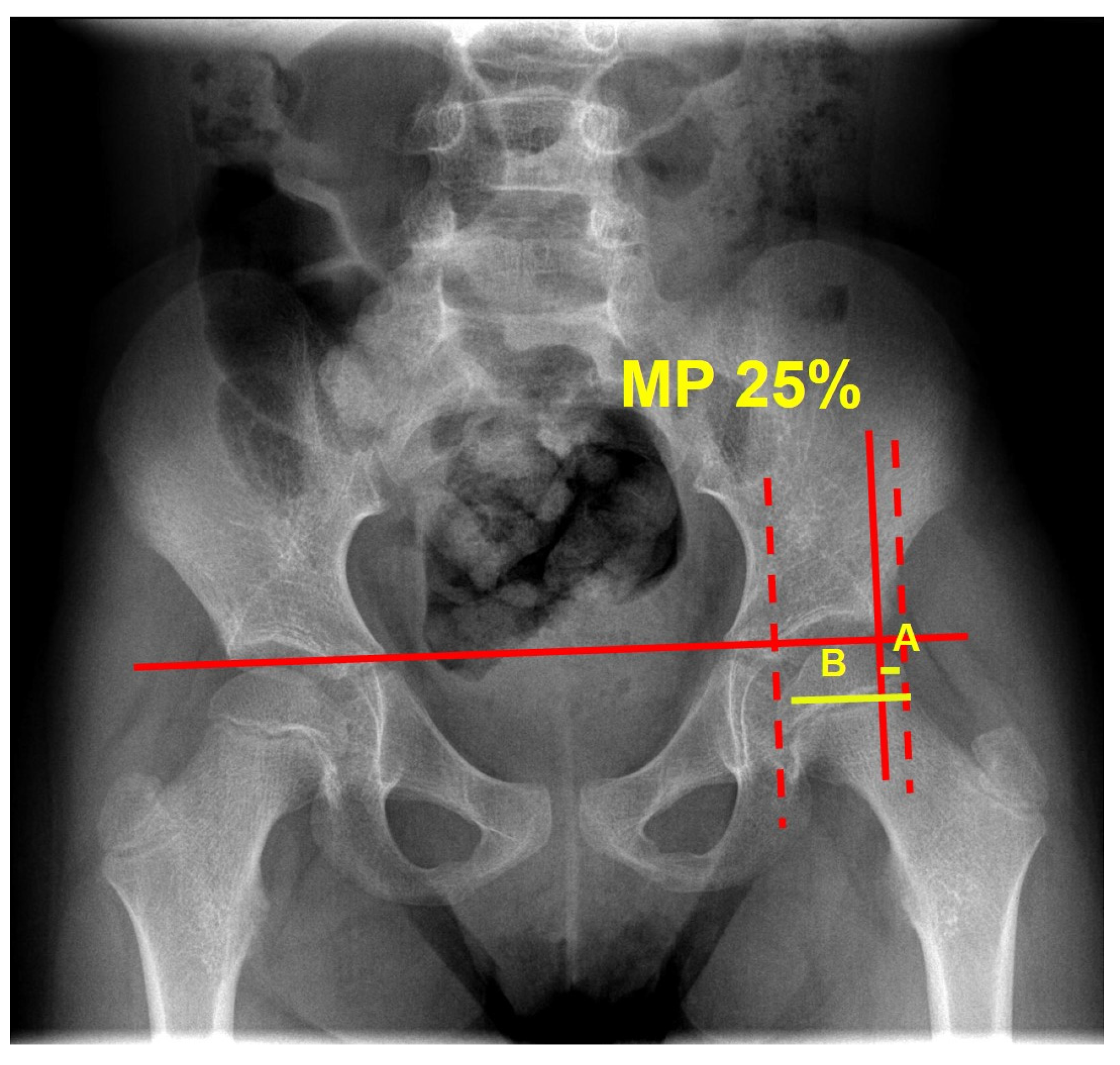
- Pelvic tilt measured by means of degrees on the antero-posterior radiograph, acquired in the supine position, with the legs parallel to each other and the vertical line;
- -
- Total number of botulinum (BoNT-A) injections in the hip muscles over the treatment period;
- -
- Seating system costs for the Italian National Health System;
- -
- Hip pain, as a dichotomous variable (pain/no pain), reported by patients or caregivers or clinically evidenced;
- -
- MRI lesions according to the MRI classification system (MRICS) [28];
- -
- Concurrent spasticity/dystonia treatments such as oral medication (baclofen, etc.), intrathecal baclofen (ITB) pump, and selective dorsal rhizotomy (SDR);
- -
- Ongoing physiotherapy;
- -
- Regular use (at least 1 h/day 5 days/week) of standing devices;
- -
- Sex, age, and CP subtype;
- -
- Costs of the seating devices, as declared in the estimate of the orthopedic workshop.
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robin, J.; Graham, H.K.; Baker, R.; Selber, P.; Simpson, P.; Symons, S.; Thomason, P. A classification system for hip disease in cerebral palsy. Dev. Med. Child. Neurol. 2008, 51, 183–192. [Google Scholar] [CrossRef]
- Terjesen, T. The natural history of hip development in cerebral palsy. Dev. Med. Child. Neurol. 2012, 54, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Larnert, P.; Risto, O.; Hägglund, G.; Wagner, P. Hip displacement in relation to age and gross motor function in children with cerebral palsy. J. Child. Orthop. 2014, 8, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, G.; Alriksson-Schmidt, A.; Lauge-Pedersen, H.; Rodby-Bousquet, E.; Wagner, P.; Westbom, L. Prevention of dislocation of the hip in children with cerebral palsy: 20-year results of a population-based prevention programme. Bone Joint J. 2014, 96-B, 1546–1552. [Google Scholar]
- Faccioli, S.; Sassi, S.; Ferrari, A.; Corradini, E.; Toni, F.; Kaleci, S.; Lombardi, F.; Benedetti, M.G. Hip subluxation in Italian cerebral palsy children and its determinants: A retrospective cohort study. Int. J. Rehabil. Res. 2022, 45, 319–328. [Google Scholar] [CrossRef]
- Wynter, M.; Gibson, N.; Willoughby, K.L.; Love, S.; Kentish, M.; Thomason, P.; Graham, H.K.; National Hip Surveillance Working Group. Australian hip surveillance guidelines for children with cerebral palsy: 5-year review. Dev. Med. Child. Neurol. 2015, 57, 808–820. [Google Scholar] [CrossRef] [PubMed]
- AACPDM Care Pathways. Hip Surveillance Care Pathway Team. Hip Surveillance: Bottom Line ‘Evidence-Informed’ Recommendations for the Hip Surveillance in Individuals with Cerebral Palsy. September. 2017. Available online: https://www.aacpdm.org/UserFiles/file/hip-surveillance-care-pathway.pdf (accessed on 26 May 2020).
- Kiapekos, N.; Broström, E.; Hägglund, G.; Åstrand, P. Primary surgery to prevent hip dislocation in children with cerebral palsy in Sweden: A minimum 5-year follow-up by the national surveillance program (CPUP). Acta Orthop. 2019, 90, 495–500. [Google Scholar] [CrossRef]
- Agarwal, K.N.; Chen, C.; Scher, D.M.; Dodwell, E.R. Migration percentage and odds of recurrence/subsequent surgery after treatment for hip subluxation in pediatric cerebral palsy: A meta-analysis and systematic review. J. Child. Orthop. 2019, 13, 582–592. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Spasticity in under 19s: Management. Clinical Guideline [CG145]. 2016. Available online: https://www.nice.org.uk/guidance/cg145 (accessed on 14 September 2023).
- Wordie, S.J.; Bugler, K.E.; Bessell, P.R.; Robb, J.E.; Gaston, M.S. Hip displacement in children with cerebral palsy. Bone Joint J. 2021, 103-B, 411–414. [Google Scholar] [CrossRef]
- Faccioli, S.; Sassi, S.; Corradini, E.; Toni, F.; Kaleci, S.; Lombardi, F.; Benedetti, M.G. A retrospective cohort study about the hip luxation in non-ambulatory cerebral palsy patients: The point of no return. J. Child. Orthop. 2022, 16, 227–232. [Google Scholar] [CrossRef]
- Picciolini, O.; Consonni, D.; Cozzaglio, M.; Porro, M.; Gasparroni, V.; Panou, A.; Mosca, F.; Portinaro, N.M. Can we prevent hip dislocation in children with cerebral palsy? Effects of postural management. Eur. J. Phys. Rehabil. Med. 2016, 52, 682–690. [Google Scholar]
- Miller, S.D.; Juricic, M.; Hesketh, K.; Mclean, L.; Magnuson, S.; Gasior, S.; Schaeffer, E.; O’Donnell, M.; Mulpuri, K. Prevention of hip displacement in children with cerebral palsy: A systematic review. Dev. Med. Child. Neurol. 2017, 59, 1130–1138. [Google Scholar] [CrossRef]
- Gmelig Meyling, C.; Ketelaar, M.; Kuijper, M.A.; Voorman, J.; Buizer, A.I. Effects of Postural Management on Hip Migration in Children With Cerebral Palsy: A Systematic Review. Pediatr. Phys. Ther. 2018, 30, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Park, D.; Ko, J.Y.; Ryu, J.S. Are Seating Systems With a Medial Knee Support Really Helpful for Hip Displacement in Children With Spastic Cerebral Palsy GMFCS IV and V? Arch. Phys. Med. Rehabil. 2019, 100, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kerr Graham, H.; Boyd, R.; Carlin, J.B.; Dobson, F.; Lowe, K.; Nattrass, G.; Thomason, P.; Wolfe, R.; Reddihough, D. Does botulinum toxin a combined with bracing prevent hip displacement in children with cerebral palsy and “hips at risk”? A randomized, controlled trial. J. Bone Joint Surg. Am. 2008, 90, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, K.; Ang, S.G.; Thomason, P.; Graham, H.K. The impact of botulinum toxin A and abduction bracing on long-term hip development in children with cerebral palsy. Dev. Med. Child. Neurol. 2012, 54, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Martinsson, C.; Himmelmann, K. Abducted Standing in Children With Cerebral Palsy: Effects on Hip Development After 7 Years. Pediatr. Phys. Ther. 2021, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Lespargot, A. La luxation postéro-externe de la hanche chez l’enfant IMC ou polyhandicapé. Mot. Cérébrale 1991, 12, 37–61. [Google Scholar]
- Palisano, R.J.; Rosenbaum, P.; Bartlett, D.; Livingston, M.H. Content validity of the expanded and revised Gross Motor Function Classification System. Dev. Med. Child. Neurol. 2008, 50, 744–750. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 2 May 2020).
- Narayanan, U.G.; Fehlings, D.L.; Weir, S.; Knights, S.; Kiran, S.; Campbell, K. Caregiver Priorities & Child Health Index of Life with Disabilities: Initial development and validation of an outcome measure of health status and well-being in children with severe cerebral palsy. Dev. Med. Child. Neurol. 2006, 48, 804–812. [Google Scholar]
- Sproccati, N.; Bertana, S.; Battisti, N.; Feliciangeli, A.; Baroncini, C.; Zenesini, C.; Cersosimo, A. Italian translation and cross-cultural adaptation of the questionnaire for the assessment of quality of life in children with cerebral palsy: Caregiver priorities and child health index of life with disabilities. Minerva Med. 2021, 112, 651–653. [Google Scholar] [CrossRef]
- Demers, L.; Weiss-Lambrou, R.; Ska, B. Item analysis of the Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST). Assist. Technol. 2000, 12, 96–105. [Google Scholar] [CrossRef]
- Galeoto, G.; Colucci, M.; Guarino, D.; Esposito, G.; Cosma, E.; De Santis, R.; Grifoni, G.; Valente, D.; Tofani, M. Exploring Validity, Reliability, and Factor Analysis of the Quebec User Evaluation of Satisfaction with Assistive Technology in an Italian Population: A Cross-Sectional Study. Occup. Ther. Health Care 2018, 32, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Himmelmann, K.; Horber, V.; De La Cruz, J.; Horridge, K.; Mejaski-Bosnjak, V.; Hollody, K.; Krägeloh-Mann, I.; SCPE Working Group. MRI classification system (MRICS) for children with cerebral palsy: Development, reliability, and recommendations. Dev. Med. Child. Neurol. 2017, 59, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sutter, E.N.; Francis, L.S.; Francis, S.M.; Lench, D.H.; Nemanich, S.T.; Krach, L.E.; Sukal-Moulton, T.; Gillick, B.T. Disrupted Access to Therapies and Impact on Well-Being During the COVID-19 Pandemic for Children With Motor Impairment and Their Caregivers. Am. J. Phys. Med. Rehabil. 2021, 100, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Shore, B.J.; Martinkevich, P.; Riazi, M.; Baird, E.; Encisa, C.; Willoughby, K.; Narayanan, U.G. CHOP Investigative Team. Reliability of Radiographic Assessments of the Hip in Cerebral Palsy. J. Pediatr. Orthop. 2019, 39, e536–e541. [Google Scholar] [CrossRef] [PubMed]
- Wawrzuta, J.; Willoughby, K.L.; Molesworth, C.; Ang, S.G.; Shore, B.J.; Thomason, P.; Graham, H.K. Hip health at skeletal maturity: A population-based study of young adults with cerebral palsy. Dev. Med. Child. Neurol. 2016, 58, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Pruszczynski, B.; Sees, J.; Miller, F. Risk Factors for Hip Displacement in Children With Cerebral Palsy: Systematic Review. J. Pediatr. Orthop. 2016, 36, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelli, C.; Altamura, P.; Vieira, E.R.; Bertoncelli, D.; Solla, F. Predicting hip dysplasia in teenagers with cerebral palsy in order to optimize prevention and rehabilitation. A longitudinal descriptive study. Dev. Neurorehabil. 2021, 24, 166–172.39. [Google Scholar]
- Okuno, K.; Kitai, Y.; Shibata, T.; Arai, H. Risk factors for hip dislocation in dyskinetic cerebral palsy. J. Orthop. Surg. 2021, 29, 23094990211001196. [Google Scholar] [CrossRef]
- Hägglund, G. Association between pelvic obliquity and scoliosis, hip displacement and asymmetric hip abduction in children with cerebral palsy: A cross-sectional registry study. BMC Musculoskelet. Disord. 2020, 21, 464. [Google Scholar] [CrossRef]
- Ramstad, K.; Jahnsen, R.B.; Terjesen, T. Severe hip displacement reduces health-related quality of life in children with cerebral palsy. Acta Orthop. 2017, 88, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Marcström, A.; Hägglund, G.; Alriksson-Schmidt, A.I. Hip pain in children with cerebral palsy: A population-based registry study of risk factors. BMC Musculoskelet. Disord. 2019, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, G.; Wagner, P. Range of hip abduction after preventive and reconstructive surgery in cerebral palsy: A longitudinal registry study of 307 children. Acta Orthop. 2022, 93, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, B.M.; Bar-On, L.; O’Brien, T.D.; Maganaris, C.N. Stretching Interventions in Children With Cerebral Palsy: Why Are They Ineffective in Improving Muscle Function and How Can We Better Their Outcome? Front. Physiol. 2020, 11, 131. [Google Scholar] [CrossRef] [PubMed]
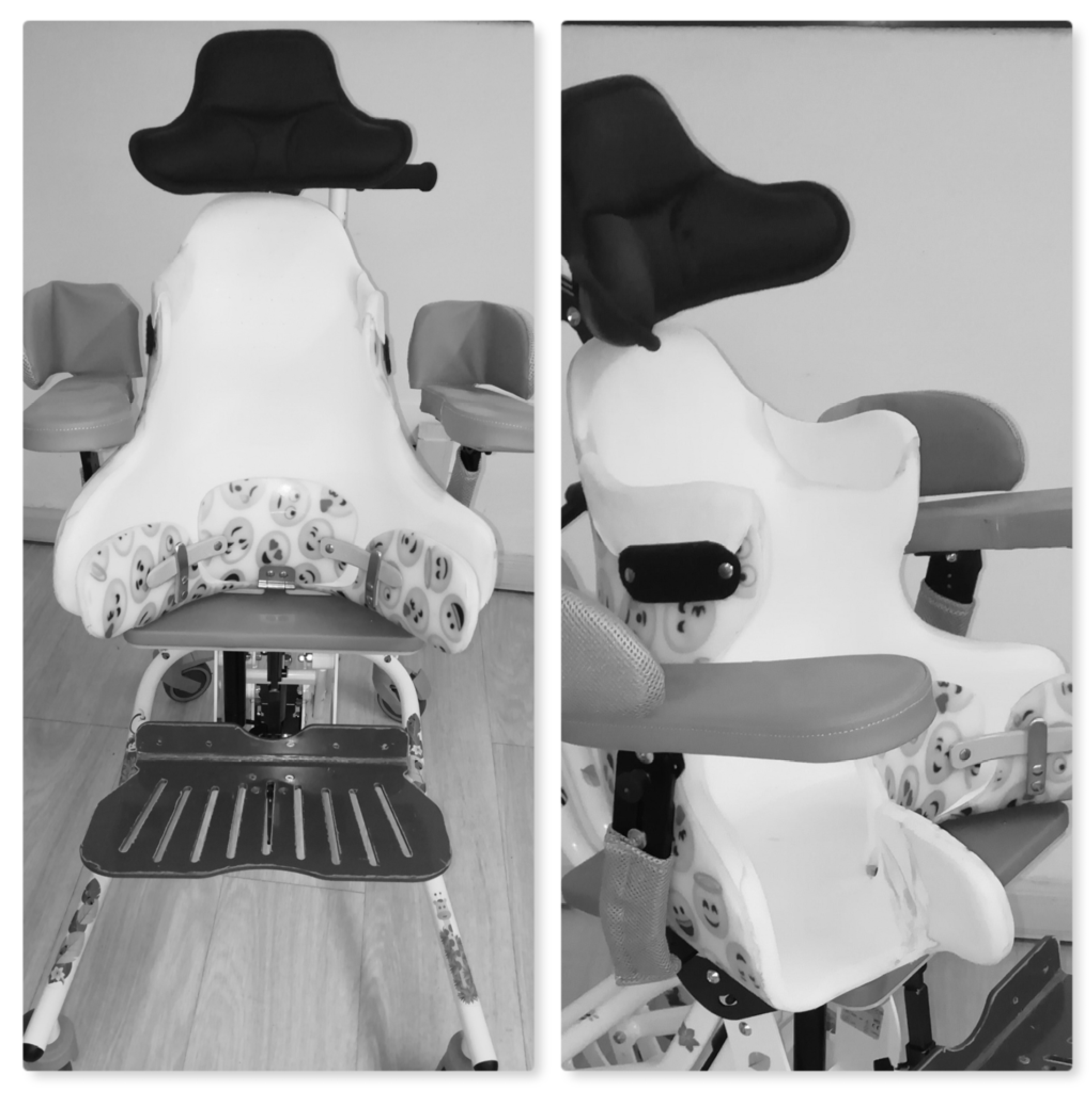

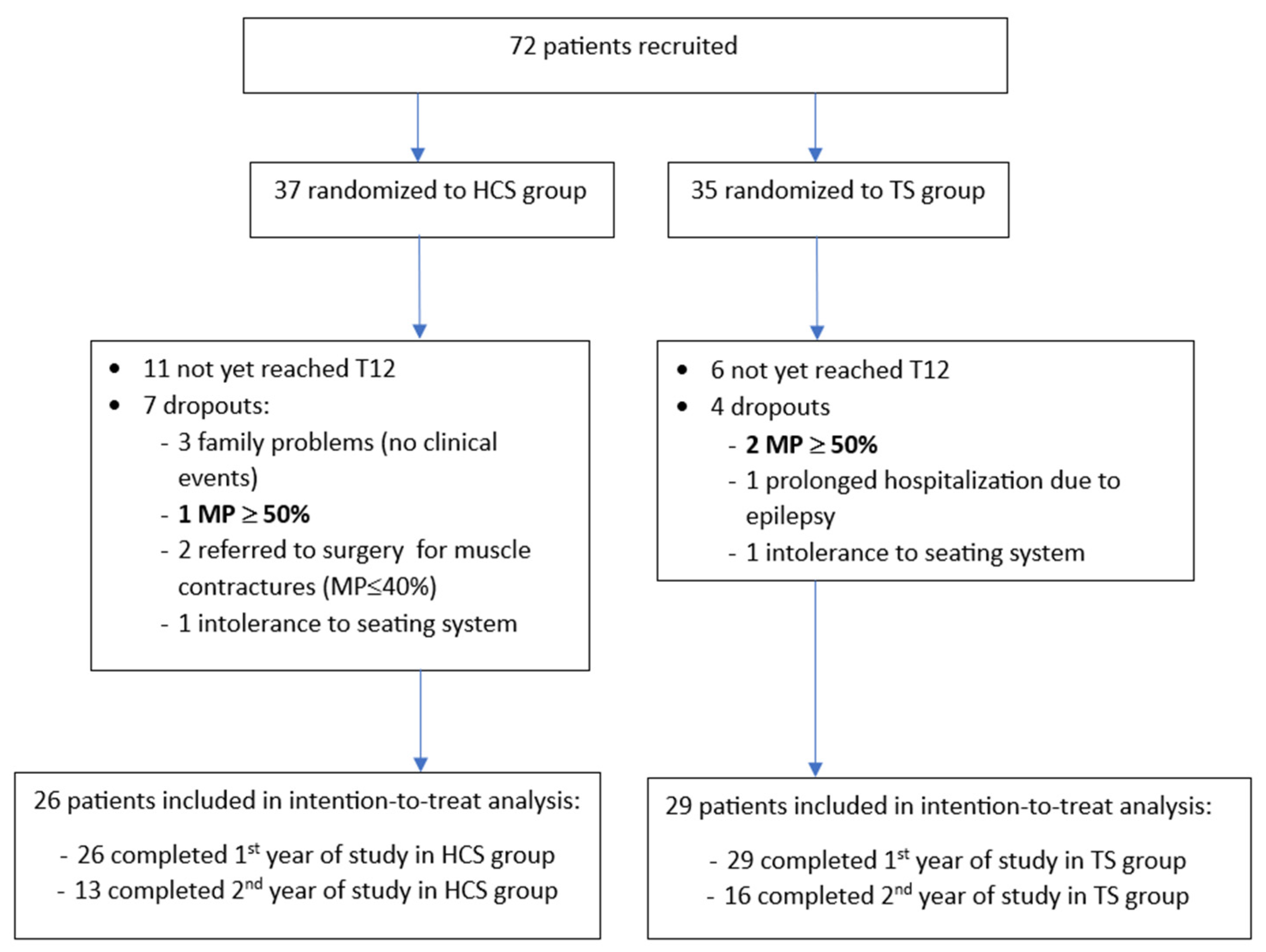
| Characteristics | Total, n = 55 | TS, n = 29 (52.7) | HCS, n = 26 (47.3) | p-Value |
|---|---|---|---|---|
| Age a | 3.7 ± 1.6 (1–6.9) | 4.0 ± 1.6 (1.5–6.9) | 3.4 ± 1.6 (1–6.9) | 0.212 |
| Sex b | ||||
| Female | 15 (27.3) | 3 (10.3) | 12 (46.2) | 0.003 |
| Male | 40 (72.7) | 26 (89.7) | 14 (53.8) | |
| MRICS b | ||||
| Maldevelopment | 7 (12.7) | 4 (13.8) | 3 (11.5) | 0.898 |
| Predominant white matter injury | 27 (49.1) | 13 (44.8) | 14 (53.9) | |
| Predominant grey matter injury | 8 (14.6) | 5 (17.3) | 3 (11.5) | |
| Miscellaneous | 13 (23.6) | 7 (24.1) | 6 (23.1) | |
| CP subtype b | ||||
| Dystonic | 15 (27.3) | 9 (31.0) | 6 (23.1) | 0.480 |
| Spastic | 39 (70.9) | 19 (65.5) | 20 (76.9) | |
| Choreoathetosis | 1 (1.8) | 1 (3.5) | 0 (0.0) | |
| GMFCS b | ||||
| IV | 29 (52.7) | 14 (48.3) | 15 (57.7) | 0.485 |
| V | 26 (47.3) | 15 (51.7) | 11 (42.3) | |
| Drug-resistant epilepsy b | ||||
| No | 47 (85.5) | 24 (82.8) | 23 (88.5) | 0.549 |
| Yes | 8 (14.5) | 5 (17.2) | 3 (11.5) | |
| Antidystonic/antispastic drugs b | ||||
| No | 49 (89.1) | 25 (86.2) | 24 (92.3) | 0.469 |
| Yes | 6 (10.9) | 4 (13.8) | 2 (7.7) | |
| ITB b | 0 | 0 | 0 | - |
| CPCHILD c | 55.7 ± 12.7 (23.7–83.8) | 53.7 ± 13.4 (23.7–83.8) | 57.9 ± 11.6 (32.3–78.0) | 0.220 |
| Characteristics of the Individual Hips | Total Hips, n = 110 | TS, n = 58 (52.7) | HCS, n = 52 (47.3) | p-Value |
|---|---|---|---|---|
| MP mean value a | 24.8 ± 8.5 (0–39.5) | 24.8 ± 8.2 (0–39.5) | 24.8 ± 8.9 (0–39) | 0.989 |
| MP median value b | 25.0 (19.1–31.4) | 24.2 (20–31.1) | 26.2 (18.9–32.6) | 0.574 |
| Pelvic tilt c | 0 ± 3.5 (−11–11) | 0 ± 3.7 (−11–11) | 0 ± 3.3 (−8–8) | 1.000 |
| pROM hip abd c | 48.5 ± 16.2 (10–90) | 45.7 ± 18.3 (10–90) | 51.6 ± 12.9 (20–80) | 0.054 |
| pROM gracilis c | 27.2 ± 12.3 (10–70) | 25.2 ± 12.3 (10–70) | 29.4 ± 12.1 (10–60) | 0.073 |
| pROM knee ext c | −4.5 ± 6.6 (−30–0) | −5.0 ± 7.8 (−30–0) | −4.0 ± 4.8 (−15–0) | 0.420 |
| pROM Thomas c | 1.9 ± 4.4 (0–20) | 2.5 ± 5.3 (0–20) | 1.1 ± 3.1 (0–15) | 0.091 |
| Outcomes | Total Hips, n = 110 | TS, n = 58 (52.7) | HCS, n = 52 (47.3) | p-Value, TS vs. HCS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T12 | T24 | p-Value | T0 | T12 | T24 | p-Value | T0 | T12 | T24 | p-Value | T0; T12; T24 | |
| MP progression Mean value a | - | 3.5 ± 9.4 (−13–42.7) | 3.9 ± 7.9 (−1–34.7) | 0.788 | - | 3.7 ± 9.5 (−11.3–42.7) | 4.1 ± 5.7 (−8.0–14) | 0.850 | - | 3.2 ± 9.3 (−13–32.5) | 3.6 ± 10.0 (−12–34.7) | 0.838 | 0.778; 0.852 |
| MP progression Median value b | - | 1.6 (−2.6–8.0) | 2.5 (−0.5–8.5) | 0.264 | - | 2.2 (−2.4–8.2) | 2.5 (−0.5–8.6) | 0.878 | - | 0.7 (−2.2–5.7) | 3.3 (−1.9–8.5) | 0.241 | 0.230; 1.000 |
| MP°, mean value a | 24.8 ± 8.5 (0–39.5) | 27.8 ± 10.4 (6–77.7) | 26.4 ± 7.1 (11.6–44.7) | 0.052 | 24.8 ± 8.2 (0–39.5) | 28.5 ± 11.4 (6–77.7) | 27.7 ± 6.4 (16.5–44.7) | 0.087 | 24.8 ± 8.9 (0–39) | 26.8 ± 8.9 (9.1–55.2) | 24.9 ± 7.7 (11.6–40.5) | 0.465 | 0.989; 0.435; 0.159 |
| MP, median value b | 25.0 (19.1–31.4) | 26.0 (21.5–33.1) | 26.5 (21.5–31.6) | * | 24.2 (20–31.1) | 25.8 (22–35.8) | 27.8 (23.3–32) | ° | 26.2 (18.9–32.6) | 26.0 (21.5–32.5) | 23.8 (20.8–30) | £ | 0.574; 0.956; 0.108 |
| Hip MP > 40 c | 0 (0.0) | 6 (5.4) | 2 (1.8) | 0.041 | 0 (0.0) | 4 (6.9) | 1 (1.7) | 0.124 | 0 (0.0) | 2 (3.8) | 1 (1.9) | 0.314 | -; 0.617; 0.918 |
| Hip MP ≥ 50 c | 0 (0.0) | 4 (3.6) | 0 (0.0) | 0.037 | 0 (0.0) | 3 (5.2) | 0 (0.0) | 0.098 | 0 (0.0) | 1 (1.9) | 0 (0.0) | 0.409 | -; 0.455; - |
| Painful hip c | 2 (1.8) | 4 (3.6) | 1 (1.5) | 0.492 | 2 (3.4) | 4 (6,9) | 1 (2.6) | 0.543 | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 0.177; 0.082; 0.381 |
| Pelvic tilt d | 0.0 ± 3.5 (−11–11) | 0.1 ± 3.3 (−10–10) | 0.0 ± 3.1 (−7–7) | 0.986 | 0.0 ± 3.7 (−11–11) | 0.0 ± 3.3 (−10–10) | 0.0 ± 2.9 (−7–7) | 1.000 | 0.0 ± 3.3 (−8–8) | 0.2 ± 3.3 (−6.5–7) | 0.0 ± 3.4 (−7–7) | 0.968 | 1.000; 0.811; 1.000 |
| pROM hip abd d | 48.5 ± 16.2 (10–90) | 49.5 ± 15.7 (10–90) | 47.1 ± 10.8 (25–70) | 0.623 | 45.7 ± 18.3 (10–90) | 47.1 ± 17.7 (10–90) | 46.8 ± 11.6 (25–70) | 0.897 | 51.6 ± 12.9 (20–80) | 52.8 ± 11.8 (30–80) | 47.4 ± 10.1 (27–60) | 0.180 | 0.054; 0.071; 0.852 |
| pROM gracilis d | 27.2 ± 12.3 (10–70) | 27.2 ± 11.9 (10–70) | 25.2 ± 15.1 (10–70) | 0.596 | 25.2 ± 12.3 (10–70) | 25.5 ± 11.8 (10–70) | 22.8 ± 15.0 (10–70) | 0.603 | 29.4 ± 12.1 (10–60) | 29.6 ± 11.8 (10–60) | 28.3 ± 14.9 (10–55) | 0.908 | 0.073; 0.092; 0.173 |
| pROM knee ext d | −4.5 ± 6.6 (−30–0) | −5.2 ± 7.1 (−30–0) | −3.1 ± 6.0 (−20–0) | 0.063 | −5.0 ± 7.8 (−30–0) | −6.5 ± 7.9 (−30–0) | −3.8 ± 6.6 (−20–0) | 0.145 | −4.0 ± 4.8 (−15–0) | −3.6 ± 5.5 (−20–0) | −2.3 ± 5.2 (−20–0) | 0.240 | 0.420; 0.029; 0.200 |
| pROM Thomas d | 1.9 ± 4.4 (0–20) | 2.7 ± 6.6 (0–30) | 2.6 ± 5.6 (0–20) | 0.528 | 2.5 ± 5.3 (0–20) | 4.3 ± 8.3 (0–30) | 3.0 ± 5.6 (0–20) | 0.332 | 1.1 ± 3.1 (0–15) | 0.4 ± 1.2 (0–5) | 2.1 ± 5.7 (0–20) | 0.126 | 0.091; 0.002; 0.563 |
| CPCHILD e | 55.7 ± 12.6 (23.7–83.8) | 57.0 ± 10.7 (35.4–79.2) | 59.1 ± 11.1 (37.9–85.1) | 0.200 | 53.7 ± 13.3 (23.7–83.8) | 56.1 ± 12.0 (35.4–77.5) | 58.4 ± 11.9 (44.7–85.1) | 0.220 | 57.9 ± 11.5 (32.3–78.0) | 58.2 ± 8.6 (42.7–79.2) | 59.8 ± 10.3 (37.9–73.3) | 0.720 | 0.079; 0.325; 0.627 |
| QUEST e | - | 4.1 ± 0. (2.0–5.0) | 3.8 ± 0.7 (2.4–5.0) | 0.057 | - | 4.1 ± 0.7 (2.0–5.0) | 3.9 ± 0.7 (2.4–5.0) | 0.583 | - | 4.2 ± 0.6 (2.8–5.0) | 3.7 ± 0.7 (2.5–4.7) | 0.022 | -; 0.516; 0.301 |
| Physiotherapy c | 106 (96.4) | 95 (86.4) | 50 (45.4) | 0.281 | - | 54 (93.1) | 26 (44.8) | 0.319 | - | 41 (78.8) | 24 (46.1) | 0.600 | -; 0.637; 0.496 |
| BTX-A injections per hip | 48 | 95 | 147 | <0.001 | 33 | 74 | 97 | <0.001 | 15 | 21 | 50 | <0.001 | 0.123; 0.005; 0.250 |
| Regular use of a stander c | 58 (52.7) | 52 (47.8) | 34 (30.9) | 0.459 | 30 (51.7) | 22 (37.9) | 0.139 | 22 (42.3) | 12 (23.1) | 0.618 | -; 0.980; 0.082 | ||
| Sitting system costs f | 4197.6 ± 1777.2 (731.0–9069.4) | 4901.0 ± 1558.8 (2963.9–9069.4) | 3413.1 ± 1686.6 (731.0–6580.8) | -; -; <0.001 | |||||||||
| Univariate Analysis | ||||
|---|---|---|---|---|
| MP Progression T0-T12 | MP Progression T0-T24 | |||
| Characteristics | B (95% CI) | p-Value | B (95% CI) | p-Value |
| Arm | ||||
| HCS | ref. | ref. | ||
| TS | 0.53 (−3.2–4.2) | 0.778 | 0.40 (−3.9–4.70) | 0.852 |
| Age | −2.38 (−3.44–(−1.32)) | <0.001 | −2.65 (−3.71–(−1.58)) | <0.001 |
| Sex | ||||
| Female | ref. | ref. | ||
| Male | −1.51 (−5.85–2.83) | 0.490 | −4.32 (−9.13–0.48) | 0.077 |
| MRICS | ||||
| Maldevelopment | ref. | ref. | ||
| Predominant white matter injury | −6.44 (−12.31–(−0.58)) | 0.032 | 1.86 (−5.57–9.30) | 0.617 |
| Predominant grey matter injury | −3.30 (−10.27–3.66) | 0.349 | 12.33 (4.03–20.63) | 0.004 |
| Miscellaneous | −7.45 (−13.90–(−1.00)) | 0.024 | 2.59 (−5.70–10.89) | 0.553 |
| CP subtype | ||||
| Dystonic | ref. | ref. | ||
| Spastic | −2.85 (−16.94–1.24) | 0.177 | −5.46 (−10.25–(−0.66) | 0.026 |
| Choreoathetosis | −12.01 (−25.46–1.45) | 0.080 | −6.54 (−18.21–5.13) | 0.266 |
| GMFCS | ||||
| IV | ref. | ref. | ||
| V | 5.32 (1.78–8.87) | 0.004 | 5.44 (1.37–9.52) | 0.010 |
| Drug-resistant epilepsy | ||||
| No | ref. | ref. | ||
| Yes | 9.12 (4.06–14.18) | 0.001 | 13.69 (6.24–21.14) | 0.001 |
| Antidystonic/antispastic drugs | ||||
| No | ref. | |||
| Yes | −0.87 (−6.61–4.85) | 0.762 | 4.92 (−1.06–10.90) | 0.105 |
| ITB | - | - | - | - |
| CPCHILD | −0.04 (−0.22–0.13) | 0.616 | −0.15 (−0.34–0.03) | 0.104 |
| Quest | 1.80 (−0.88–4.48) | 0.186 | −2.74 (−5.51–0.02) | 0.052 |
| Pelvic tilt | 0.24 (0.32–0.81) | 0.398 | 0.03 (−0.66–0.72) | 0.930 |
| pROM hip abd | −0.07 (−0.19–0.04) | 0.244 | 0.03 (−0.18–0.24) | 0.770 |
| pROM gracilis | 0.05 (−0.10–0.21) | 0.473 | −0.07 (−0.24–0.09) | 0.357 |
| pROM knee ext | 0.12 (−0.13–0.38) | 0.339 | 0.24 (−0.04–0.53) | 0.090 |
| pROM Thomas | 0.05 (−0.23–0.33) | 0.721 | −0.27 (−0.66–0.12) | 0.172 |
| Hip pain | −0.3 (−9.92–9.28) | 0.948 | - | - |
| BTX-A injections | −0.05 (−1.27–1.16) | 0.930 | 0.38 (−0.43–1.21) | 0.346 |
| Regular use of a stander | 3.10 (−0.63–6.85) | 0.103 | 1.12 (−3.28–5.54) | 0.611 |
| Multivariate Analysis | ||||
|---|---|---|---|---|
| MP Progression T0-T12 | MP Progression T0-T24 | |||
| Characteristics | B (95% CI) | p-Value | B (95% CI) | p-Value |
| Arm | ||||
| HCS | ref. | ref. | ||
| TS | 1.51 (−1.89–4.91) | 0.381 | 2.28 (−0.72–6.37) | 0.116 |
| Age | −2.23 (−3.30–(−1.15)) | <0.001 | −2.23 (−3.30–(−1.15)) | <0.001 |
| Drug-resistant epilepsy | ||||
| No | ref. | ref. | ||
| Yes | 7.13 (2.28–11.98) | 0.004 | 7.64 (0.48–14.79) | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccioli, S.; Maggi, I.; Pagliano, E.; Migliorini, C.; Michelutti, A.; Guerra, L.; Ronchetti, A.; Cristella, G.; Battisti, N.; Mancini, L.; et al. Sitting Postural Management to Prevent Migration Percentage Progression in Non-Ambulatory Children with Cerebral Palsy: Randomized Controlled Trial Preliminary Data. J. Clin. Med. 2024, 13, 3129. https://doi.org/10.3390/jcm13113129
Faccioli S, Maggi I, Pagliano E, Migliorini C, Michelutti A, Guerra L, Ronchetti A, Cristella G, Battisti N, Mancini L, et al. Sitting Postural Management to Prevent Migration Percentage Progression in Non-Ambulatory Children with Cerebral Palsy: Randomized Controlled Trial Preliminary Data. Journal of Clinical Medicine. 2024; 13(11):3129. https://doi.org/10.3390/jcm13113129
Chicago/Turabian StyleFaccioli, Silvia, Irene Maggi, Emanuela Pagliano, Claudia Migliorini, Arianna Michelutti, Liliana Guerra, Anna Ronchetti, Giovanna Cristella, Nicoletta Battisti, Lara Mancini, and et al. 2024. "Sitting Postural Management to Prevent Migration Percentage Progression in Non-Ambulatory Children with Cerebral Palsy: Randomized Controlled Trial Preliminary Data" Journal of Clinical Medicine 13, no. 11: 3129. https://doi.org/10.3390/jcm13113129
APA StyleFaccioli, S., Maggi, I., Pagliano, E., Migliorini, C., Michelutti, A., Guerra, L., Ronchetti, A., Cristella, G., Battisti, N., Mancini, L., Picciolini, O., Alboresi, S., Trabacca, A., & Kaleci, S. (2024). Sitting Postural Management to Prevent Migration Percentage Progression in Non-Ambulatory Children with Cerebral Palsy: Randomized Controlled Trial Preliminary Data. Journal of Clinical Medicine, 13(11), 3129. https://doi.org/10.3390/jcm13113129






