Abstract
Antiphospholipid syndrome (APS), also known as Hughes syndrome, is an acquired autoimmune and procoagulant condition that predisposes individuals to recurrent thrombotic events and obstetric complications. Central is the role of three types of antiphospholipid antibodies that target phospholipid-binding proteins: lupus anticoagulant (LAC), anti-β2-glycoprotein I (β2-GPI-Ab), and anti-cardiolipin (aCL). Together with clinical data, these antibodies are the diagnostic standard. However, the diagnosis of APS in older adults may be challenging and, in the diagnostic workup of thromboembolic complications, it is an underestimated etiology. The therapeutic management of APS requires distinguishing two groups with differential risks of thromboembolic complications. The standard therapy is based on low-dose aspirin in the low-risk group and vitamin K antagonists in the high-risk group. The value of direct oral anticoagulants is currently controversial. The potential role of monoclonal antibodies is investigated. For example, rituximab is currently recommended in catastrophic antiphospholipid antibody syndrome. Research is ongoing on other monoclonal antibodies, such as daratumumab and obinutuzumab. This narrative review illustrates the pathophysiological mechanisms of APS, with a particular emphasis on cardiovascular complications and their impact in older adults. This article also highlights advancements in the diagnosis, risk stratification, and management of APS.
1. Introduction
Antiphospholipid syndrome (APS), also known as Hughes syndrome, is a multifaceted autoimmune disorder characterized by the presence of a procoagulant state that predisposes individuals to recurrent thrombotic events and obstetric complications. Central to the pathogenesis of APS are three types of antiphospholipid antibodies (aPLs): lupus anticoagulant (LAC), anti-β2-glycoprotein I (β2-GPI-Ab), and anti-cardiolipin (aCL) [1]. These antibodies specifically target proteins that bind to phospholipids and act on endothelial cells to increase platelet adhesion and influence the balance of coagulation and anticoagulation molecules. This action results in a hypercoagulable state, leading to intravascular thrombosis and subsequent multiorgan damage, including harm to the cardiovascular system [2].
The estimated incidence of APS is 1–2 cases per 100,000 individuals, with a prevalence ranging from 40 to 50 cases per 100,000. Among patients with pregnancy disorders, the prevalence of thrombotic events, whether arterial or venous, is approximately 9–10%, and the detection of antiphospholipid antibodies in lab tests is around 6–9%. Over the past few decades, the mortality rate has decreased significantly from 50 to 80% [3,4] to around 20% [5]. This improvement is primarily due to advances in therapy, particularly the use of anticoagulants, corticosteroids, plasma exchanges, and intravenous immunoglobulins [5].
APS can be classified into two clinical subtypes: primary and secondary. Primary APS involves the detection of antiphospholipid antibodies without any other systemic diseases [6]. Secondary APS, on the other hand, is associated with other disorders, with systemic lupus erythematosus (SLE) being the most common, followed by other autoimmune diseases like Sjögren’s syndrome, rheumatoid arthritis, and vasculitis as well as solid and hematological malignancies, infections, and more. About half of patients with SLE exhibit clinical manifestations of APS despite the presence of antiphospholipid antibodies [6]. In 2023, the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) updated the “Sapporo—2006 revised criteria” (or Sydney criteria) [1] with new diagnostic tools that encompass both clinical and laboratory domains, improving specificity and providing better diagnostic accuracy [6].
The aim of this review is to illustrate the pathophysiological mechanisms of APS, with a particular emphasis on cardiovascular complications and their impact in older adults. This review highlights diagnostic methods and discusses advancements in the risk stratification and management of APS.
2. Cardiovascular Complications of Antiphospholipid Syndrome: Why Does It Matter?
Recurrent thromboses, primarily venous and often in the lower extremities, are a prominent feature of APS. However, thromboses can also occur in other sites, including the pelvic, pulmonary, hepatic, portal, renal, and inferior vena cava veins, and sometimes even in the superficial venous circulation [7]. Arterial thromboses are most frequently observed in the cerebral circulation, leading to strokes or transient ischemic attacks. Other arteries, such retinal, coronary, renal, and mesenteric arteries, can also be involved [8]. Other relatively common signs of APS include thrombocytopenia, autoimmune hemolytic anemia, microangiopathic syndrome, pulmonary hypertension, and livedo reticularis [9]. A rare but severe manifestation, known as catastrophic antiphospholipid antibody syndrome (CAPS), may result in multiorgan failure [10].
Cardiovascular manifestations are prominent in APS, with valvular disease being prevalent. This includes thickening and non-bacterial valvular vegetations (Libman–Sacks endocarditis) affecting about 30% of patients and significantly increasing stroke risk [9,11]. The mitral and aortic valves are most commonly affected [12]. Moreover, the available literature on heart valve surgery and prosthetic valve management in APS indicates that there is a heightened risk of morbidity and mortality, both in the short and long term [13,14,15,16]. Patients with APS also face a higher risk of coronary artery disease and left ventricular dysfunction, pulmonary thromboembolism, microvascular thrombosis, and the rupture of atherosclerotic plaques [17,18]. The risks of valvular involvement and coronary artery disease are heightened in the presence of aCL and LAC [19,20]. The cardiac complications of APS can manifest as angina, cardiomyopathy, graft thrombosis after coronary artery bypass surgery, and intracardiac thrombi, emphasizing the broad impact of this syndrome on cardiovascular health [9] (Figure 1).
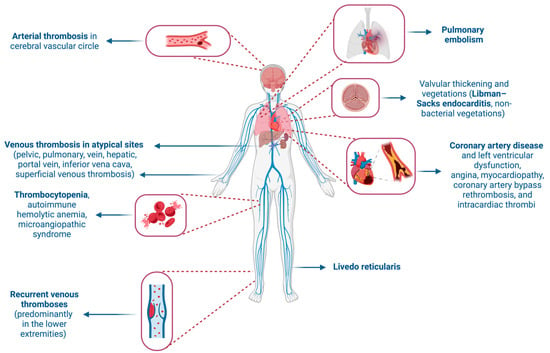
Figure 1.
Clinical manifestations of antiphospholipid syndrome. Created with BioRender.com (accessed on 2 May 2024).
3. Pathophysiology of Antiphospholipid Syndrome with a Focus on the Cardiovascular System
aPLs not only act as indicators for APS but also play a vital role in its development and progression. Studies in mice demonstrated that administering aPLs induced manifestations resembling those of APS, indicating that the presence of aPLs is necessary but not sufficient to cause blood clot formation [21,22]. This has led to the hypothesis of a “two-hit” pathophysiological mechanism [2]. The first hit entails the presence of aPLs, while the second involves disturbances in endothelial homeostasis, potentially triggered by factors like pregnancy, infections, and surgery [2]. This theory is supported by findings in CAPS, where a “second hit” is detectable in up to 80% of cases [23].
The prothrombotic effects of aPLs stem from their ability to disrupt the balance between prothrombotic and anticoagulant systems, affecting various cells and pathways such as endothelial cells, platelets, monocytes, neutrophils, the complement system, fibrinolysis, and coagulation pathways. Endothelial cells play a pivotal role in the pathophysiology of APS. In vitro studies highlighted the importance of the activation of Apo E receptor 2 (Apoer2), which in turn triggers the nuclear factor kB (NF-kB) and P38 pathways [24,25,26,27]. Following this activation sequence, a downregulation of anti-inflammatory and vascular protective genes occurs together with enhanced interaction between immune cells and the endothelial layer, a reduction in the local production of nitric oxide leading to vasoconstriction, and the release of tissue factor [24,25,26,27]. Platelets have a dual role in APS. They are well known for their role in thrombus formation, but they also interact with lymphocytes, forming circulating complexes in patients with APS [27]. This interaction fosters a proinflammatory state and continuous mild platelet activation, predisposing to thrombosis, particularly in the presence of a second hit [2]. In monocytes, aPLs induce the overexpression of tissue factor and proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 1-beta (IL-1β), enhancing the prothrombotic environment [28].
The complement system also plays a critical role in APS. Evidence suggests the presence of a persistent, mild activation of the complement system in patients with APS, reinforcing the link between inflammation and coagulation [29]. This is particularly supported by studies in animal models [30,31]. Moreover, aPLs have been shown to inhibit fibrinolysis, potentially through a direct action on fibrinolytic factors, further contributing to the thrombotic imbalance in APS [32]. All these mechanisms contribute to the pathophysiology of cardiovascular complications in APS, including valvular diseases, atherosclerosis, myocardial ischemia, pulmonary thromboembolism, hypertension, myocardial dysfunction, and rare cases of intracavitary thrombosis. Immunoglobulin deposits, especially IgG, on valve leaflets can initiate an inflammatory process leading to the formation of sterile vegetations (Libman–Sacks endocarditis), evolving into fibrosis and valvular deformation [33].
The interaction between aPLs and the endothelium not only fosters a proinflammatory and procoagulant state conducive to atherosclerosis but also involves an indirect mechanism through autoantibody cross-reaction by oxidative stress [34]. Complexes formed between β2-GPI-Ab and oxidized low-density lipoproteins may contribute to chronic vascular inflammation, affecting nitric oxide generation, monocyte adhesion, vasodilation inhibition, and local oxidative stress [34,35]. This proatherogenic environment, coupled with prothrombotic activity, can precipitate cardiac ischemia, with traditional risk factors exacerbating this risk [36]. The prevalence of myocardial infarction (usually ST-elevation myocardial infarction) in patients with APS is 1.2% and is further elevated in those with concurrent SLE (3.8%), underlining the significant contribution of thromboembolic activity in acute coronary syndromes in APS [34]. A number of studies have highlighted the association between APS and myocardial infarction with non-obstructive coronary arteries (MINOCA) [37,38,39,40,41], with APS that may be diagnosed in up to 15.5% of patients with MINOCA [42].
Pulmonary arterial hypertension (PH) is a relatively frequent complication of APS with a prevalence ranging between 1.8 and 3.5%. PH is frequently induced by pulmonary embolism (usually related to deep vein thrombosis of the lower limbs), defined as chronic thromboembolic pulmonary hypertension (CTEPH), the most common form of PH in APS. aPLs could lead to PH by promoting remodeling of pulmonary vessel cells through endothelin-1, thereby causing precapillary pulmonary hypertension [43,44].
4. Diagnosis of Antiphospholipid Syndrome with Cardiovascular Manifestations
A side-to-side comparison of the main diagnostic criteria established for APS can be found in Table 1. Similar to the Sydney criteria, the updated APS classification criteria released by ACR/EULAR are not designed to be diagnostic, but are often used to identify patients with homogeneous characteristics who can be included in clinical studies on APS [6]. These criteria consist of a primary inclusion criterion, which requires at least one positive assay of aPLs within three years of detection of a clinical criterion, in addition to other criteria grouped into six clinical domains and two laboratory domains. The six clinical domains are (1) macrovascular venous thromboembolism (VTE), (2) macrovascular arterial thrombosis, (3) microvascular presentations (skin, lung, renal, myocardial, and adrenal), (4) obstetric pathology (such as fetal death, preeclampsia), (5) cardiac valve involvement (thickening or vegetation), and (6) unexplained thrombocytopenia. The two laboratory domains are (1) LAC positivity and (2) aCL and β2-GPI-Ab positivity; the antibody titers of the latter must be determined with a standardized ELISA test. Each criterion is assigned a score from one to seven, and to diagnose APS, it is necessary to reach at least three points from each domain (clinical and laboratory).

Table 1.
Main diagnostic criteria for antiphospholipid syndrome.
ACR/EULAR criteria, in their dual role of diagnosis and classification, categorize patients into risk classes, thereby aiding in the clinical management of these patients. Accordingly, individuals with APS are classified into low- and high-risk groups. Low-risk individuals, also called asymptomatic aPL carriers, may be identified during diagnostic investigations for rheumatologic disease, in-depth analysis of recurrent miscarriages, unexplained prolongation of activated partial thromboplastin time (aPTT), or in the absence of any episode of thrombosis (arterial or venous) or obstetric APS [6]. This group has a marginally increased risk of thrombosis compared with the general population, estimated as 1.3% per year according to a study by Pengo et al. [45]. High-risk individuals are those with a history of thrombotic episodes (arterial or venous) or obstetric APS. They often have an antibody profile characterized LAC positivity (confirmed in two or more measurements at least twelve weeks apart), multiple aPL positivity (double or triple), and persistently high aPL titers. This group has an estimated 37% incidence of thromboembolic complications over 10 years [45,46].
Diagnosing APS with only cardiovascular involvement is particularly challenging, as it first necessitates ruling out other more common and potential secondary causes of cardiovascular damage. Valvular involvement is the most common cardiac manifestation of APS, and it is defined as the coexistence of aPLs along with the echocardiographic (transthoracic or transesophageal) identification of a valve lesion and/or moderate to severe regurgitation and/or stenosis of the mitral or aortic valve. Valve vegetation is characterized by leaflet thickness greater than 3 mm in the proximal or middle part of the cusps, or the presence of irregular nodules on the atrial area of the mitral valve or the vascular side of the aortic valve. As mentioned, other potential causes of valvular degeneration, such as a history of rheumatic disease or infective endocarditis, must be excluded [1].
Intracardiac thrombosis is one of the possible manifestations of APS. In the general population, it is more frequently associated with reduced left ventricular ejection fraction, cardiac wall aneurysms, and akinesia of certain high-risk segments such as the cardiac apex. The simultaneous involvement of two or three cardiac chambers, in the absence of the anatomical context mentioned above, diagnosed by transthoracic or transesophageal echocardiogram and confirmed at cardiac magnetic resonance imaging or autopsy examination, could suggest a direct role of autoantibodies (mainly aCL IgG), independent of ventricular dysfunction [47,48]. A study found early diastolic changes and high left ventricular filling pressures in young patients with APS, without other cardiovascular risk factors, who subsequently developed heart failure [49]. Further studies are needed to assess whether echocardiogram should be used as an early screening tool for myocardial involvement in APS. Another study correlated the presence of a high blood titer of aCL IgM with manifest heart failure [47]. More research is needed to establish whether high aCL antibody titers can serve as a predictive marker for heart failure in APS.
In conclusion, for younger patients with cardiovascular diseases, more common secondary causes must be excluded first; once ruled out, these patients should be screened for and monitored according to EULAR criteria for APS over a three-year period, along with other causes of thrombophilia. A high thrombotic burden, even in the presence of other more plausible secondary causes, may indicate the coexistence of APS.
5. Management Strategies for Antiphospholipid Syndrome with Cardiovascular Complications
5.1. Current Recommendations for the Management of Antiphospholipid Syndrome
General management measures for APS, regardless of risk classification, include controlling cardiovascular risk factors such as hypertension, dyslipidemia, diabetes, smoking, and physical inactivity. Additionally, regular screening including Doppler ultrasounds is recommended for the early detection of complications. In circumstances such as immobilization, hospitalization, surgery, and puerperium, anticoagulation therapy is necessary, even in cases considered to be low-risk. Measures to be implemented also include patient education on adherence to therapy, discontinuation of oral contraceptives or postmenopausal hormone therapy, and intensification of monitoring during pregnancy or the postpartum period.
In low-risk individuals (asymptomatic carriers of aPLs who do not fulfill any criteria for classification of vascular or obstetric APS) with a high-risk aPL profile, such as those diagnosed with SLE [50,51], prophylactic treatment with low-dose aspirin (LDA) (75–100 mg daily) is recommended (level of evidence 2a) (Figure 2) [6].
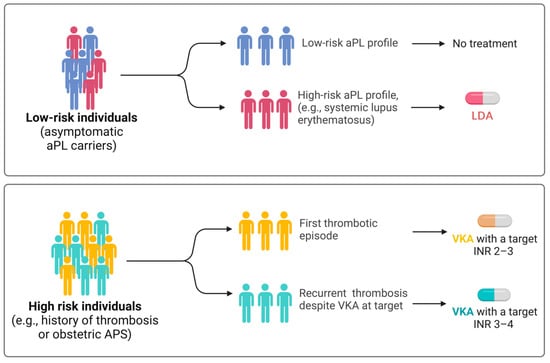
Figure 2.
Recommended treatment approaches according to the latest recommendations by the European League Against Rheumatism (EULAR). In case of recurrent thrombosis despite anticoagulant therapy in the therapeutic range, it is recommended to increase the INR in the 3–4 range. Abbreviations: APS, antiphospholipid syndrome; INR, international normalized ratio; VKA, vitamin K antagonist. Created with BioRender.com (accessed on 2 May 2024).
LDA treatment may also be considered in individuals with a low-risk aPL profile (level of evidence 2b). A meta-analysis of observational studies involving a total of 497 patients with isolated antiphospholipid antibody positivity demonstrated a reduced risk of thromboembolism in patients treated with LDA (adjusted hazard ratio: 0.43, 95% confidence interval: 0.25–0.75) [52]. In women who are not pregnant but have had a previous episode of obstetric APS (with or without a diagnosis of SLE), treatment with LDA as prophylaxis is recommended (level of evidence 2a).
5.2. Role of Anticoagulant Therapy and Related Challenges
In high-risk individuals, such as those experiencing their first episode of deep vein thrombosis, treatment with vitamin K antagonists (VKAs) targeting an INR of 2 to 3 is mandatory (level of evidence 1b) (Figure 2). If the episode of venous thrombosis is unprovoked, anticoagulant therapy should be extended long-term (level of evidence 2b). A retrospective study conducted by Rosove et al. [53] has shown that recurrent thromboembolism occurs at a rate of 30% annually in individuals with APS who have discontinued anticoagulant therapy. If the episode of venous thrombosis is provoked (i.e., attributable to immobilization, surgery, concomitant estrogen–progestin contraceptive, or postmenopausal therapy), anticoagulant treatment should be continued according to international guidelines for VTE for a duration equal to that recommended for patients without APS (level of evidence 5). Extending anticoagulant therapy may be considered if other risk factors coexist. Some studies have investigated, often with conflicting results, the use of direct oral anticoagulants (DOACs) if contraindications to VKAs are present (allergies, intolerances) or when a target INR cannot be achieved despite adherence to therapy [54,55]. A randomized clinical trial was conducted to determine whether rivaroxaban was noninferior to VKAs for preventing recurrent thrombosis in 190 patients with APS and arterial or venous thrombosis [56]. There were more episodes of recurrent thrombosis in the rivaroxaban-treated group (6.3% of VKA-treated participants versus 11.6% of those receiving rivaroxaban; risk ratio: 1.83; 95% confidence interval: 0.71–4.76), with comparable bleeding risk.
A systematic review with a comparative analysis of the international guidelines by Pastori et al. [57] addressed that while VKAs are the main treatment for patients with arterial thrombosis or triple aPL positivity, DOACs may be preferred in patients with venous thrombosis and single or double aPL positivity. Another systematic review with meta-analysis demonstrated that dabigatran or apixaban yielded a similar rate of recurrent thrombosis and major bleedings to VKAs, while rivaroxaban was associated with a higher risk of recurrent arterial thromboses [58]. A more recent study by Bakow et al. [59] found that the incidence rate of recurrent venous thrombosis in APS patients with single aPL positivity was comparable to that of patients without APS who had previously experienced venous thrombosis and did not vary depending on the anticoagulation regimen (DOACs or VKAs), suggesting that single-positive APS patients may not require extended anticoagulation with VKAs. Therefore, although the guidelines for the management of APS strictly recommend the use of VKAs, evidence suggests that alternative regimens may be considered in patients with APS with an estimated low risk of recurrent VTE to overcome the contraindications linked with VKA administration. Nevertheless, there is the need to conduct further clinical trials in order to explore the efficacy and safety profiles of DOACs or discontinuation of anticoagulation in low-risk aPL syndrome.
There is evidence on the challenges associated with INR fluctuations in patients with concomitant APS and a mechanical valve [60,61]. The current guidelines recommend targeting the INR based on both patient-related risk factors and the thrombogenicity of the mechanical valve. Furthermore, they advise considering a bioprosthesis in patients at increased risk of thromboembolism, including APS [62]. In light of these complexities, a tailored approach to anticoagulation management in APS patients with valvular heart disease is essential. This approach should consider individual risk factors and include vigilant monitoring in order to optimize outcomes and minimize complications.
5.3. Specific Considerations for Managing Cardiovascular Complications
In the event of a first episode of arterial thrombosis (such as ischemic stroke or myocardial infarction), treatment with VKA targeting an international normalized ratio (INR) of two to three is recommended [39] (Figure 2). The currently available evidence suggests that regular-dose aspirin (325 mg) or dual antiplatelet therapy may have equivalent efficacy to VKAs in the treatment of a first episode of arterial thrombosis; however, the results must be interpreted in light of significant limitations in the available evidence [63].
The use of DOACs is contraindicated in patients with triple aPL positivity and arterial events, as it is associated with a high risk of recurrence (level of evidence 1b) [6].
In patients with APS and recurrent episodes of deep vein thrombosis or arterial thrombosis despite VKA treatment, after ensuring proper adherence to prescribed therapy and maintenance of INR at target, the addition of LDA (level of evidence 4d) and the increase in the INR target to 3–4 or switching to low-molecular-weight heparin (LMHW) may be considered (Figure 2). A systematic review based on retrospective studies observed a reduction in thrombotic events if the INR target was maintained above three (3.8%; 4% arterial and 1% venous thrombosis) compared with an INR below three (23%; 13% arterial and 16% venous thrombosis) [64].
Although definitive studies on this topic are lacking, aPL-positive patients with valve involvement should receive LDA. If the risk of embolization increases (e.g., valve vegetations) or in case of myocardial infarction, anticoagulation should be considered. In individuals with APS-related valvular involvement, valve lesions do not recede with antiplatelet therapy and VKA. However, this treatment regimen appears to prevent the occurrence of embolic events [65]. There are no clear recommendations for patients who, despite anticoagulant therapy, have a thromboembolic event secondary to cardiac vegetations. The use of alternative therapies, such as immunomodulatory agents [66] including rituximab for hematologic manifestations (e.g., thrombocytopenia) [67] and hydroxychloroquine and eculizumab in refractory APS [68], has been explored. However, further research is warranted to establish their efficacy and safety.
6. Cardiovascular Implications of Antiphospholipid Syndrome in Older Adults
6.1. Clinical Manifestations of Antiphospholipid Syndrome in Older Adults
APS in advanced age poses a significant concern owing to frequent atypical presentations and a lack of evidence on the management of APS in older adults [69]. Notably, older patients are diagnosed with single-positive APS more often than their younger counterparts (82.8% vs. 59.8%) [70], presenting more frequently with primary APS (72.7% vs. 53.1%) [71]. Studies indicate a peak in APS incidence late in life, particularly in individuals over 55 years, with a higher prevalence in those older than 75 [72,73]. Contrary to young adults, in old age, the male sex prevails in terms of prevalence (46.6% vs. 18.6%) [9,70,74] and is associated with an increased risk of cardiovascular complications [75,76].
Late-onset APS has a peculiar disease profile [77]. Older adults are more prone to myocardial infarction [70], stroke, and pulmonary embolism [71] compared with younger individuals. The frequency of lower-limb deep vein thrombosis has been reported to be higher in one study [70] and lower in another investigation [71] relative to younger adults. Thrombotic relapse-free survival is shortened in older adults with APS, which is associated with reduced overall survival, possibly due to recurrent arterial and venous thrombosis events [70].
The presence of aPLs in older adults may increase the risk of cardiovascular events [78,79]. However, mixed findings have been reported, with studies showing a link between aCL and dementia and stroke [80,81], while others suggest that aCL and β2-GPI-Ab may be incidental findings in the older population [82,83,84]. Indeed, the prevalence of aPL positivity increases with age, both in healthy individuals [74] and in those with a variety of conditions (e.g., rheumatoid factor positivity [85,86], advanced renal or hepatic failure [87,88], giant-cell arteritis [89], and cancer [81,90]). However, aPL IgG with a titer above 40 units has been shown to be an independent risk factor for thrombosis [81]. LAC is a significant biological marker of APS in older adults, with a higher prevalence in those over 65 years [74], and it is often implicated in vascular events related to APS in this age group [84]. Malignancies, whose incidence increases with aging [91], pose special challenges due to their potential presentations as venous thrombosis [92] and aPL positivity. Hence, an underlying malignancy should be considered in older adults with thrombosis and high aPL titers [69,93,94].
6.2. Special Considerations for the Treatment of Antiphospholipid Syndrome in Older Adults
The management of APS in older adults requires special attention owing to their distinct disease profile and treatment-related risks. Efforts should be made to achieve an optimal control of modifiable cardiovascular risk factors. LDA is recommended by EULAR for primary thromboprophylaxis in asymptomatic aPL individuals with high-risk profiles regardless of age [6]. However, special caution is needed in older adults in whom primary prevention with LDA is associated with a high risk of major bleeding and questionable benefits [95]. Anticoagulation with VKAs is the mainstay therapy for cardiovascular complications of APS in older adults [96]. Given the increased bleeding risk in old patients on long-term anticoagulation [97,98], targeting an INR of two to three is recommended in this age group [99]. In individuals with a high thrombotic risk profile who require a higher target INR, a close monitoring of bleeding risk as well as cardiovascular risk factors is necessary [71].
7. Future Perspectives in Antiphospholipid Syndrome Research and Management
7.1. Emerging Biomarkers
Several autoantibodies, including anti-β2 GPI domain I [100], antiphosphatidylserine/prothrombin [101], antilysobisphosphatidic/endothelial protein C receptor antibodies [102], anti-β2-GPI complexed with HLA class II molecules [103], and antineutrophil extracellular trap antibodies [104], have been proposed as diagnostic markers of APS. However, none of them have a defined role in clinical settings [105]. Some micro-RNAs (miRNAs) have been found to be underexpressed in neutrophils from APS and SLE [106]. Teruel et al. [107] showed that the expressions of miR-19b and miR-20a, two miRNAs belonging to the miR-17-92 cluster that binds directly to tissue factor mRNA and suppresses its translation, were reduced by about 30% in patients with APS and SLE compared with healthy controls. This finding suggests that increased tissue factor expression in the setting of APS may result from altered miRNA signaling. Wu et al. [108] found that treating endothelial cells with β2-GPI-Ab isolated from APS patients induced the secretion of extracellular vesicles with a different miRNA content than those generated after treatment with non-immune IgG. Hence, miRNA alterations can alter the ability of extracellular vesicles exposed to β2-GPI-Ab to activate other endothelial cells. Circulating miRNAs may also serve as biomarkers in obstetric APS [109]. Further evaluation of these miRNAs could enable their use for risk stratification in different clinical manifestations of APS, such as thrombotic events, pregnancy morbidity, and triple aPL positivity. Additionally, miRNAs have the potential to serve as biomarkers for disease progression.
7.2. Potential New Treatments and Their Implication in Cardiovascular Complications
Existing prevention and treatment strategies are estimated to fail in approximately 20–30% of obstetric APS and more than 30% of thrombotic APS cases [110]. In recent years, there has been an increase in the understanding of the pathogenesis of APS, which has stimulated research on new targeted therapies [110].
Rituximab, a monoclonal anti-CD20 antibody, is recommended for use as a second-line treatment in APS [111], particularly in cases of refractory CAPS [112]. Potential pitfalls of rituximab include uncertainties regarding its long-term efficacy, adverse effects (e.g., prothrombotic state), and high costs [113]. Furthermore, rituximab may lead to insufficient suppression of aPL-producing plasma cells in patients with APS [114], highlighting the need for combination therapies. In recent years, daratumumab, a monoclonal antibody directed against CD38, has been employed in the management of autoimmune-mediated diseases, including APS [115]. A combination therapy with rituximab to simultaneously target CD20 and CD38 has been tested in preclinical models showing a high degree of synergism [116]. Obinutuzumab, a humanized glycoengineered type II monoclonal antibody targeting CD20, was shown to be more effective than rituximab at inducing B-cell depletion during in vitro whole blood assays, even in the presence of excess B-cell activating factor (BAFF) [117,118]. This suggests that obinutuzumab might serve as a treatment option for APS cases that are resistant to rituximab. Belimumab [119], eculizumab [120,121], and TNF-α blockers such as adalimumab [122,123] have been reported to be effective in some cases, but additional data on their efficacy and safety profiles are needed for their routine clinical use.
8. Conclusions
The management of cardiovascular complications in APS, particularly in older adults, remains a significant challenge. In these patients, it is essential to carefully balance the risks and benefits of antiplatelet and anticoagulant therapies due to an increased risk of bleeding. While current treatments primarily include LDA and VKAs, safer and more targeted treatments are highly sought after. Diagnostic approaches are also evolving, with emerging biomarkers like circulating miRNAs proposed to aid in early detection and risk stratification. This is especially relevant in older adults in whom APS may have atypical presentations and lead to severe outcomes.
Author Contributions
Conceptualization, M.B., L.S. and S.C.; methodology, M.B., L.S., E.M. and S.C.; validation, T.M., G.B.-Z. and P.S.; investigation, S.A., A.P., G.F., C.I., A.E.F., C.-E.H. and K.K.-W.; writing—original draft preparation, M.B., L.S., S.A., A.P., G.F., C.I., A.E.F. and K.K.-W.; writing—review and editing, C.-E.H., T.M., E.M. and S.C.; supervision, E.M. and S.C.; funding acquisition, E.M. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Università Cattolica del Sacro Cuore [D1.2020, D1.2022, and D1.2023] and the nonprofit research foundation “Centro Studi Achille e Linda Lorenzon” [N/A]. The authors also acknowledge co-funding from Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8—Project Age-It: “Ageing Well in an Ageing Society”. This resource was co-financed by Next Generation EU [DM 1557 11 October 2022]. The views and opinions expressed are only those of the authors and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them.
Conflicts of Interest
G.B.-Z. has consulted for Amarin, Balmed, Cardionovum, Crannmedical, Endocore Lab, Eukon, Guidotti, Innovheart, Meditrial, Microport, Opsens Medical, Terumo, and Translumina, outside of the present work. P.S. has received speaker fees from AstraZeneca, Amgen, Axis TV, BMS, Les laboratoires Servier, Novartis, Novonordisk, Sanofi, and Vifor, outside of the present work. E.M. has received speaker fees from Abbot, Difass International, Nestlè, and Nutricia and consulting fees from Cepton Strategies and Pfizer, outside of the present work. All other authors report no conflicts of interest.
References
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Kanthi, Y. Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin. Immunopathol. 2022, 44, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Dabit, J.Y.; Valenzuela-Almada, M.O.; Vallejo-Ramos, S.; Duarte-Garcia, A. Epidemiology of Antiphospholipid Syndrome in the General Population. Curr. Rheumatol. Rep. 2022, 23, 8510. [Google Scholar] [CrossRef]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; de Ramon, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Bucciarelli, S.; Asherson, R.A.; Cervera, R. Morbidity and mortality in the catastrophic antiphospholipid syndrome: Pathophysiology, causes of death, and prognostic factors. Semin. Thromb. Hemost. 2008, 34, 290–294. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. The 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis Rheumatol. 2023, 75, 1687–1702. [Google Scholar] [CrossRef] [PubMed]
- Sayar, Z.; Moll, R.; Isenberg, D.; Cohen, H. Thrombotic antiphospholipid syndrome: A practical guide to diagnosis and management. Thromb. Res. 2021, 198, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Matyja-Bednarczyk, A.; Swadzba, J.; Iwaniec, T.; Sanak, M.; Dziedzina, S.; Cmiel, A.; Musial, J. Risk factors for arterial thrombosis in antiphospholipid syndrome. Thromb. Res. 2014, 133, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Piette, J.C.; Font, J.; Khamashta, M.A.; Shoenfeld, Y.; Camps, M.T.; Jacobsen, S.; Lakos, G.; Tincani, A.; Kontopoulou-Griva, I.; et al. Antiphospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002, 46, 1019–1027. [Google Scholar] [CrossRef]
- Bitsadze, V.; Yakubova, F.; Khizroeva, J.; Lazarchuk, A.; Salnikova, P.; Vorobev, A.; Tretyakova, M.; Degtyareva, N.; Grigoreva, K.; Gashimova, N.; et al. Catastrophic Antiphospholipid Syndrome. Int. J. Mol. Sci. 2024, 25, 668. [Google Scholar] [CrossRef]
- Farzaneh-Far, A.; Roman, M.J.; Lockshin, M.D.; Devereux, R.B.; Paget, S.A.; Crow, M.K.; Davis, A.; Sammaritano, L.; Levine, D.M.; Salmon, J.E. Relationship of antiphospholipid antibodies to cardiovascular manifestations of systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 3918–3925. [Google Scholar] [CrossRef] [PubMed]
- Zuily, S.; Huttin, O.; Mohamed, S.; Marie, P.Y.; Selton-Suty, C.; Wahl, D. Valvular heart disease in antiphospholipid syndrome. Curr. Rheumatol. Rep. 2013, 15, 320. [Google Scholar] [CrossRef] [PubMed]
- Pons, I.; Louro, J.; Sitges, M.; Vidal, B.; Cervera, R.; Espinosa, G. Heart Valve Involvement in Patients with Antiphospholipid Syndrome: A Long-Term Follow-Up Study of a Single Centre. J. Clin. Med. 2023, 12, 2996. [Google Scholar] [CrossRef] [PubMed]
- Colli, A.; Mestres, C.A.; Espinosa, G.; Plasin, M.A.; Pomar, J.L.; Font, J.; Cervera, R. Heart valve surgery in patients with the antiphospholipid syndrome: Analysis of a series of nine cases. Eur. J. Cardiothorac. Surg. 2010, 37, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Berkun, Y.; Elami, A.; Meir, K.; Mevorach, D.; Naparstek, Y. Increased morbidity and mortality in patients with antiphospholipid syndrome undergoing valve replacement surgery. J. Thorac. Cardiovasc. Surg. 2004, 127, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Gorki, H.; Malinovski, V.; Stanbridge, R.D. The antiphospholipid syndrome and heart valve surgery. Eur. J. Cardiothorac. Surg. 2008, 33, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Denas, G.; Jose, S.P.; Bracco, A.; Zoppellaro, G.; Pengo, V. Antiphospholipid syndrome and the heart: A case series and literature review. Autoimmun. Rev. 2015, 14, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Nevras, V.; Milaras, N.; Katsioulis, C.; Sotiriou, Z.; Tsalamandris, S.; Gkounti, G.; Skevos, S. Acute Coronary Syndromes in Antiphospholipid Syndrome-above Suspicion: A Systematic Review. Curr. Probl. Cardiol. 2023, 48, 101503. [Google Scholar] [CrossRef] [PubMed]
- Zuily, S.; Regnault, V.; Selton-Suty, C.; Eschwege, V.; Bruntz, J.F.; Bode-Dotto, E.; De Maistre, E.; Dotto, P.; Perret-Guillaume, C.; Lecompte, T.; et al. Increased risk for heart valve disease associated with antiphospholipid antibodies in patients with systemic lupus erythematosus: Meta-analysis of echocardiographic studies. Circulation 2011, 124, 215–224. [Google Scholar] [CrossRef]
- Chighizola, C.B.; Andreoli, L.; de Jesus, G.R.; Banzato, A.; Pons-Estel, G.J.; Erkan, D.; Aps, A. The association between antiphospholipid antibodies and pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: A critical review of the literature. Lupus 2015, 24, 980–984. [Google Scholar] [CrossRef]
- Blank, M.; Cohen, J.; Toder, V.; Shoenfeld, Y. Induction of anti-phospholipid syndrome in naive mice with mouse lupus monoclonal and human polyclonal anti-cardiolipin antibodies. Proc. Natl. Acad. Sci. USA 1991, 88, 3069–3073. [Google Scholar] [CrossRef] [PubMed]
- Pierangeli, S.S.; Barker, J.H.; Stikovac, D.; Ackerman, D.; Anderson, G.; Barquinero, J.; Acland, R.; Harris, E.N. Effect of human IgG antiphospholipid antibodies on an in vivo thrombosis model in mice. Thromb. Haemost. 1994, 71, 670–674. [Google Scholar] [CrossRef]
- Cervera, R.; Bucciarelli, S.; Plasin, M.A.; Gomez-Puerta, J.A.; Plaza, J.; Pons-Estel, G.; Shoenfeld, Y.; Ingelmo, M.; Espinos, G.; Catastrophic Antiphospholipid Syndrome Registry Project, G. Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of a series of 280 patients from the “CAPS Registry”. J. Autoimmun. 2009, 32, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, V.; Gelber, S.E.; Vukelic, M.; Sacharidou, A.; Herz, J.; Urbanus, R.T.; de Groot, P.G.; Natale, D.R.; Harihara, A.; Redecha, P.; et al. ApoE Receptor 2 Mediation of Trophoblast Dysfunction and Pregnancy Complications Induced by Antiphospholipid Antibodies in Mice. Arthritis Rheumatol. 2016, 68, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Urbanus, R.T.; Pennings, M.T.; Derksen, R.H.; de Groot, P.G. Platelet activation by dimeric beta2-glycoprotein I requires signaling via both glycoprotein Ibalpha and apolipoprotein E receptor 2′. J. Thromb. Haemost. 2008, 6, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Romay-Penabad, Z.; Aguilar-Valenzuela, R.; Urbanus, R.T.; Derksen, R.H.; Pennings, M.T.; Papalardo, E.; Shilagard, T.; Vargas, G.; Hwang, Y.; de Groot, P.G.; et al. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood 2011, 117, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Vega-Ostertag, M.; Casper, K.; Swerlick, R.; Ferrara, D.; Harris, E.N.; Pierangeli, S.S. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum. 2005, 52, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Martini, F.; Farsi, A.; Gori, A.M.; Boddi, M.; Fedi, S.; Domeneghetti, M.P.; Passaleva, A.; Prisco, D.; Abbate, R. Antiphospholipid antibodies (aPL) increase the potential monocyte procoagulant activity in patients with systemic lupus erythematosus. Lupus 1996, 5, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.D.; Brey, R.L. Antiphospholipid antibodies and complement activation in patients with cerebral ischemia. Clin. Exp. Rheumatol. 1992, 10, 455–460. [Google Scholar]
- Carrera-Marin, A.; Romay-Penabad, Z.; Papalardo, E.; Reyes-Maldonado, E.; Garcia-Latorre, E.; Vargas, G.; Shilagard, T.; Pierangeli, S. C6 knock-out mice are protected from thrombophilia mediated by antiphospholipid antibodies. Lupus 2012, 21, 1497–1505. [Google Scholar] [CrossRef]
- Salmon, J.E.; Girardi, G. Antiphospholipid antibodies and pregnancy loss: A disorder of inflammation. J. Reprod. Immunol. 2008, 77, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Cugno, M.; Cabibbe, M.; Galli, M.; Meroni, P.L.; Caccia, S.; Russo, R.; Bottasso, B.; Mannucci, P.M. Antibodies to tissue-type plasminogen activator (tPA) in patients with antiphospholipid syndrome: Evidence of interaction between the antibodies and the catalytic domain of tPA in 2 patients. Blood 2004, 103, 2121–2126. [Google Scholar] [CrossRef]
- Hojnik, M.; George, J.; Ziporen, L.; Shoenfeld, Y. Heart valve involvement (Libman-Sacks endocarditis) in the antiphospholipid syndrome. Circulation 1996, 93, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Jara, L.J.; Medina, G.; Vera-Lastra, O.; Amigo, M.C. Accelerated atherosclerosis, immune response and autoimmune rheumatic diseases. Autoimmun. Rev. 2006, 5, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, E.; Kobayashi, K.; Inoue, K.; Lopez, L.R.; Shoenfeld, Y. Oxidized LDL/beta2-glycoprotein I complexes: New aspects in atherosclerosis. Lupus 2005, 14, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Pons-Estel, G.J.; Andreoli, L.; Scanzi, F.; Cervera, R.; Tincani, A. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J. Autoimmun. 2017, 76, 10–20. [Google Scholar] [CrossRef]
- Ciliberti, G.; Bergamaschi, L.; Paolisso, P.; Zilio, F.; Pizzi, C. Ten questions about myocardial infarction with non-obstructed coronary arteries. G. Ital. Cardiol. 2024, 25, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, H.; Ahmed, N.; Spevack, D.M. Prevalence of myocardial infarction with non-obstructive coronary arteries (MINOCA) amongst acute coronary syndrome in patients with antiphospholipid syndrome. Int. J. Cardiol. Heart Vasc. 2019, 22, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.; Nowak, K.; Wypasek, E.; Zalewski, J.; Undas, A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: Comparison with cryptogenic stroke. Int. J. Cardiol. 2019, 290, 1–6. [Google Scholar] [CrossRef]
- Svenungsson, E.; Spaak, J.; Strandberg, K.; Wallen, H.N.; Agewall, S.; Brolin, E.B.; Collste, O.; Daniel, M.; Ekenback, C.; Frick, M.; et al. Antiphospholipid antibodies in patients with myocardial infarction with and without obstructive coronary arteries. J. Intern. Med. 2022, 291, 327–337. [Google Scholar] [CrossRef]
- Ramjas, V.; Jain, A.; Lee, R.D.M.; Fioni, F.; Tawfik, N.; Sandhu, O.; Hamid, P. Unraveling the Association Between Myocardial Infarction of Nonobstructive Coronary Arteries and Antiphospholipid Syndrome. Cureus 2021, 13, e17002. [Google Scholar] [CrossRef]
- Kruchinova, S. Prevalence of thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries and cryptogenic stroke. Eur. Heart J. 2020, 41. [Google Scholar] [CrossRef]
- Coletto, L.A.; Gerosa, M.; Valentini, M.; Cimaz, R.; Caporali, R.; Meroni, P.L.; Chighizola, C.B. Myocardial involvement in anti-phospholipid syndrome: Beyond acute myocardial infarction. Autoimmun. Rev. 2022, 21, 102990. [Google Scholar] [CrossRef]
- Yeo, J.; Shin, N.; Ahn, K.J.; Seo, M.; Jang, A.Y.; Kim, M.; Chung, W.J. Pulmonary arterial hypertension due to antiphospholipid syndrome initially mimicking chronic thromboembolic pulmonary hypertension. Clin. Hypertens. 2022, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Testa, S.; Martinelli, I.; Ghirarduzzi, A.; Legnani, C.; Gresele, P.; Passamonti, S.M.; Bison, E.; Denas, G.; Jose, S.P.; et al. Incidence of a first thromboembolic event in carriers of isolated lupus anticoagulant. Thromb. Res. 2015, 135, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, P.; Lehtonen, K.V.; Javela, K.; Puurunen, M. Persistent antiphospholipid antibody (aPL) in asymptomatic carriers as a risk factor for future thrombotic events: A nationwide prospective study. Lupus 2014, 23, 1468–1476. [Google Scholar] [CrossRef]
- Djokovic, A.; Stojanovich, L.; Kontic, M.; Stanisavljevic, N.; Radovanovic, S.; Marisavljevic, D. Association between cardiac manifestations and antiphospholipid antibody type and level in a cohort of Serbian patients with primary and secondary antiphospholipid syndrome. Isr. Med. Assoc. J. 2014, 16, 162–167. [Google Scholar]
- Martinuzzo, M.E.; Pombo, G.; Forastiero, R.R.; Cerrato, G.S.; Colorio, C.C.; Carreras, L.O. Lupus anticoagulant, high levels of anticardiolipin, and anti-beta2-glycoprotein I antibodies are associated with chronic thromboembolic pulmonary hypertension. J. Rheumatol. 1998, 25, 1313–1319. [Google Scholar]
- Kolitz, T.; Shiber, S.; Sharabi, I.; Winder, A.; Zandman-Goddard, G. Cardiac Manifestations of Antiphospholipid Syndrome with Focus on Its Primary Form. Front. Immunol. 2019, 10, 941. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Laskari, K.; Panagiotakos, D.B.; Moutsopoulos, H.M. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum. 2009, 61, 29–36. [Google Scholar] [CrossRef]
- Somers, E.; Magder, L.S.; Petri, M. Antiphospholipid antibodies and incidence of venous thrombosis in a cohort of patients with systemic lupus erythematosus. J. Rheumatol. 2002, 29, 2531–2536. [Google Scholar] [PubMed]
- Arnaud, L.; Mathian, A.; Devilliers, H.; Ruffatti, A.; Tektonidou, M.; Forastiero, R.; Pengo, V.; Lambert, M.; Lefevre, G.; Martinez-Zamora, M.A.; et al. Patient-level analysis of five international cohorts further confirms the efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies. Autoimmun. Rev. 2015, 14, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Rosove, M.H.; Brewer, P.M. Antiphospholipid thrombosis: Clinical course after the first thrombotic event in 70 patients. Ann. Intern. Med. 1992, 117, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Hunt, B.J.; Efthymiou, M.; Arachchillage, D.R.; Mackie, I.J.; Clawson, S.; Sylvestre, Y.; Machin, S.J.; Bertolaccini, M.L.; Ruiz-Castellano, M.; et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): A randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016, 3, e426–e436. [Google Scholar] [CrossRef] [PubMed]
- Dufrost, V.; Risse, J.; Zuily, S.; Wahl, D. Direct Oral Anticoagulants Use in Antiphospholipid Syndrome: Are These Drugs an Effective and Safe Alternative to Warfarin? A Systematic Review of the Literature. Curr. Rheumatol. Rep. 2016, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Ordi-Ros, J.; Sáez-Comet, L.; Pérez-Conesa, M.; Vidal, X.; Riera-Mestre, A.; Castro-Salomó, A.; Cuquet-Pedragosa, J.; Ortiz-Santamaria, V.; Mauri-Plana, M.; Solé, C.; et al. Rivaroxaban Versus Vitamin K Antagonist in Antiphospholipid Syndrome: A Randomized Noninferiority Trial. Ann. Intern. Med. 2019, 171, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Menichelli, D.; Cammisotto, V.; Pignatelli, P. Use of Direct Oral Anticoagulants in Patients with Antiphospholipid Syndrome: A Systematic Review and Comparison of the International Guidelines. Front. Cardiovasc. Med. 2021, 8, 715878. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, S.; Yu, B.; He, T. Comparing the efficacy and safety of direct oral anticoagulants versus Vitamin K antagonists in patients with antiphospholipid syndrome: A systematic review and meta-analysis. Blood Coagul. Fibrinolysis 2022, 33, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Bakow, B.R.; Yanek, L.; Crowther, M.A.; Chaturvedi, S. Low recurrent thrombosis rates in single positive antiphospholipid syndrome regardless of type of anticoagulation. Thromb. Res. 2024, 237, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Emara, O.; Ramos, M.; Aller, C.B. Anticoagulation in a patient with antiphospholipid syndrome and a mechanical heart valve: A case study. Blood Coagul. Fibrinolysis 2018, 29, 472–475. [Google Scholar] [CrossRef]
- Endara, S.A.; Davalos, G.A.; Fierro, C.H.; Ullauri, V.E.; Molina, G.A. Antiphospholipid syndrome and valvular heart disease, a complex scenario of thrombotic events, a case report. J. Cardiothorac. Surg. 2020, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Quattrocchi, G.; Tohidi-Esfahani, I.; Sayar, Z.; Chandratheva, A.; Cohen, H. Antiphospholipid syndrome, antiphospholipid antibodies, and stroke. Int. J. Stroke 2023, 18, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Irastorza, G.; Hunt, B.J.; Khamashta, M.A. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum. 2007, 57, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, N.E.; Montes, R.M.; Soto, M.E.; Vanzzini, N.A.; Amigo, M.C. Primary antiphospholipid syndrome: A 5-year transesophageal echocardiographic followup study. J. Rheumatol. 2004, 31, 2402–2407. [Google Scholar] [PubMed]
- Sciascia, S.; Khamashta, M.A.; D’Cruz, D.P. Targeted therapy in antiphospholipid syndrome. Curr. Opin. Rheumatol. 2014, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, J.; Stevens, R. Rituximab therapy for recurrent thromboembolic disease in antiphospholipid syndrome. Lupus 2013, 22, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Tarantino, M.D.; Chaturvedi, S.; McCrae, K.R.; Roberts, J.C. Eculizumab for refractory thrombosis in antiphospholipid syndrome. Blood Adv. 2022, 6, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Piette, J.C.; Cacoub, P. Antiphospholipid syndrome in the elderly: Caution. Circulation 1998, 97, 2195–2196. [Google Scholar] [CrossRef]
- Masson, C.; Nguyen, T.T.A.; Dufrost, V.; Audrain, M.; Hémont, C.; Agard, C.; Artifoni, M.; Connault, J.; Fouassier, M.; Hamidou, M.; et al. Antiphospholipid syndrome in patients over 65 years: A comparative study of clinical and biological features and thrombotic relapses. Lupus 2022, 31, 1816–1823. [Google Scholar] [CrossRef]
- Grimaud, F.; Yelnik, C.; Pineton de Chambrun, M.; Amoura, Z.; Arnaud, L.; Costedoat Chalumeau, N.; Hachulla, E.; Lambert, M.; Roriz, M.; Sibilia, J.; et al. Clinical and immunological features of antiphospholipid syndrome in the elderly: A retrospective national multicentre study. Rheumatology 2019, 58, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Duarte-García, A.; Pham, M.M.; Crowson, C.S.; Amin, S.; Moder, K.G.; Pruthi, R.K.; Warrington, K.J.; Matteson, E.L. The Epidemiology of Antiphospholipid Syndrome: A Population-Based Study. Arthritis Rheumatol. 2019, 71, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Shin, S.H.; Kim, Y.J.; Oh, Y.M.; Lee, S.D.; Kim, Y.H.; Choi, C.W.; Lee, J.S. Epidemiology of Antiphospholipid Syndrome in Korea: A Nationwide Population-based Study. J. Korean Med. Sci. 2020, 35, e35. [Google Scholar] [CrossRef] [PubMed]
- Quéméneur, T.; Lambert, M.; Hachulla, E.; Dubucquoi, S.; Caron, C.; Fauchais, A.L.; Devulder, B.; Hatron, P.Y. Significance of persistent antiphospholipid antibodies in the elderly. J. Rheumatol. 2006, 33, 1559–1562. [Google Scholar] [PubMed]
- Pengo, V.; Ruffatti, A.; Legnani, C.; Testa, S.; Fierro, T.; Marongiu, F.; De Micheli, V.; Gresele, P.; Tonello, M.; Ghirarduzzi, A.; et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: A multicenter prospective study. Blood 2011, 118, 4714–4718. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.A., Jr.; Spencer, F.A. Risk factors for venous thromboembolism. Circulation 2003, 107, I-9–I-16. [Google Scholar] [CrossRef] [PubMed]
- Bountola, S.; Perifanis, V.; Kazantzi, A.; Keramidioti, C.; Hassapopoulou-Matami, E.; Hatzitolios, A.; Girtovitis, F. PS1585 Elderly with Antiphospholipid Syndrome Present with Distinct Clinical Profile: A Retrospective Single Center Study. HemaSphere 2019, 3, 730–731. [Google Scholar] [CrossRef]
- Tanne, D.; D’Olhaberriague, L.; Trivedi, A.M.; Salowich-Palm, L.; Schultz, L.R.; Levine, S.R. Anticardiolipin antibodies and mortality in patients with ischemic stroke: A prospective follow-up study. Neuroepidemiology 2002, 21, 93–99. [Google Scholar] [CrossRef]
- Piette, J.C. 1996 diagnostic and classification criteria for the antiphospholipid/cofactors syndrome: A mission impossible? Lupus 1996, 5, 354–363. [Google Scholar] [CrossRef]
- de Godoy, J.M.; de Godoy, M.R.; Cipulo, J.P.; Tognola, V.A. Vascular dementia and anticardiolipin antibodies. Med. Sci. Monit. 2005, 11, Cr430–Gr433. [Google Scholar]
- Finazzi, G.; Brancaccio, V.; Moia, M.; Ciaverella, N.; Mazzucconi, M.G.; Schinco, P.C.; Ruggeri, M.; Pogliani, E.M.; Gamba, G.; Rossi, E.; et al. Natural history and risk factors for thrombosis in 360 patients with antiphospholipid antibodies: A four-year prospective study from the Italian Registry. Am. J. Med. 1996, 100, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Manoussakis, M.N.; Tzioufas, A.G.; Silis, M.P.; Pange, P.J.; Goudevenos, J.; Moutsopoulos, H.M. High prevalence of anti-cardiolipin and other autoantibodies in a healthy elderly population. Clin. Exp. Immunol. 1987, 69, 557–565. [Google Scholar]
- Richaud-Patin, Y.; Cabiedes, J.; Jakez-Ocampo, J.; Vidaller, A.; Llorente, L. High prevalence of protein-dependent and protein-independent antiphospholipid and other autoantibodies in healthy elders. Thromb. Res. 2000, 99, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Luciani, D.; Bertolini, G.; Barbui, T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: A systematic review of the literature. Blood 2003, 101, 1827–1832. [Google Scholar] [CrossRef]
- Guerin, J.; Feighery, C.; Sim, R.B.; Jackson, J. Antibodies to beta2-glycoprotein I--a specific marker for the antiphospholipid syndrome. Clin. Exp. Immunol. 1997, 109, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Krause, I.; Cohen, J.; Blank, M.; Bakimer, R.; Cartman, A.; Hohmann, A.; Valesini, G.; Asherson, R.A.; Khamashta, M.A.; Hughes, G.R.; et al. Distribution of two common idiotypes of anticardiolipin antibodies in sera of patients with primary antiphospholipid syndrome, systemic lupus erythematosus and monoclonal gammopathies. Lupus 1992, 1, 91–96. [Google Scholar] [CrossRef]
- Piette, J.C.; Cacoub, P.; Wechsler, B. Renal manifestations of the antiphospholipid syndrome. Semin. Arthritis Rheum. 1994, 23, 357–366. [Google Scholar] [CrossRef]
- Quintarelli, C.; Ferro, D.; Valesini, G.; Basili, S.; Tassone, G.; Violi, F. Prevalence of lupus anticoagulant in patients with cirrhosis: Relationship with beta-2-glycoprotein I plasma levels. J. Hepatol. 1994, 21, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, K.; Pountain, G.; Merry, P.; Byron, M.; Hazleman, B.; Scott, D.G. A longitudinal study of anticardiolipin antibody in polymyalgia rheumatica and giant cell arteritis. J. Rheumatol. 1995, 22, 1694–1697. [Google Scholar]
- Ramos-Casals, M.; García-Carrasco, M.; Brito, M.P.; López-Soto, A.; Font, J. Autoimmunity and geriatrics: Clinical significance of autoimmune manifestations in the elderly. Lupus 2003, 12, 341–355. [Google Scholar] [CrossRef]
- Thakkar, J.P.; McCarthy, B.J.; Villano, J.L. Age-specific cancer incidence rates increase through the oldest age groups. Am. J. Med. Sci. 2014, 348, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Bessis, D.; Sotto, A.; Viard, J.P.; Bérard, M.; Ciurana, A.J.; Boffa, M.C. Trousseau’s syndrome with nonbacterial thrombotic endocarditis: Pathogenic role of antiphospholipid syndrome. Am. J. Med. 1995, 98, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Tincani, A.; Taraborelli, M.; Cattaneo, R. Antiphospholipid antibodies and malignancies. Autoimmun. Rev. 2010, 9, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Schved, J.F.; Dupuy-Fons, C.; Biron, C.; Quére, I.; Janbon, C. A prospective epidemiological study on the occurrence of antiphospholipid antibody: The Montpellier Antiphospholipid (MAP) Study. Haemostasis 1994, 24, 175–182. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Wolfe, R.; Woods, R.L.; Tonkin, A.M.; Donnan, G.A.; Nelson, M.R.; Reid, C.M.; Lockery, J.E.; Kirpach, B.; Storey, E.; et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Khamashta, M.A.; Shoenfeld, Y.; Camps, M.T.; Jacobsen, S.; Kiss, E.; Zeher, M.M.; Tincani, A.; Kontopoulou-Griva, I.; Galeazzi, M.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 5-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2009, 68, 1428–1432. [Google Scholar] [CrossRef]
- Palareti, G.; Leali, N.; Coccheri, S.; Poggi, M.; Manotti, C.; D’Angelo, A.; Pengo, V.; Erba, N.; Moia, M.; Ciavarella, N.; et al. Bleeding complications of oral anticoagulant treatment: An inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet 1996, 348, 423–428. [Google Scholar] [CrossRef]
- Torn, M.; Bollen, W.L.; van der Meer, F.J.; van der Wall, E.E.; Rosendaal, F.R. Risks of oral anticoagulant therapy with increasing age. Arch. Intern. Med. 2005, 165, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Tumian, N.R.; Hunt, B.J. Clinical Management of Thrombotic Antiphospholipid Syndrome. J. Clin. Med. 2022, 11, 735. [Google Scholar] [CrossRef]
- de Laat, B.; Pengo, V.; Pabinger, I.; Musial, J.; Voskuyl, A.E.; Bultink, I.E.; Ruffatti, A.; Rozman, B.; Kveder, T.; de Moerloose, P.; et al. The association between circulating antibodies against domain I of beta2-glycoprotein I and thrombosis: An international multicenter study. J. Thromb. Haemost. 2009, 7, 1767–1773. [Google Scholar] [CrossRef]
- Sciascia, S.; Radin, M.; Cecchi, I.; Rubini, E.; Scotta, A.; Rolla, R.; Montaruli, B.; Pergolini, P.; Mengozzi, G.; Muccini, E.; et al. Reliability of Lupus Anticoagulant and Anti-phosphatidylserine/prothrombin Autoantibodies in Antiphospholipid Syndrome: A Multicenter Study. Front. Immunol. 2019, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Müller-Calleja, N.; Hollerbach, A.; Royce, J.; Ritter, S.; Pedrosa, D.; Madhusudhan, T.; Teifel, S.; Meineck, M.; Häuser, F.; Canisius, A.; et al. Lipid presentation by the protein C receptor links coagulation with autoimmunity. Science 2021, 371, eabc0956. [Google Scholar] [CrossRef]
- Tanimura, K.; Jin, H.; Suenaga, T.; Morikami, S.; Arase, N.; Kishida, K.; Hirayasu, K.; Kohyama, M.; Ebina, Y.; Yasuda, S.; et al. β2-Glycoprotein I/HLA class II complexes are novel autoantigens in antiphospholipid syndrome. Blood 2015, 125, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Gudjonsson, J.E.; Kahlenberg, J.M.; Joseph McCune, W.; Bockenstedt, P.L.; Karp, D.R.; Knight, J.S. Anti-Neutrophil Extracellular Trap Antibodies and Impaired Neutrophil Extracellular Trap Degradation in Antiphospholipid Syndrome. Arthritis Rheumatol. 2020, 72, 2130–2135. [Google Scholar] [CrossRef]
- Vandevelde, A.; Chayoua, W.; de Laat, B.; Moore, G.W.; Musiał, J.; Zuily, S.; Wahl, D.; Devreese, K.M.J. Added value of antiphosphatidylserine/prothrombin antibodies in the workup of thrombotic antiphospholipid syndrome: Communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost. 2022, 20, 2136–2150. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Shi, H.; Li, C.; Knight, J.S. Antiphospholipid syndrome: A clinical perspective. Chin. Med. J. 2020, 133, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Teruel, R.; Pérez-Sánchez, C.; Corral, J.; Herranz, M.T.; Pérez-Andreu, V.; Saiz, E.; García-Barberá, N.; Martínez-Martínez, I.; Roldán, V.; Vicente, V.; et al. Identification of miRNAs as potential modulators of tissue factor expression in patients with systemic lupus erythematosus and antiphospholipid syndrome. J. Thromb. Haemost. 2011, 9, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Barnard, J.; Kundu, S.; McCrae, K.R. A novel pathway of cellular activation mediated by antiphospholipid antibody-induced extracellular vesicles. J. Thromb. Haemost. 2015, 13, 1928–1940. [Google Scholar] [CrossRef]
- Gysler, S.M.; Mulla, M.J.; Guerra, M.; Brosens, J.J.; Salmon, J.E.; Chamley, L.W.; Abrahams, V.M. Antiphospholipid antibody-induced miR-146a-3p drives trophoblast interleukin-8 secretion through activation of Toll-like receptor 8. Mol. Hum. Reprod. 2016, 22, 465–474. [Google Scholar] [CrossRef]
- Garcia, D.; Erkan, D. Diagnosis and Management of the Antiphospholipid Syndrome. N. Engl. J. Med. 2018, 378, 2010–2021. [Google Scholar] [CrossRef]
- Uthman, I.; Noureldine, M.H.A.; Ruiz-Irastorza, G.; Khamashta, M. Management of antiphospholipid syndrome. Ann. Rheum. Dis. 2019, 78, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Erkan, D. Expert Perspective: Management of Microvascular and Catastrophic Antiphospholipid Syndrome. Arthritis Rheumatol. 2021, 73, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Legault, K.; Schunemann, H.; Hillis, C.; Yeung, C.; Akl, E.A.; Carrier, M.; Cervera, R.; Crowther, M.; Dentali, F.; Erkan, D.; et al. McMaster RARE-Bestpractices clinical practice guideline on diagnosis and management of the catastrophic antiphospholipid syndrome. J. Thromb. Haemost. 2018, 16, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Hisada, R.; Kato, M.; Sugawara, E.; Kanda, M.; Fujieda, Y.; Oku, K.; Bohgaki, T.; Amengual, O.; Horita, T.; Yasuda, S.; et al. Circulating plasmablasts contribute to antiphospholipid antibody production, associated with type I interferon upregulation. J. Thromb. Haemost. 2019, 17, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.; Walsh, A.; Yin, X.; Wechalekar, M.D.; Smith, M.D.; Proudman, S.M.; Veale, D.J.; Fearon, U.; Pitzalis, C.; Humby, F.; et al. Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res. Ther. 2018, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Tommy Gambles, M.; Li, J.; Christopher Radford, D.; Sborov, D.; Shami, P.; Yang, J.; Kopeček, J. Simultaneous crosslinking of CD20 and CD38 receptors by drug-free macromolecular therapeutics enhances B cell apoptosis in vitro and in vivo. J. Control Release 2022, 350, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; Klein, C.; Isenberg, D.A.; Glennie, M.J.; Cambridge, G.; Cragg, M.S.; Leandro, M.J. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology 2017, 56, 1227–1237. [Google Scholar] [CrossRef]
- Reddy, V.R.; Pepper, R.J.; Shah, K.; Cambridge, G.; Henderson, S.R.; Klein, C.; Kell, L.; Taylor, S.J.; Isenberg, D.A.; Cragg, M.S.; et al. Disparity in peripheral and renal B-cell depletion with rituximab in systemic lupus erythematosus: An opportunity for obinutuzumab? Rheumatology 2022, 61, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Yoshizuka, R.; Hasegawa, H.; Kamiya, M.; Umezawa, N.; Yasuda, S. Refractory antiphospholipid antibody syndrome-induced thrombocytopaenia successfully treated with belimumab. Lupus 2022, 31, 624–627. [Google Scholar] [CrossRef]
- Shapira, I.; Andrade, D.; Allen, S.L.; Salmon, J.E. Brief report: Induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012, 64, 2719–2723. [Google Scholar] [CrossRef]
- Canaud, G.; Kamar, N.; Anglicheau, D.; Esposito, L.; Rabant, M.; Noël, L.H.; Guilbeau-Frugier, C.; Sberro-Soussan, R.; Del Bello, A.; Martinez, F.; et al. Eculizumab improves posttransplant thrombotic microangiopathy due to antiphospholipid syndrome recurrence but fails to prevent chronic vascular changes. Am. J. Transplant. 2013, 13, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Soto, M.J.; Cuadrado, M.J.; Khamashta, M.A. Autoimmune diseases induced by TNF-targeted therapies. Best. Pract. Res. Clin. Rheumatol. 2008, 22, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Cheemalavagu, S.; McCoy, S.S.; Knight, J.S. Digital ischaemia secondary to adalimumab-induced antiphospholipid syndrome. BMJ Case Rep. 2020, 13, e232907. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).