Comparing Outcomes of Community-Acquired Pneumonia Patients Discharged from General Medicine and Respiratory Units in Australia: A Propensity Score-Matched Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Outcomes
2.2. Statistical Analysis
2.2.1. Propensity Score Methods
2.2.2. Sensitivity Analysis
3. Results
3.1. Patient Characteristics
3.2. Outcomes
Unadjusted Analysis
3.3. PSM
3.4. Sensitivity Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsirgiotis, E.; Ruffin, R. Community acquired pneumonia. A perspective for general practice. Aust. Fam. Physician 2000, 29, 639–645. [Google Scholar]
- Lim, W.S.; Baudouin, S.V.; George, R.C.; Hill, A.T.; Jamieson, C.; Le Jeune, I.; Macfarlane, J.T.; Read, R.C.; Roberts, H.J.; Levy, M.L.; et al. BTS guidelines for the management of community acquired pneumonia in adults: Update 2009. Thorax 2009, 64 (Suppl. 3), iii1–iii55. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Mandal, P.; Singanayagam, A.; Akram, A.R.; Choudhury, G.; Short, P.M.; Hill, A.T. Severity assessment tools to guide ICU admission in community-acquired pneumonia: Systematic review and meta-analysis. Intensive Care Med. 2011, 37, 1409–1420. [Google Scholar] [CrossRef]
- Sharma, Y.; Sumanadasa, S.; Shahi, R.; Thompson, C. Role of vitamin C in treatment of community-acquired pneumonia in adult patients requiring hospitalisation: A systematic review protocol. BMJ Open 2024, 14, e082257. [Google Scholar] [CrossRef]
- Campling, J.; Wright, H.F.; Hall, G.C.; Mugwagwa, T.; Vyse, A.; Mendes, D.; Slack, M.P.E.; Ellsbury, G.F. Hospitalization costs of adult community-acquired pneumonia in England. J. Med. Econ. 2022, 25, 912–918. [Google Scholar] [CrossRef]
- Horwood, C.M.; Hakendorf, P.; Thompson, C.H. Comparison of specialist and generalist care. Aust. Health Rev. 2018, 42, 579–583. [Google Scholar] [CrossRef]
- Robert, L.; Mark, V.; Moayed, A.; Nivashen, A.; Vinod, R.; Sophie, P.; Mohamed, E.W.; Rusheng, C. Antimicrobial prescribing and outcomes of community-acquired pneumonia in Australian hospitalized patients: A cross-sectional study. J. Int. Med. Res. 2021, 49, 3000605211058366. [Google Scholar] [CrossRef]
- SA Health. Community Acquired Pneumonia (Adults) Clinical Guideline 2021. Available online: https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/resources/policies/community+acquired+pneumonia+%28adults%29+clinical+guideline (accessed on 19 January 2024).
- Surme, S.; Balkan, I.I.; Bayramlar, O.F.; Ali, R.K.; Mete, B.; Tabak, F.; Saltoglu, N. Predictors of Long-term Outcomes in the Older Adults with Community-Acquired Pneumonia. J. Infect. Dev. Ctries. 2021, 15, 1910–1916. [Google Scholar] [CrossRef]
- Laing, R.; Coles, C.; Chambers, S.; Frampton, C.; Jennings, L.; Karalus, N.; Mills, G.; Town, G.I. Community-acquired pneumonia: Influence of management practices on length of hospital stay. Intern. Med. J. 2004, 34, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-H.; Chen, J.; Zhu, R.-X. The relationship between frailty and community-acquired pneumonia in older patients. Aging Clin. Exp. Res. 2023, 35, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Kenzaka, T.; Kumabe, A.; Mabuchi, M.; Goda, K.; Yahata, S. A comparison of pneumonia care quality between general physicians and pulmonologists. J. Gen. Fam. Med. 2018, 19, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, M.; Trevisan, R.; Demsar, M.; Lattuada, L.; Longo, C.; Cifaldi, R.; Jevnikar, M.; Santagiuliana, M.; Pelusi, L.; Pistelli, R. Opening of a respiratory intermediate care unit in a general hospital: Impact on mortality and other outcomes. Respiration 2015, 90, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Independent Health and Aged Care Pricing Authority (IHACPA). Chronicle of The International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification (ICD-10-AM) First Edition to Eleventh Edition; Independent Health and Aged Care Pricing Authority (IHACPA): Darlinghurst, NSW, Australia, 2019.
- Sharma, Y.; Horwood, C.; Hakendorf, P.; Shahi, R.; Thompson, C. External Validation of the Hospital Frailty-Risk Score in Predicting Clinical Outcomes in Older Heart-Failure Patients in Australia. J. Clin. Med. 2022, 11, 2193. [Google Scholar] [CrossRef] [PubMed]
- Campling, J.; Jones, D.; Chalmers, J.; Jiang, Q.; Vyse, A.; Madhava, H.; Ellsbury, G.; Rabe, A.; Slack, M. Clinical and financial burden of hospitalised community-acquired pneumonia in patients with selected underlying comorbidities in England. BMJ Open Respir. Res. 2020, 7, e000703. [Google Scholar] [CrossRef] [PubMed]
- Blanc, E.; Chaize, G.; Fievez, S.; Féger, C.; Herquelot, E.; Vainchtock, A.; Timsit, J.F.; Gaillat, J. The impact of comorbidities and their stacking on short- and long-term prognosis of patients over 50 with community-acquired pneumonia. BMC Infect. Dis. 2021, 21, 949. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.B.; Bates, D.W.; Ngo, L.; Ufberg, J.W.; Shapiro, N.I. Charlson Index is associated with one-year mortality in emergency department patients with suspected infection. Acad. Emerg. Med. 2006, 13, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Thompson, C.; Kaambwa, B.; Shahi, R.; Miller, M. Validity of the Malnutrition Universal Screening Tool (MUST) in Australian hospitalized acutely unwell elderly patients. Asia Pac. J. Clin. Nutr. 2017, 26, 994–1000. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat. Methods Med. Res. 2017, 26, 1654–1670. [Google Scholar] [CrossRef]
- Cabre, M.; Bolivar, I.; Pera, G.; Pallares, R. Factors influencing length of hospital stay in community-acquired pneumonia: A study in 27 community hospitals. Epidemiol. Infect. 2004, 132, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tu, J.; She, Q.; Li, M.; Wang, K.; Zhao, W.; Huang, P.; Chen, B.; Wu, J. Prognostic significance of frailty in hospitalized elderly patients with community-acquired pneumonia: A retrospective cohort study. BMC Geriatr. 2023, 23, 308. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.; Mazur, E.; Charney, P.; Wang, Y.; Fine, J. Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J. Gen. Intern. Med. 2007, 22, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Hecimovic, A.; Matijasevic, V.; Frost, S.A. Characteristics and outcomes of patients receiving Hospital at Home Services in the South West of Sydney. BMC Health Serv. Res. 2020, 20, 1090. [Google Scholar] [CrossRef] [PubMed]
- Fine, M.J.; Pratt, H.M.; Obrosky, D.; Lave, J.R.; McIntosh, L.J.; E Singer, D.; Coley, C.M.; Kapoor, W.N. Relation between length of hospital stay and costs of care for patients with community-acquired pneumonia. Am. J. Med. 2000, 109, 378–385. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.; Fine, M.J.; Coley, C.M.; Marrie, T.J.; Lave, J.R.; Obrosky, D.; Kapoor, W.N.; Singer, D.E. Variation in length of hospital stay in patients with community-acquired pneumonia: Are shorter stays associated with worse medical outcomes? Am. J. Med. 1999, 107, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Pothirat, C.; Liwsrisakun, C.; Bumroongkit, C.; Deesomchok, A.; Theerakittikul, T.; Limsukon, A. Comparative study on health care utilization and hospital outcomes of severe acute exacerbation of chronic obstructive pulmonary disease managed by pulmonologists vs internists. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Jong, P.; Gong, Y.; Liu, P.P.; Austin, P.C.; Lee, D.S.; Tu, J.V. Care and outcomes of patients newly hospitalized for heart failure in the community treated by cardiologists compared with other specialists. Circulation 2003, 108, 184–191. [Google Scholar] [CrossRef]
- Sharma, Y.; Horwood, C.; Hakendorf, P.; Thompson, C. Trends in Frailty and Use of Evidence-Based Pharmacotherapy for Heart Failure in Australian Hospitalised Patients: An Observational Study. J. Clin. Med. 2021, 10, 5780. [Google Scholar] [CrossRef]
- IMSANZ. What Is a General Physician? Available online: https://www.imsanz.org.au/about-us/what-is-a-general-physician (accessed on 26 November 2023).
| Total | General Medicine | Respiratory | p Value | |
|---|---|---|---|---|
| No. (%) | 3004 | 2673 (71.8) | 331 (9.1) | |
| Age years, mean (SD) | 73.4 (17.4) | 74.5 (17.1) | 64.2 (17.6) | <0.05 |
| Sex male n (%) | 1646 (54.8) | 1454 (54.4) | 192 (58.0) | 0.213 |
| CCI mean (SD) | 2.3 (2.7) | 2.4 (2.8) | 1.9 (2.3) | <0.05 |
| CURB-65 mean (SD) | 1.8 (1.1) | 1.8 (1.1) | 1.4 (1.1) | <0.05 |
| HFRS mean (SD) | 5.4 (4.8) | 5.5 (4.9) | 4.0 (3.8) | <0.05 |
| MUST score mean (SD) | 0.6 (1.1) | 0.7 (1.1) | 0.6 (1.2) | 0.872 |
| Chronic lung disease n (%) | 1109 (36.9) | 943 (35.3) | 166 (50.2) | <0.05 |
| CAD n (%) | 269 (8.9) | 250 (9.4) | 19 (5.7) | <0.05 |
| CKD n (%) | 427 (14.2) | 404 (15.1) | 23 (6.9) | <0.05 |
| Cancer n (%) | 354 (11.8) | 304 (11.4) | 50 (15.1) | 0.05 |
| Smokers n (%) | 145 (4.8) | 114 (4.3) | 31 (9.4) | <0.05 |
| Alcohol n (%) | 165 (5.5) | 148 (5.5) | 17 (5.1) | 0.763 |
| Positive bacterial culture n (%) | 326 (10.9) | 247 (9.2) | 79 (23.9) | <0.05 |
| Haemoglobin g/L mean (SD) | 120.6 (20.4) | 120.5 (20.5) | 120.9 (19.9) | 0.739 |
| WBC × 109/L mean (SD) | 12.8 (7.5) | 12.8 (7.5) | 12.9 (6.9) | 0.816 |
| CRP mg/L mean (SD) | 109.5 (104.3) | 108.4 (102.4) | 118.8 (119.1) | 0.096 |
| Creatinine mmol/L mean (SD) | 102.8 (75.3) | 104.7 (77.8) | 87.2 (47.7) | <0.05 |
| Albumin g/L mean (SD) | 29.6 (5.5) | 29.7 (5.5) | 28.5 (5.8) | <0.05 |
| INR mean (SD) | 1.4 (0.7) | 1.4 (0.7) | 1.3 (0.6) | 0.352 |
| MET calls n (%) | 432 (14.4) | 376 (14.1) | 56 (16.9) | 0.163 |
| ICU admission n (%) | 138 (4.6) | 94 (3.5) | 44 (13.3) | <0.05 |
| ICU Length of stay in hrs median IQR | 74.5 (44, 140.5) | 75 (41, 134) | 75.5 (53, 148.5) | 0.394 |
| High-flow oxygen n (%) | 68 (2.3) | 41 (1.5) | 27 (8.2) | <0.05 |
| NIV n (%) | 33 (1.1) | 13 (0.5) | 20 (6.0) | <0.05 |
| Mechanical ventilation n (%) | 14 (0.5) | 13 (0.5) | 1 (0.3) | 0.642 |
| Vasopressor support n (%) | 113 (3.8) | 90 (3.4) | 23 (6.9) | <0.05 |
| Outcome | Overall | General Medicine | Respiratory | p Value |
|---|---|---|---|---|
| No. (%) | 3004 | 2673 (78.9) | 331 (11.1) | |
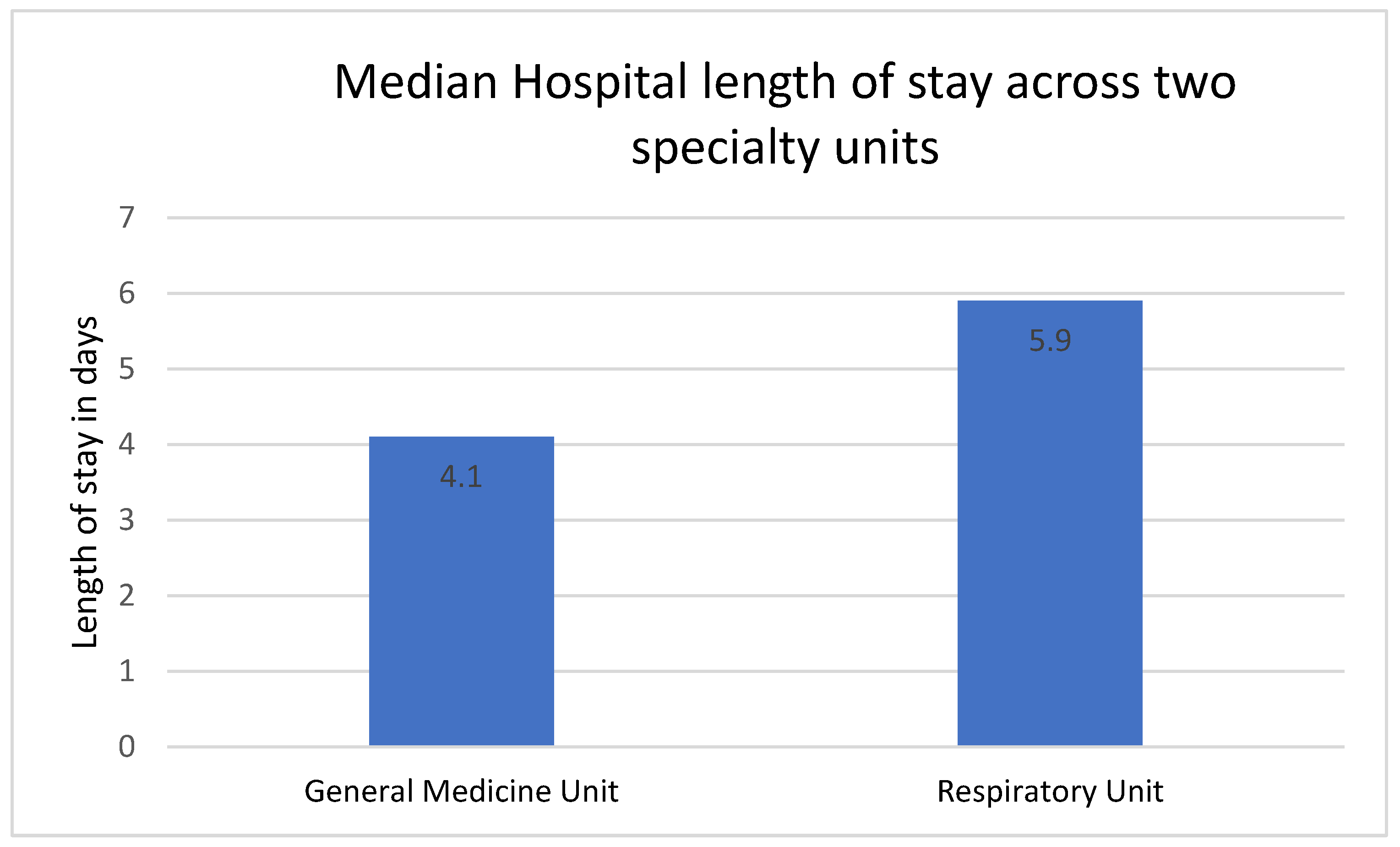
| Length of stay, median IQR | 3.9 (2, 7) | 4.1 (2.5, 7.1) | 5.9 (3.5, 9) | <0.05 |
| In-hospital mortality n (%) | 215 (7.2) | 199 (7.4) | 16 (4.8) | 0.082 |
| 30-day mortality n (%) | 378 (12.6) | 348 (13.0) | 30 (9.1) | <0.05 |
| 30-day readmissions median IQR | 481 (16.0) | 428 (16.0) | 53 (16.1) | 0.100 |
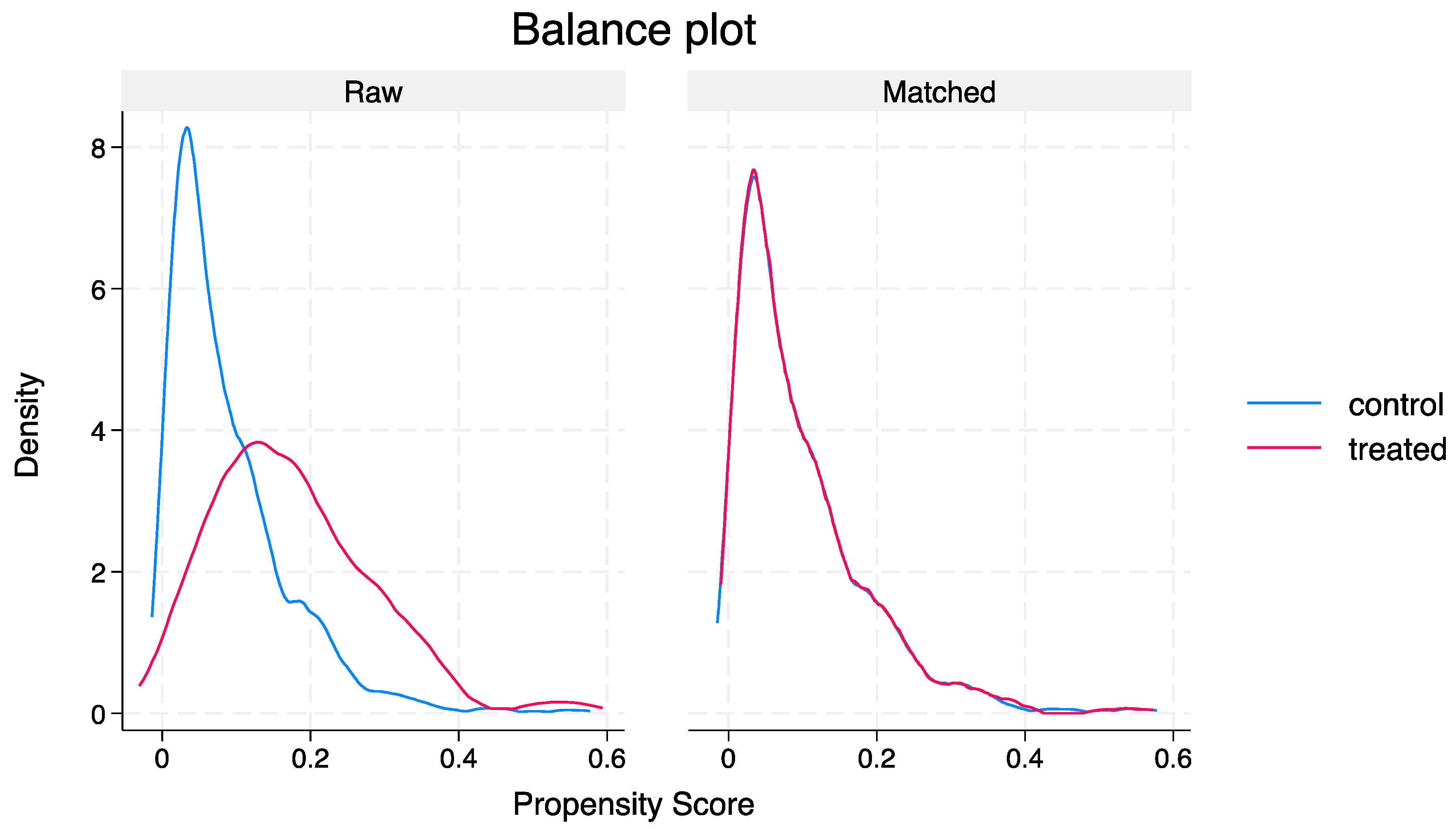
| Standardised Mean Differences | Variance Ratio | |||
|---|---|---|---|---|
| Variable | Raw | Matched | Raw | Matched |
| Age | −0.56 | −0.09 | 1.11 | 0.88 |
| Sex | 0.05 | −0.08 | 0.99 | 1.01 |
| CCI | −0.22 | 0.76 | 0.75 | 1.28 |
| CURB-65 | −0.54 | 0.01 | 0.86 | 0.96 |
| MUST_score | −0.04 | −0.06 | 1.01 | 0.84 |
| HFRS | −0.53 | −0.03 | 0.47 | 0.69 |
| Haemoglobin | 0.07 | −0.06 | 0.99 | 1.26 |
| CRP | −0.12 | 0.10 | 0.97 | 1.52 |
| Creatinine | −0.31 | 0.08 | 0.03 | 0.58 |
| Albumin | −0.11 | 0.01 | 1.35 | 1.71 |
| Chronic lung disease | 0.35 | 0.09 | 1.07 | 0.94 |
| Cancer | 0.02 | 0.04 | 1.07 | 1.10 |
| CAD | −0.11 | −0.08 | 0.70 | 0.54 |
| CKD | −0.26 | −0.03 | 0.51 | 0.94 |
| ICU admission | 0.04 | 0.09 | 0.04 | 0.09 |
| High-flow oxygen | 0.01 | 0.05 | 0.01 | 0.04 |
| NIV | 0.01 | 0.06 | 0.01 | 0.07 |
| Outcome | Odds Ratio * | 95% CI | p Value |
|---|---|---|---|
| LOS | 8.53 | 1.96–37.25 | 0.004 |
| In-hospital mortality | 1.02 | 0.97–1.07 | 0.775 |
| 30-day mortality | 0.96 | 0.91–1.02 | 0.101 |
| 30-day readmissions | 0.95 | 0.87–1.05 | 0.336 |
| Outcome | Odds Ratio * | Robust SE | 95% CI | p Value |
|---|---|---|---|---|
| LOS | 6.87 | 3.00 | 0.99–12.75 | 0.022 |
| In-hospital mortality | 1.01 | 0.02 | 0.98–1.05 | 0.461 |
| 30-day mortality | 0.98 | 0.01 | 0.96–1.07 | 0.191 |
| 30-day readmissions | 0.89 | 0.08 | 0.76–1.05 | 0.177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, Y.; Mangoni, A.A.; Shahi, R.; Horwood, C.; Thompson, C. Comparing Outcomes of Community-Acquired Pneumonia Patients Discharged from General Medicine and Respiratory Units in Australia: A Propensity Score-Matched Analysis. J. Clin. Med. 2024, 13, 3001. https://doi.org/10.3390/jcm13103001
Sharma Y, Mangoni AA, Shahi R, Horwood C, Thompson C. Comparing Outcomes of Community-Acquired Pneumonia Patients Discharged from General Medicine and Respiratory Units in Australia: A Propensity Score-Matched Analysis. Journal of Clinical Medicine. 2024; 13(10):3001. https://doi.org/10.3390/jcm13103001
Chicago/Turabian StyleSharma, Yogesh, Arduino A. Mangoni, Rashmi Shahi, Chris Horwood, and Campbell Thompson. 2024. "Comparing Outcomes of Community-Acquired Pneumonia Patients Discharged from General Medicine and Respiratory Units in Australia: A Propensity Score-Matched Analysis" Journal of Clinical Medicine 13, no. 10: 3001. https://doi.org/10.3390/jcm13103001
APA StyleSharma, Y., Mangoni, A. A., Shahi, R., Horwood, C., & Thompson, C. (2024). Comparing Outcomes of Community-Acquired Pneumonia Patients Discharged from General Medicine and Respiratory Units in Australia: A Propensity Score-Matched Analysis. Journal of Clinical Medicine, 13(10), 3001. https://doi.org/10.3390/jcm13103001







