Abstract
Introduction: Indonesia, as a developing country, has limited data on the factors associated with 30-day mortality in COVID-19 patients in Indonesia. As a matter of fact, study analyzing factors associated with 30-day mortality of COVID-19 infection in Indonesia has never been conducted. This study aims to fill this gap in the literature by conducting a large-scale analysis of factors associated with 30-day mortality in COVID-19 patients in Indonesia. Method: This study employed a single-center retrospective cohort observational design, and was conducted at Cipto Mangunkusumo National General Hospital between the years 2022 and 2023. Sampling was conducted using the consecutive sampling method. The study included patients aged 18 years and above who had been confirmed to have COVID-19 infection. Survival analysis was conducted using Kaplan–Meier and multivariate Cox regression analysis. Result: Our study included a total of 644 patients, with 120 patients (18.6%) expiring within 30 days. In the multivariate analysis using the backward Wald method, severe COVID-19 (HR: 7.024; 95% CI: 3.971–12.744; p value: <0.0001), moderate COVID-19 infection (HR: 1.660; 95% CI: 1.048–2.629; p value: 0.031), liver cirrhosis (HR: 3.422; 95% CI: 1.208–9.691; p value: 0.021), female sex (HR: 1.738; 95% CI: 1.187–2.545; p value: 0.004), old age (HR: 2.139; 95% CI: 1.279–3.577; p value: 0.004), high leukocyte (HR: 11.502; 95% CI: 1.523–86.874; p value: 0.018), high NLR (HR: 1.720; 95% CI: 1.049–2.819; p value: 0.032), high CRP (HR: 1.906; 95% CI: 1.092–3.329; p value: 0.023), high procalcitonin (HR: 3.281; 95% CI: 1.780–6.049; p value: 0.001), and high creatinine (HR: 1.863; 95% CI: 1.240–2.800; p value: 0.003) were associated with 30-day mortality from COVID-19 infection. Subgroup analysis excluding cancer patients showed that age, D-Dimer, CRP, and PCT were associated with 30-day mortality in COVID-19 patients, while steroid therapy is protective. Conclusions: This study finds that COVID-19 severity, liver cirrhosis, sex, age, leukocyte, NLR, CRP, creatinine, and procalcitonin were associated with COVID-19 mortality within 30 days. These findings underscore the multifactorial nature of COVID-19 infection mortality. It is important, therefore, that patients which exhibit these factors should be treated more aggressively to prevent mortality.
1. Introduction
As is now widely known, the COVID-19 virus, also known as SARS-CoV-2, has emerged as one of the most significant global health challenges of the 21st century. The virus was first identified in December 2019 in the city of Wuhan, China. This virus then quickly spread worldwide, leading to a pandemic declaration by the World Health Organization (WHO) in March 2020. The unprecedented scale and impact of COVID-19 have reshaped daily life, economies, and public health strategies across the globe, with over 6 million deaths and millions more people infected [1,2,3].
During the pandemic, the total number of patients requiring hospitalization increased sharply, and the demand for intensive care unit (ICU) beds increased during the pandemic [4]. This has put a strain on the healthcare system and, at that time, it was difficult to provide adequate care to all patients.
Mortality from COVID-19 is a major concern for healthcare providers. There are many risk factors of mortality from COVID-19. One of the risk factors is age, which has consistently emerged as one of the most significant risk factors for COVID-19 mortality [5]. Multiple studies have shown that older individuals face a substantially higher risk of death from COVID-19 compared to younger age groups [6,7,8]. Older age is linked to a weakening of the immune system and an increased occurrence of other health issues like heart disease, diabetes, and respiratory ailments. These factors raise the chances of experiencing severe outcomes from COVID-19 [9].
There are other risk factors such as BMI. Studies have consistently demonstrated a dose–response relationship between BMI and mortality risk, with higher BMI categories associated with increased odds of severe outcomes, including death [10,11]. Comorbidities appear to also be linked with COVID-19 mortality [12,13].
D-Dimer, a biomarker indicative of blood clot formation and breakdown, has been shown to be useful in predicting prognosis of COVID-19 patients. Elevated levels of D-Dimer have been consistently associated with an increased risk of mortality [14,15]. COVID-19 triggers a hyperinflammatory response and a propensity for thrombotic events, leading to microvascular damage and organ dysfunction.
Indonesia is a developing country with suboptimal healthcare. Furthermore, there are many barriers and challenges in conducting research in Indonesia, such as the low priority of health research and poor research culture [16]. This has resulted in limited publications in developing countries, including Indonesia. Thus, even post-pandemic, Indonesia still has no data from study on the factors associated with 30-day mortality of COVID-19 patients in the country.
Other developing countries have more established data on COVID-19. For example, a study by Ismail et al. in Malaysia demonstrated that older age, male sex, and multiple comorbidities were factors associated with COVID-19 mortality [17]. Meanwhile, a study in Thailand showed that older age, the use of high flow nasal cannula, the use of mechanical ventilation, and hydrocortisone treatment were associated with in-hospital mortality [18].
Thus, this study aims to fill this gap in the literature by conducting a large-scale analysis of factors associated with 30-day mortality in COVID-19 patients in Indonesia.
2. Method
2.1. Study Design
This study employed a single-center retrospective cohort observational design, and was conducted at Cipto Mangunkusumo National General Hospital between the years 2022 and 2023. The hospital is a national tertiary referral hospital in Indonesia. Data for this study were sourced from patient medical records covering the period from January 2020 to July 2023.
The dependent variable for this study was 30-day mortality. Meanwhile, the independent variables were the severity of the COVID-19 infection, sex, age, BMI, hemoglobin levels, leukocyte count, platelet count, neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio, C-reactive protein (CRP) levels, D-Dimer, pro-calcitonin levels, aspartate aminotransferase (AST) levels, alanine aminotransferase (ALT) levels, creatinine levels, steroid therapy, and comorbidities. Assessed comorbidities were cancer, hypertension, diabetes, chronic kidney disease, liver cirrhosis, and cardiovascular diseases.
2.2. Operational Definitions
COVID-19 infection status was determined using a PCR test and/or antigen test. The severity of COVID-19 was categorized into three groups: mild, moderate, and severe, following the guidelines provided by the Indonesian COVID-19 management (edition 4). Survival time was calculated from the day of hospital admission until the patient expired or survived. If the patient was lost to follow-up, such as discharge against medical advice (DAMA), the latest day of follow-up would be used, and the patient would be classified as censored in survival analysis.
Age was determined from the date of birth. Sex was determined using biological sex. BMI classification for this study used criteria for Asia–Pacific, which are: (1) <18.5 kg/m2 as underweight; (2) 18.5 to 22.9 kg/m2 as normal weight; (3) 23.0 to 24.9 kg/m2 as overweight; and (4) ≥25 kg/m2 as obese [19].
Laboratory parameters were collected either from the first day of hospital admission or the closest subsequent day, while comorbidities were identified through examination of the diagnoses on the medical records. All laboratory parameters were conducted at Cipto Mangunkusumo National General Hospital. The variable of cardiovascular disease included stroke, heart failure, a history of myocardial infarction, arrythmia, and peripheral artery disease. Meanwhile, the variable of cancer included all types of cancer, irrespective of the cancer stage.
2.3. Participants and Inclusion Criteria
The study included patients aged 18 years and above who had been confirmed to have a COVID-19 infection. Patients with incomplete medical record data were excluded from the study. Sampling was conducted using consecutive sampling.
2.4. Statistical Analysis
Descriptive analysis was conducted to summarize the characteristics of patients. Numerical data with a normal distribution were described using the mean and standard deviation, while numerical data with a non-normal distribution were presented using the median and minimum–maximum values. Categorical data were presented using percentages.
Comparisons between variables were conducted using a chi-square for categorical data and a t-test or Mann–Whitney test for numerical data, depending on the data normality.
Survival analysis was conducted using Kaplan–Meier analysis and Cox regression. For survival analysis, numerical variables were converted into categorical variables using median/mean as the cutoff, or using predetermined cutoffs. For the Kaplan–Meier analysis, a Log-rank test and Breslow tests were used to analyze the statistical differences in survival.
Bivariate Cox regression for factors associated with 30-day mortality was presented as a hazard ratio (HR) with 95% confidence intervals. Multivariate Cox regression was conducted using a backward Wald method.
All collected data were processed using SPSS version 26.0.
2.5. Ethical Approval
This study was approved by ethical committee of Cipto Mangukusumo National General Hospital, with approval number KET-300/UN2.F1/ETIK/PPM.00.02/2022. As this study used secondary data, no consent was determined to be required by ethical committee of Cipto Mangukusumo National General Hospital.
3. Results
Our study included a total of 644 patients hospitalized with COVID-19 infection. Their characteristics are presented in Table 1. Within our study population, 120 patients (18.6%) expired within 30 days of hospitalization. Mild, moderate, and severe COVID-19 infections constitute 35.9%, 58.9%, and 5.3% of total patients, respectively.

Table 1.
Baseline characteristics.
A total of 281 (43.6%) patients were males, and 363 (56.4%) were females. The mean age of the patients was 48.75 years, and the group that expired had statistically older patients than the group that survived (mean 53.83 vs. 47.57 years; p value: <0.0001). The group that expired also had higher proportion of cancer (65% vs. 34.7%; p value: <0.0001). Furthermore, there are differences in the proportions of patients that survived and expired, as determined by the chi-square tests, on COVID-19 severity (p value: <0.0001), BMI (p value: 0.002), chronic kidney disease (p value: <0.0001), and steroid use (p value: 0.004).
For the numerical variables, the patients that expired also had lower BMI (p value: <0.0001), lower hemoglobin (p value: <0.0001), lower hematocrit (p value: <0.0001), lower platelet (p value: <0.0001), and higher AST (p value: 0.033).
The median survival assessed by Kaplan–Meier analysis was 28 days (95% CI: 26.594–29.406), while the mean survival was 22.643 days (95% CI: 21.442–23.843) (Figure 1). Analyses conducted showed that COVID-19 severity was associated with 30-day survival (Log Rank p value: <0.0001; Breslow p value: <0.0001) (Figure 1).
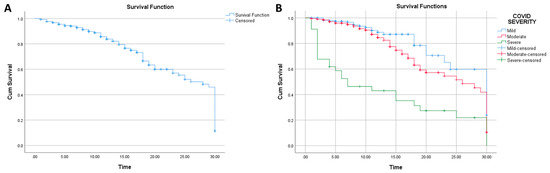
Figure 1.
(A): Kaplan–Meier analysis for survival (n: 644); (B): Kaplan–Meier analysis for survival based on COVID-19 severity (n: 644).
In Figure 1, it can be seen that the highest survival was from the group with a mild COVID-19 infection, as the survival from moderate and severe COVID-19 were lower. Noteworthy, from the figure, it appears that the group with a severe COVID-19 infection has a high proportion of patients expiring in less than 10 days when compared with other groups.
Similarly, chronic kidney disease (Log Rank p value: 0.011; Breslow p value: 0.047), age (Log Rank p value: 0.030; Breslow p value: 0.042), cancer status (Log Rank p value: 0.003; Breslow p value: <0.0001), hemoglobin (Log Rank p value: 0.051; Breslow p value: 0.002), leukocyte (Log Rank p value: <0.0001; Breslow p value: <0.0001), NLR (Log Rank p value: <0.0001; Breslow p value: <0.0001), D-Dimer (Log Rank p value: 0.006; Breslow p value: 0.001), CRP (Log Rank p value: <0.0001; Breslow p value: <0.0001), PCT (Log Rank p value: <0.0001; Breslow p value: <0.0001), AST (Log Rank p value: 0.001; Breslow p value: 0.007), and creatinine level (Log Rank p value: 0.002; Breslow p value: 0.002) were variables associated with survival in the Kaplan–Meier analysis (Figure 2).
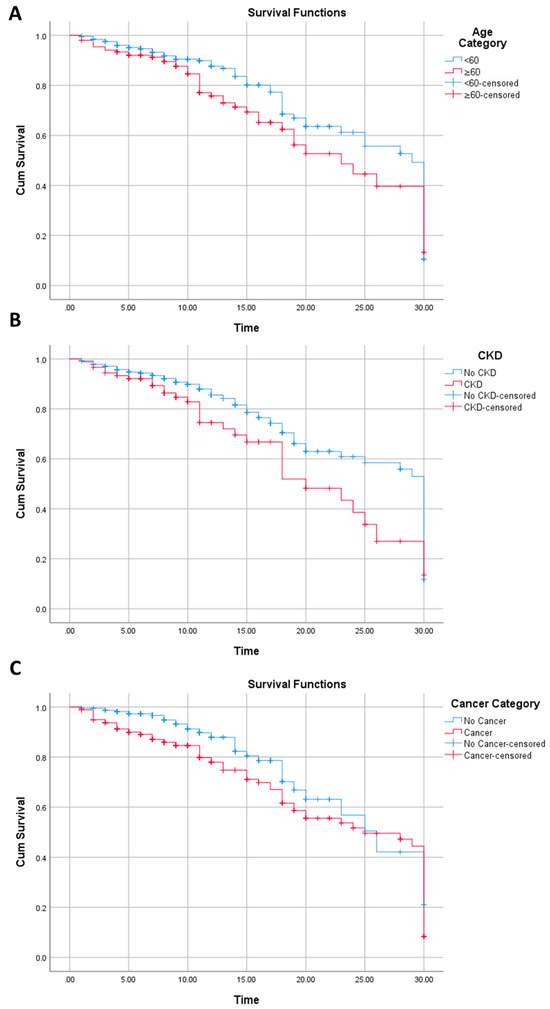
Figure 2.
Kaplan–Meier analysis for survival on age (A), chronic kidney disease (B), and cancer (C).
Per Figure 2, patients with an age of <60 years old had higher survival than those with an age of ≥60 years old from the start of the follow-up to the last follow-up. This is similar with the chronic kidney disease variable. In contrast, the survival of cancer patients was generally lower than non-cancer patients, but only until approximately 25 days of follow-up, where, subsequent to this, the survival of cancer patients is slightly higher than non-cancer patients.
Cox regression results are presented in Table 2. In the bivariate Cox regression analysis, it was found that severe COVID-19 infection, chronic kidney disease, cancer status, age, hematocrit, leukocyte, NLR, D-Dimer, CRP, procalcitonin, and AST were associated with 30-day COVID-19 mortality.

Table 2.
Bivariate and multivariate Cox regression analysis of factors associated with mortality from COVID-19 within 30 days.
In the multivariate analysis using the backward Wald method, only nine variables were found significantly associated with COVID-19 mortality. From these nine variables, severe COVID-19 infection had the highest HR (HR: 7.024; 95% CI: 3.971–12.744; p value: <0.0001). Meanwhile, moderate COVID-19 infection had a lower HR (HR: 1.660; 95% CI: 1.048–2.629; p value: 0.031).
Other variables associated with COVID-19 mortality include liver cirrhosis (HR: 3.422; 95% CI: 1.208–9.691; p value: 0.021), sex (HR: 1.738; 95% CI: 1.187–2.545; p value: 0.004), age (HR: 2.139; 95% CI: 1.279–3.577; p value: 0.004), leukocyte (HR: 11.502; 95% CI: 1.523–86.874; p value: 0.018), NLR (HR: 1.720; 95% CI: 1.049–2.819; p value: 0.032), CRP (HR: 1.906; 95% CI: 1.092–3.329; p value: 0.023), procalcitonin (HR: 3.281; 95% CI: 1.780–6.049; p value: 0.001), and creatinine (HR: 1.863; 95% CI: 1.240–2.800; p value: 0.003).
A subgroup analysis excluding cancer patients was conducted to address the potential confounding factors related to cancer, as cancer is known to cause elevated NLR, D-Dimer, CRP, and PCT (Table 3).

Table 3.
Results of multivariate Cox regression analysis excluding cancer patients (n: 384).
In this subgroup analysis, the multivariate analysis revealed that age (HR: 2.225; 95% CI: 1.184–4.182; p value: 0.013), D-Dimer (HR: 2.942; 95% CI: 1.126–7.684; p value: 0.028), CRP(HR: 4.356; 95% CI: 1.665–11.398; p value: 0.003), PCT (HR: 8.098; 95% CI: 1.913–34.278; p value: 0.004), were associated with 30-day COVID-19 mortality. Meanwhile, steroid use showed a protective effect against COVID-19 mortality (HR: 0.332; 95% CI:0.154–0.714; p value: 0.005).
4. Discussion
This study was the first to investigate the factors associated with 30-day mortality in COVID-19 patients in Indonesia. This study found that the severity of the COVID-19 infection is a major risk factor for 30-day mortality, similar to other studies [20,21,22]. Also, this finding is similar to other infections, such as pneumonia, sepsis, and meningitis, where the more severe the infection is, the higher the likelihood for complications and mortality. Therefore, it is important to identify patients with severe COVID-19 infection early on, so that they can receive the appropriate treatment.
This study also found that an age of ≥60 years is a significant risk factor for 30-day mortality (HR: 2.139). Older patients are more likely to have underlying health conditions that make them more susceptible to the complications of COVID-19 [6,23,24]. The other main factors for COVID-19 mortality in the elderly are immunosenescence and chronic inflammation [25,26].
A study conducted by Doerre et al. showed that male patients were more likely to die from COVID-19 than female patients [27]. The reasons for this are not fully understood, but it may be due to the fact males might have higher exposure to COVID-19, or have a higher likelihood of smoking [28]. In contrast, we found that female patients had a higher hazard ratio for mortality. It is possible that the female sex is related to poorer nutrition in Indonesia.
This study also showed that liver cirrhosis was associated with 30-day COVID-19 morality. Liver cirrhosis is a common chronic condition, especially in developed countries, characterized by scarring of the liver tissue, often resulting from long-term liver damage and inflammation, such as from long-term alcohol drinking or obesity. COVID-19 infection primarily affects the respiratory system, but can also have systemic effects, including on the liver. Liver cirrhosis often leads to immune system dysfunction, making individuals more susceptible to infections, including viral infections like COVID-19 [29]. A study by Wang et al. showed that liver cirrhosis is an independent predictor of COVID-19 mortality [30].
Finally, this study found that leukocytosis, high NLR, high CRP, and high PCT are all associated with 30-day mortality. These are all markers of inflammation, and it is widely known that they are indicative of more severe immune responses to COVID-19 and general infections [31,32,33].
In the multivariate analysis, D-Dimer was not found to be statistically significant, as it is confounded by cancers, which also increase D-Dimer. When a subgroup analysis was conducted excluding cancer patients, D-Dimer became statistically significant. However, there are still other factors that can influence D-Dimer other than cancer, such as diabetes, age, and various comorbidities.
D-Dimer is a fibrin degradation product that is elevated in various clinical conditions, including COVID-19. A study conducted by Ali et al. discovered that high D-Dimer levels were independently correlated with the need for invasive mechanical ventilation (IMV) in COVID-19 patients [34]. The study also found that low levels of D-Dimer could predict post-IMV survival of mechanically ventilated COVID-19 patients. Another study by Zuckier et al. found that increased levels of D-Dimer were associated with pulmonary embolism in COVID-19 patients [35]. Furthermore, a study by Wang et al. found that persistent elevation of D-Dimer levels following recovery from COVID-19 was associated with an increased risk of thrombotic events [36].
Cancers cause derailment of the immune system and inflammation in patients [37]. Cancer patients infected with COVID-19 face a significantly higher risk of severe illness and death compared to the general population. Meta-analyses by ElGohary et al. estimated an approximately 3.23 odds ratio of mortality in cancer patients [38]. In the bivariate Cox regression analysis of this study, cancer was found to be associated with 30-day COVID-19 mortality, but in the multivariate analysis, no significance was found. It is possible that that cancer indirectly increases several parameters, such as NLR, D-Dimer, and CRP, resulting in the cancer variable not being significant during analysis.
The findings of this study have important implications for the management of COVID-19 patients in Indonesia. Healthcare professionals should be aware of the risk factors for 30-day mortality so that they can identify patients who are at high risk, and provide them with appropriate treatment. Our results are largely consistent with those of previous studies on hospitalized patients.
This study has several limitations. First, the study’s retrospective design may limit the ability to establish causal relationships from the statistical associations observed. Finally, we also did not have data of mechanical ventilation use among patients, nor detailed information on the type and dosage of steroid use among the patients.
5. Conclusions
This study finds that COVID-19 severity, liver cirrhosis, sex, age, leukocyte, NLR, CRP, creatinine, and procalcitonin are associated with COVID-19 mortality within 30 days. In an analysis excluding cancer patients, it was found that age, D-Dimer, CRP, and PCT were associated with 30-day mortality in COVID-19 patients, while steroid therapy is protective. These findings underscore the multifactorial nature of COVID-19 infection mortality. It is important, therefore, that patients which exhibit these factors should be treated more aggressively to prevent mortality.
Author Contributions
Conceptualization, I.R., E.Y., W.R., C.I., L.S., A.R., N.A.M., A.M.L., F.P., R.C., D.P., A.R.A., A.S., T.D.A., A.H.R., Z.D., K.T., K.W., Y.M.S., L.W., S.P. and B.C.E.; Methodology, I.R., M.Y., A.M. and K.W.; Formal analysis, I.R., M.Y., E.Y., A.M.L., D.P., K.W. and B.C.E.; Investigation, I.R. and K.W.; Resources, I.R. and Z.D.; Data curation, I.R.; Writing—original draft, I.R. and K.W.; Writing—review & editing, I.R., M.Y., E.Y., D.P., Z.D., K.W., Y.M.S., L.W., S.P. and B.C.E.; Supervision, K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Cipto Mangukusumo National General Hospital (protocol code KET-300/UN2.F1/ETIK/PPM.00.02/2022 and 28 March 2022).
Informed Consent Statement
Patient consent was waived by ethical committee of Cipto Mangukusumo National General Hospital due to the study used secondary data.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Ikhwan Rinaldi, upon reasonable request.
Acknowledgments
The authors would like to express their gratitude toward Marcello Mikhael Kadharusman and Muhammad Alifian Remifta Putra for their assistance in proofreading the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martín Sánchez, F.J.; Martínez-Sellés, M.; Molero García, J.M.; Guillén, S.M.; Rodríguez-Artalejo, F.; Ruiz-Galiana, J.; Cantón, R.; De Lucas Ramos, P.; García-Botella, A.; García-Lledó, A.; et al. Insights for COVID-19 in 2023. Rev. Esp. Quimioter. Publ. Soc. Esp. Quimioter. 2023, 36, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Miyah, Y.; Benjelloun, M.; Lairini, S.; Lahrichi, A. COVID-19 Impact on Public Health, Environment, Human Psychology, Global Socioeconomy, and Education. Sci. World J. 2022, 2022, 5578284. [Google Scholar] [CrossRef]
- Naseer, S.; Khalid, S.; Parveen, S.; Abbass, K.; Song, H.; Achim, M.V. COVID-19 outbreak: Impact on global economy. Front. Public Health 2023, 10, 1009393. [Google Scholar] [CrossRef] [PubMed]
- Janke, A.T.; Mei, H.; Rothenberg, C.; Becher, R.D.; Lin, Z.; Venkatesh, A.K. Analysis of Hospital Resource Availability and COVID-19 Mortality Across the United States. J. Hosp. Med. 2021, 16, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Metheny, H.; LeMasters, K.; Brinkley-Rubinstein, L. Age and COVID-19 Mortality in the United States: A Comparison of the Prison and General Population. Int. J. Prison. Health 2022, 19, 35–46. [Google Scholar] [CrossRef]
- Elo, I.T.; Luck, A.; Stokes, A.C.; Hempstead, K.; Xie, W.; Preston, S.H. Evaluation of Age Patterns of COVID-19 Mortality by Race and Ethnicity from March 2020 to October 2021 in the US. JAMA Netw. Open 2022, 5, e2212686. [Google Scholar] [CrossRef] [PubMed]
- Bonanad, C.; García-Blas, S.; Tarazona-Santabalbina, F.; Sanchis, J.; Bertomeu-González, V.; Fácila, L.; Ariza, A.; Núñez, J.; Cordero, A. The Effect of Age on Mortality in Patients with COVID-19: A Meta-Analysis with 611,583 Subjects. J. Am. Med. Dir. Assoc. 2020, 21, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.K.; Petermann-Rocha, F.; Gray, S.R.; Jani, B.D.; Katikireddi, S.V.; Niedzwiedz, C.L.; Foster, H.; Hastie, C.E.; Mackay, D.F.; Gill, J.M.R.; et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS ONE 2020, 15, e0241824. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Alonzi, T.; Alter, G.; Noonan, D.M.; Landay, A.L.; Albini, A.; Goletti, D. Impact of aging on immunity in the context of COVID-19, HIV, and tuberculosis. Front. Immunol. 2023, 14, 1146704. [Google Scholar] [CrossRef] [PubMed]
- Gabbrielli, R.; Pugno, N.M. The impact of mean body mass index on reported mortality from COVID-19 across 181 countries. Front. Public Health 2023, 11, 1106313. [Google Scholar] [CrossRef]
- Singh, R.; Rathore, S.S.; Khan, H.; Karale, S.; Chawla, Y.; Iqbal, K.; Bhurwal, A.; Tekin, A.; Jain, N.; Mehra, I.; et al. Association of Obesity with COVID-19 Severity and Mortality: An Updated Systemic Review, Meta-Analysis, and Meta-Regression. Front. Endocrinol. 2022, 13, 780872. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Nalla, L.V.; Sharma, M.; Sharma, N.; Singh, A.A.; Malim, F.M.; Ghatage, M.; Mukarram, M.; Pawar, A.; Parihar, N.; et al. Association of COVID-19 with Comorbidities: An Update. ACS Pharmacol. Transl. Sci. 2023, 6, 334–354. [Google Scholar] [CrossRef] [PubMed]
- Marc, M.S.; Rosca, D.; Bratosin, F.; Fira-Mladinescu, O.; Oancea, C.; Pescaru, C.C.; Velescu, D.; Wellmann, N.; Motofelea, A.C.; Ciuca, I.M.; et al. The Effect of Comorbidities and Complications on COVID-19 Mortality: A Detailed Retrospective Study in Western Romania. J. Pers. Med. 2023, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Nemec, H.M.; Ferenczy, A.; Christie, B.D., III; Ashley, D.W.; Montgomery, A. Correlation of D-dimer and Outcomes in COVID-19 Patients. Am. Surg. 2022, 88, 2115. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.; Poudel, Y.; Adhikari, A.; Aryal, B.B.; Dangol, D.; Bajracharya, T.; Maharjan, A.; Gautam, R. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS ONE 2021, 16, e0256744. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ghoshal, U.C.; Ragunath, K.; Jenkins, G.; Edwards, C.; Hasan, M.; Taylor-Robinson, S.D. Biomedical research in developing countries: Opportunities, methods, and challenges. Indian J. Gastroenterol. 2020, 39, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.N.; Abdul Halim Zaki, I.; Noordin, Z.M.; Hussin, N.S.M.; Ming, L.C.; Zulkifly, H.H. Clinical characteristics and risk factors for mortality in patients with COVID-19, A retrospective nationwide study in Malaysia. Proc. Singap. Healthc. 2022, 31, 20101058221085743. [Google Scholar] [CrossRef]
- Naorungroj, T.; Viarasilpa, T.; Tongyoo, S.; Detkaew, A.; Pinpak, T.; Wimolwattanaphan, R.; Ratanarat, R.; Promsin, P.; Thamrongpiroj, P.; Phumpichet, A.; et al. Characteristics, outcomes, and risk factors for in-hospital mortality of COVID-19 patients: A retrospective study in Thailand. Front. Med. 2022, 9, 1061955. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.-H.; Yeh, W.-T. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: An extension of Asian-Pacific recommendations. Asia Pac. J. Clin. Nutr. 2008, 17, 370–374. [Google Scholar]
- Sharma, J.; Rajput, R.; Bhatia, M.; Arora, P.; Sood, V. Clinical Predictors of COVID-19 Severity and Mortality: A Perspective. Front. Cell. Infect. Microbiol. 2021, 11, 674277. [Google Scholar] [CrossRef]
- Mehri, A.; Sotoodeh Ghorbani, S.; Farhadi-Babadi, K.; Rahimi, E.; Barati, Z.; Taherpour, N.; Izadi, N.; Shahbazi, F.; Mokhayeri, Y.; Seifi, A.; et al. Risk Factors Associated with Severity and Death from COVID-19 in Iran: A Systematic Review and Meta-Analysis Study. J. Intensive Care Med. 2023, 38, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, X.; Liu, G.; Gao, Y. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-J.; Jung, S.I. Age-Related Morbidity and Mortality among Patients with COVID-19. Infect. Chemother. 2020, 52, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, T. Improving Preparedness for and Response to Coronavirus Disease 19 (COVID-19) in Long-Term Care Hospitals in Korea. Infect. Chemother. 2020, 52, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.C. Clinical relevance of age-related immune dysfunction. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 31, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Quiros-Roldan, E.; Biasiotto, G.; Zanella, I. Letter to the Editor on ‘Bonafè M, Prattichizzo F, Giuliani A, Storci G, Sabbatinelli J, Olivieri F. Inflamm-aging: Why older men are the most susceptible to SARS-CoV-2 complicated outcomes. Cytokine Growth Factor Rev’. Cytokine Growth Factor Rev. 2020, 54, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Doerre, A.; Doblhammer, G. The influence of gender on COVID-19 infections and mortality in Germany: Insights from age- and gender-specific modeling of contact rates, infections, and deaths in the early phase of the pandemic. PLoS ONE 2022, 17, e0268119. [Google Scholar] [CrossRef]
- Patanavanich, R.; Siripoon, T.; Amponnavarat, S.; Glantz, S.A. Active Smokers Are at Higher Risk of COVID-19 Death: A Systematic Review and Meta-analysis. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2023, 25, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Brozat, J.F.; Hanses, F.; Haelberger, M.; Stecher, M.; Dreher, M.; Tometten, L.; Ruethrich, M.M.; Vehreschild, J.J.; Trautwein, C.; Borgmann, S.; et al. COVID-19 mortality in cirrhosis is determined by cirrhosis-associated comorbidities and extrahepatic organ failure: Results from the multinational LEOSS registry. United Eur. Gastroenterol. J. 2022, 10, 409–424. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Yang, H. Cirrhosis is an independent predictor for COVID-19 mortality: A meta-analysis of confounding cofactors-controlled data. J. Hepatol. 2023, 78, e28–e31. [Google Scholar] [CrossRef]
- Feng, T.; James, A.; Doumlele, K.; White, S.; Twardzik, W.; Zahid, K.; Sattar, Z.; Ukponmwan, O.; Nakeshbandi, M.; Chow, L.; et al. Procalcitonin Levels in COVID-19 Patients Are Strongly Associated with Mortality and ICU Acceptance in an Underserved, Inner City Population. Medicina 2021, 57, 1070. [Google Scholar] [CrossRef] [PubMed]
- Rathod, B.D.; Amle, D.; Khot, R.S.; Prathipati, K.K.; Joshi, P.P. Neutrophil-to-Lymphocyte Ratio as a Predictor of Disease Severity and Mortality in Coronavirus Disease 2019: Prospective Study From Central India. Cureus 2022, 14, e23696. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, Y.; Ye, L.; Sun, H.; Du, S.; Huang, H.; Zeng, F.; Chen, X.; Deng, G. Clinical Significance of Plasma D-Dimer in COVID-19 Mortality. Front. Med. 2021, 8, 638097. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Liang, W.; Abdelhafiz, A.S.; Saleh, M.M.; Salem, H.; Moazen, E.M.; Elmazny, M.I.; Rakha, M.A.; Elfeky, S.E.F. Elevation of D-dimer levels are associated with early need for mechanical ventilation support in patients with COVID-19. BMC Pulm. Med. 2023, 23, 283. [Google Scholar] [CrossRef] [PubMed]
- Zuckier, L.S.; Moadel, R.M.; Haramati, L.B.; Freeman, L.M. Diagnostic Evaluation of Pulmonary Embolism During the COVID-19 Pandemic. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Sang, L.; Ye, F.; Ruan, S.; Zhong, B.; Song, T.; Alshukairi, A.N.; Chen, R.; Zhang, Z.; et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J. Clin. Investig. 2020, 130, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- ElGohary, G.M.; Hashmi, S.; Styczynski, J.; Kharfan-Dabaja, M.A.; Alblooshi, R.M.; de la Cámara, R.; Mohmed, S.; Alshaibani, A.; Cesaro, S.; El-Aziz, N.A.; et al. The Risk and Prognosis of COVID-19 Infection in Cancer Patients: A Systematic Review and Meta-Analysis. Hematol. Oncol. Stem Cell Ther. 2022, 15, 45–53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).