The Clinical Management of Electrical Stimulation Therapies in the Rehabilitation of Individuals with Spinal Cord Injuries
Abstract
1. Introduction
2. Methods
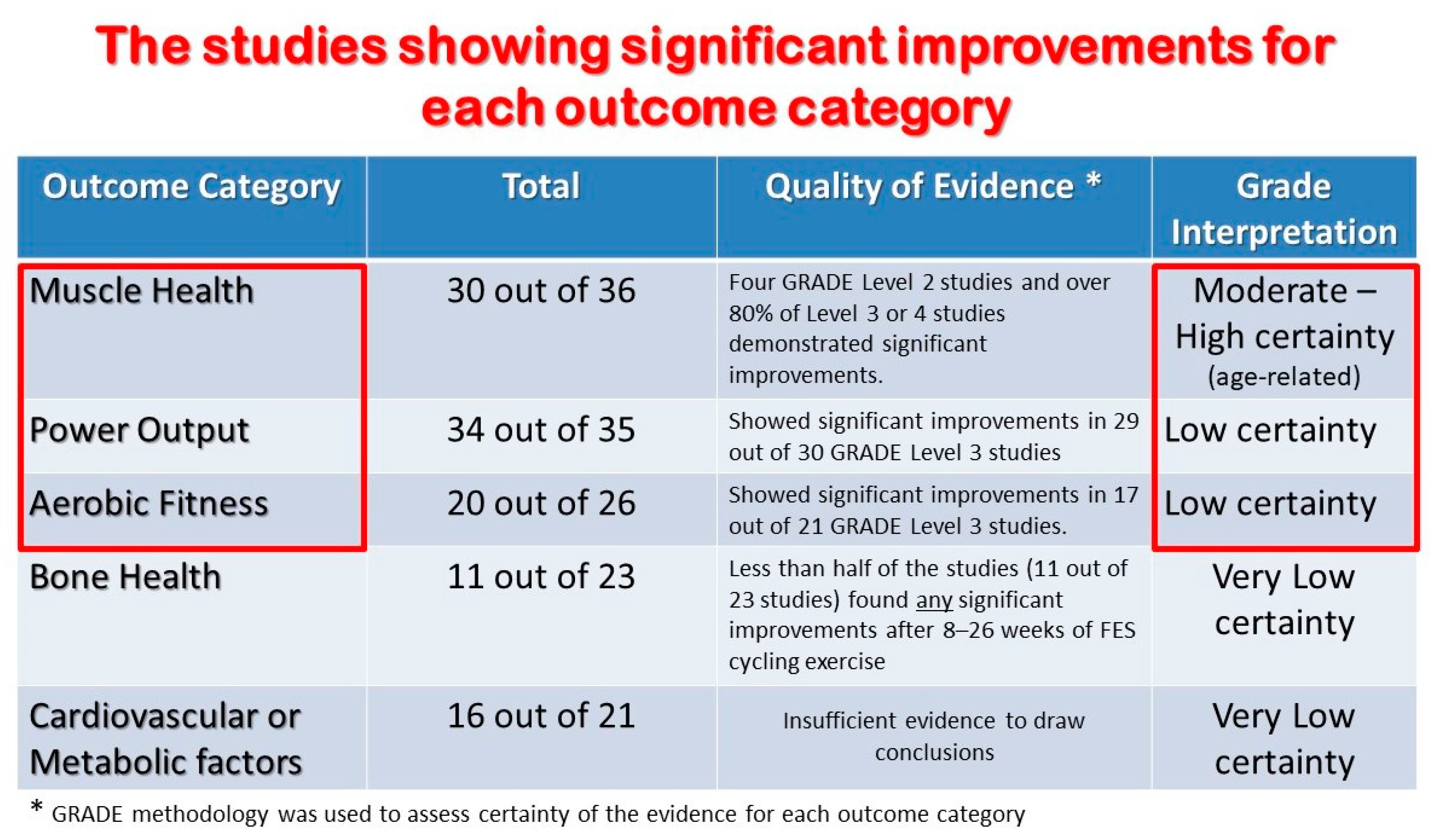
3. Results
3.1. Cardiovascular and Metabolic Health (Table 1)
| Source | Participants | Treatments | Results |
|---|---|---|---|
| Farkas et al. [36] | n = 13 Chronic SCI | 6—FES-LCE 5x/week for 16 weeks 7—ACE 5x/week for 16 weeks | FES +2.5% VO2peak ACE +20% VO2peak |
| Hasnan et al. [37] | n = 9 Chronic SCI | 9—completed ACE, FES-LCE, FES-LCE, and outside hybrid cycling at 40%, 60%, and 80% of VO2peak. | FES-LCE + ACE and outside hybrid cycling resulted in significantly higher VO2peak, PO, and cardiac output than FES-LCE during all three submaximal intensities |
| Johnston et al. [39] | n = 30 Pediatric SCI (age 5–13) | 10—FES-LCE 10—passive cycling 10—NMES | FES-LCE +16% VO2peak Passive cycling −27% VO2peak NMES −17% cholesterol level |
| Brurok et al. [40] | n = 6 Chronic SCI | 6—hybrid HIIT-FES-LCE 3x/week for 8 weeks after a 7-week control period | +24% VO2peak +33% stroke volume Decreased cardiovascular disease risk |
| Hasnan et al. [41] | n = 8 Chronic SCI | 8—hybrid HIIT FES-LCE + virtual reality 2–3 times per week (96 min per week) | +33% POpeak +20% VO2peak Blood lipids and glucose (no change) |
| Gorgey et al. [42] | n = 27 Chronic SCI | 17—NMES-RT + FES-LCE 16-PMT + FES-LCE | NMES-RT + FES-LCE +29% VO2 PMT + FES-LCE +16% VO2 |
| Griffin et al. [45] | n = 18 Chronic SCI | 18—FES-LCE 2–3 times per week for 10 weeks | Cholesterol −1% Triglyceride −4% CRP −19% IL-6 −22% TNF-α −4% Insulin levels at 60 and 120 min during oral glucose tolerance test. |
| Gorgey et al. [43] | n = 9 Chronic SCI | 11—NMES-RT 2 times/week for 16 weeks plus 2–6mg/day testosterone 11—testosterone only. | NMES grp Triglyceride −38% Cholesterol/HDL −14% Free fatty acids −24% Diet-only group Free fatty acids −20% |
Summary
3.2. Muscle Strength and Mass (Table 2)
| Source | Participants | Treatment | Results |
|---|---|---|---|
| Farkas et al. [36] | n = 13 chronic SCI | 6—FES-LCE 5x/week for 16 weeks 7—ACE 5x/week for 16 weeks | FES +4% LM FES +7% legs LM ACE +2% LM FES −4% BF% ACE −5% BF% |
| Gorgey et al. [42] | n = 27 chronic SCI | 17—NMES-RT + FES-LCE 16-PMT + FES-LCE | NMES-RT + FES-LCE +30–33% proximal Quadriceps CSA, 29–32% middle quadriceps CSA, 26–28% distal quadriceps CSA |
| Rosley et al. [46] | n = 23 chronic ‘incomplete’ SCI | 10—FES-LCE + PRT 1 session PRT and 2 sessions FES-LCE weekly over 12 weeks 13—FES-LCE 3 sessions/weekly over 12 weeks | FES-LCE + PRT left hamstring peak torque +45% change, higher than FES-LCE FES-LCE + PRT right quadricep peak torque +31% change, greater than the FES-LCE FES-LCE + PRT Muscle volume +7% increase |
| Dolbow et al. [47] | n = 10 chronic SCI | 5—interval HIIT-FES cycling 3x/week for 8 weeks and diet 5—diet alone | HIIT-FES cycling group Legs LM +7% Total BF% −2.5% Diet-only group No changes |
| Gorgey et al. [43] | n = 9 chronic SCI | 11—NMES-RT 2 times/week for 16 weeks plus 2–6mg/day testosterone 11—testosterone only | NMES grp Thigh CSA +28% Knee ext CSA +35% Knee flexor +16% |
| Gorgey et al. [49] | n= 22 chronic SCI | 11—NMES-RT 2 times/week for 16 weeks plus 2–6mg/day testosterone | NMES-RT Plus T grp (CSA) Prox knee ext +34% Mid knee ext +32% Low knee ext +30% -IMF Prox knee ext +43% Mid knee ext +34% Low knee ext +33% |
| Johnston et al. [39] | n = 17 chronic SCI | Low cadence 20 rpm High cadence 50 rpm 9—low cadence 8—high cadence 3x/week for 6 months | Low cadence +19% LM High cadence +10% LM |
Summary
3.3. Bone Mass (Table 3)
| Source | Participants | Treatment | Results |
|---|---|---|---|
| Holman et al. [54] | n = 10 chronic SCIs | NMES-RT 2x/week | Distal femur—small trabecular increase Proximal tibia—medium trabecular increase |
| Frotzler et al. [55] | n = 11 chronic SCIs | FES-LCE 3–4x/week for 1 year | +14% BMD trabecular bone (distal femur) +7% BMD total bone (distal femur) |
| Shields and Dudley-Javorski, [56] | n = 7 (6 weeks post-SCI) | FES to plantar flexor muscles of one leg. The other leg was the control | +31% BMD (distal tibia) |
| Johnston et al. [48] | n = 17 chronic SCIs | Low cadence 20 rpm High cadence 50 rpm 9—low cadence 8—high cadence 3x/week for 6 months | +7% trabecular bone in distal femur |
Summary
3.4. Diagnosis, Prognosis, and Treatment for Upper Limbs (Table 4)
| Source | Participants | Testing or Treatment | Results |
|---|---|---|---|
| Bersch et al. [58] | n = 32 Tetraplegia | Retrospective analysis Defined motor points and wrist and finger activities to detect UMN/LMN Lesions | 16 hands developed tenodesis grasp all with LMN of EDC 24: no tightness of finger flexors |
| Bersch et al. [59] | n = 24 Tetraplegia | Tested forearms for LMN/UMN lesions 44 arms analyzed | FDPIII—26 arms with UMN lesion 10 arms with partial denervation 5 arms with denervation FPL—16 arms with UMN lesion 12 arms with partial denervation 14 arms with denervation |
| Jung et al. [60] | n = 37 Tetraplegia (preserved wrist extension with paralysis in fingers) | Assessment of passive tenodesis grasp (open and closed) GRASSP testing | Those with 4–5/5 muscle strength showed higher GRASSP scores than those with 3/5 wrist extension |
| Bersch et al. [62] | n = 220 Tetraplegia Data Base | Retrospective analysis of AIS and MMT of arm and hand muscles at different time points | Hand and arm function predicted by MP and AIS and used as the basis for providing an individualized treatment plan |
| Koch-Boner et al. [63] | n = 82 159 hands Tetraplegia | Divided into 3 thumb positions (key pinch, slack thumb, and thumb-in-palm). Muscle testing and motor point testing | Muscles showed a different expression of MP and the MMT values between key punch and thumb slack positions. MMT of FPL was greater in the group “thumb-in-palm” compared with the “key pinch” position |
| Bersch & Friden [64] | n = 22 Tetraplegia ECU and 1st dorsal interosseous denervated | Electrical stimulation 33 min, 5x/wk, 12 wks | ECU +27% muscle thickness +71% pennation angle 1st dorsal interosseus +46% muscle thickness +100% pennation angle |
Summary
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deley, G.; Denuziller, J.; Babault, N. Functional electrical stimulation: Cardiorespiratory adaptations and applications for training in paraplegia. Sports Med. 2015, 45, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.A.; Krassioukov, A.V. Contemporary cardiovascular concerns after spinal cord injury: Mechanisms, maladaptations, and management. J. Neurotrauma 2015, 32, 1927–1942. [Google Scholar] [CrossRef] [PubMed]
- Ginis, K.A.M.; Latimer, A.E.; Arbour-Nicitopoulos, K.P.; Buchholz, A.C.; Bray, S.R.; Craven, B.C.; Hayes, K.C.; Hicks, A.L.; McColl, M.A.; Potter, P.J.; et al. Leisure time physical activity in a population-based sample of people with spinal cord injury part I: Demographic and injury-related correlates. Arch. Phys. Med. Rehabil. 2010, 91, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Colley, R.C.; Garriguet, D.; Janssen, I.; Craig, C.L.; Clarke, J.; Tremblay, M.S. Physical activity of Canadian adults: Accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2011, 22, 7–14. [Google Scholar] [PubMed]
- Soriano, J.E.; Squair, J.W.; Cragg, J.J.; Thompson, J.; Sanguinetti, R.; Vaseghi, B.; Emery, C.A.; Grant, C.; Charbonneau, R.; Larkin-Kaiser, K.A.; et al. A national survey of physical activity after spinal cord injury. Sci. Rep. 2022, 12, 4405. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.; Datta, N.; Jimsheleishvili, G.; Gater, D.R., Jr. Pathophysiology, Classification and Comorbidities after Traumatic Spinal Cord Injury. J. Pers. Med. 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gater, D.R., Jr.; Farkas, G.J.; Tiozzo, E. Pathophysiology of neurogenic obesity after spinal cord injury. Top. Spinal Cord Inj. Rehabil. 2021, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Farkas, G.J.; Gater, D.R. Neurogenic obesity and systemic inflammation following spinal cord injury: A review. J. Spinal Cord Med. 2018, 41, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.S.; Farkas, G.J.; Gater, D.R., Jr. Neurogenic Obesity-Induced Insulin Resistance and Type 2 Diabetes Mellitus in Chronic Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2021, 27, 36–56. [Google Scholar] [CrossRef]
- Nash, M.S.; Groah, S.L.; Gater, D.R.; Dyson-Hudson, T.A.; Lieberman, J.A.; Myers, J.; Sabharwal, S.; Taylor, A.J. Identification and management of cardiometabolic risk after spinal cord injury. J. Spinal Cord Med. 2019, 42, 643–677. [Google Scholar] [CrossRef]
- Shin, J.W.; Kim, T.; Lee, B.S.; Kim, O. Factors Affecting Metabolic Syndrome in Individuals with Chronic Spinal Cord Injury. Ann. Rehabil. Med. 2022, 46, 24–32, Correction in Ann. Rehabil. Med. 2022, 46, 109. [Google Scholar] [CrossRef]
- Faghri, P.D.; Yount, J.P.; Pesce, W.J.; Seetharama, S.; Votto, J.J. Circulatory hypokinesis and functional electric stimulation during standing in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2001, 82, 1587–1595. [Google Scholar] [CrossRef]
- Furlan, J.C.; Fehlings, M.G. Cardiovascular complications after acute spinal cord injury: Pathophysiology, diagnosis, and management. Neurosurg. Focus 2008, 25, E13. [Google Scholar] [CrossRef] [PubMed]
- Wecht, J.M.; Harel, N.Y.; Guest, J.; Kirshblum, S.C.; Forrest, G.F.; Bloom, O.; Ovechkin, A.V.; Harkema, S. Cardiovascular autonomic dysfunction in spinal cord injury: Epidemiology, diagnosis, and management. Semin. Neurol. 2020, 40, 550–559. [Google Scholar] [CrossRef]
- Eschlböck, S.; Wenning, G.; Fanciulli, A. Evidence-based treatment of neurogenic orthostatic hypotension and related symptoms. J. Neural Transm. 2017, 124, 1567–1605. [Google Scholar] [CrossRef] [PubMed]
- Mathias, C.J. Orthostatic hypotension and paroxysmal hypertension in humans with high spinal cord injury. Prog. Brain Res. 2006, 152, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Krassioukov, A.; Linsenmeyer, T.A.; Beck, L.A.; Elliott, S.; Gorman, P.; Kirshblum, S.; Vogel, L.; Wecht, J.; Clay, S. Evaluation and management of autonomic dysreflexia and other autonomic dysfunctions: Preventing the highs and lows: Management of blood pressure, sweating, and temperature dysfunction. Top. Spinal Cord Inj. Rehabil. 2021, 27, 225–290. [Google Scholar] [CrossRef]
- Eldahan, K.C.; Rabchevsky, A.G. Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management. Auton. Neurosci. 2018, 209, 59–70. [Google Scholar] [CrossRef]
- Tweedy, S.M.; Beckman, E.M.; Geraghty, T.J.; Theisen, D.; Perret, C.; A Harvey, L.; Vanlandewijck, Y.C. Exercise and sports science Australia (ESSA) position statement on exercise and spinal cord injury. J. Sci. Med. Sport 2017, 20, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.S.; Bilzon, J.L.J. Guideline Approaches for Cardioendocrine Disease Surveillance and Treatment Following Spinal Cord Injury. Curr. Phys. Med. Rehabil. Rep. 2018, 6, 264–276. [Google Scholar] [CrossRef]
- Cowan, R.E.; Nash, M.S.; Anderson-Erisman, K. Perceived exercise barriers and odds of exercise participation among persons with SCI living in high-income households. Top. Spinal Cord Inj. Rehabil. 2012, 18, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xu, H.; Zuo, Y.; Liu, X.; All, A.H. A Review of Functional Electrical Stimulation Treatment in Spinal Cord Injury. Neuromolecular Med. 2020, 22, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Sadowsky, C.L.; Hammond, E.R.; Strohl, A.B.; Commean, P.K.; Eby, S.A.; Damiano, D.L.; Wingert, J.R.; Bae, K.T.; McDonald, J.W. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J. Spinal Cord Med. 2013, 36, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Karamian, B.A.; Siegel, N.; Nourie, B.; Serruya, M.D.; Heary, R.F.; Harrop, J.S.; Vaccaro, A.R. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J. Orthop. Traumatol. 2022, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Hayek, R. Role of electrical stimulation for rehabilitation and regeneration after spinal cord injury: An overview. Eur. Spine J. 2008, 17, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Bochkezanian, V.; Newton, R.U.; Trajano, G.S.; Blazevich, A.J. Effects of Neuromuscular Electrical Stimulation in People with Spinal Cord Injury. Med. Sci. Sports Exerc. 2018, 50, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Dudley-Javoroski, S.; Shields, R.K. Muscle and bone plasticity after spinal cord injury: Review of adaptations to disuse and to electrical muscle stimulation. J. Rehabil. Res. Dev. 2008, 45, 283–296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahoney, E.T.; Bickel, C.S.; Elder, C.; Black, C.; Slade, J.M.; Apple, D., Jr.; Dudley, G.A. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2005, 86, 1502–1504. [Google Scholar] [CrossRef]
- de Freitas, G.R.; Szpoganicz, C.; Ilha, J. Does Neuromuscular Electrical Stimulation Therapy Increase Voluntary Muscle Strength After Spinal Cord Injury? A Systematic Review. Top. Spinal Cord Inj. Rehabil. 2018, 24, 6–17. [Google Scholar] [CrossRef]
- Atkins, K.D.; Bickel, C.S. Effects of functional electrical stimulation on muscle health after spinal cord injury. Curr. Opin. Pharmacol. 2021, 60, 226–231. [Google Scholar] [CrossRef]
- Bickel, C.S.; Yarar-Fisher, C.; Mahoney, E.T.; McCully, K.K. Neuromuscular Electrical Stimulation-Induced Resistance Training After SCI: A Review of the Dudley Protocol. Top. Spinal Cord Inj. Rehabil. 2015, 21, 294–302. [Google Scholar] [CrossRef][Green Version]
- Ho, C.H.; Triolo, R.J.; Elias, A.L.; Kilgore, K.L.; DiMarco, A.F.; Bogie, K.; Vette, A.H.; Audu, M.L.; Kobetic, R.; Chang, S.R.; et al. Functional electrical stimulation and spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 631–654. [Google Scholar] [CrossRef]
- Lu, X.; Battistuzzo, C.R.; Zoghi, M.; Galea, M.P. Effects of training on upper limb function after cervical spinal cord injury: A systematic review. Clin. Rehabil. 2015, 29, 3–13. [Google Scholar] [CrossRef]
- van der Scheer, J.W.; Goosey-Tolfrey, V.L.; Valentino, S.E.; Davis, G.M.; Ho, C.H. Functional electrical stimulation cycling exercise after spinal cord injury: A systematic review of health and fitness-related outcomes. J. NeuroEng. Rehabil. 2021, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Street, T.; Davis, G.M. New Clinical Practice Guidelines for Functional Electrical Stimulation (FES) Cycling and Walking. Forward 2022, 166, 30–31. [Google Scholar]
- Farkas, G.J.; Gorgey, A.S.; Dolbow, D.R.; Berg, A.S.; Gater, D.R., Jr. Energy expenditure, cardiorespiratory fitness, and body composition following arm cycling or functional electrical stimulation exercises in spinal cord injury: A 16-week randomized controlled trial. Top. Spinal Cord Inj. Rehabil. 2021, 27, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Hasnan, N.; Ektas, N.; Tanhoffer, A.I.; Tanhoffer, R.; Fornusek, C.; Middleton, J.W.; Husain, R.; Davis, G.M. Exercise responses during functional electrical stimulation cycling in individuals with spinal cord injury. Med. Sci. Sports Exerc. 2013, 45, 1131–1138. [Google Scholar] [CrossRef]
- Davis, G.M. RehabWeek, Presentation. Discipline of Exercise and Sport Sciences, Sydney School of Health Sciences, Faculty of Medicine and Health, The University of Sydney, Camperdown, NSW 2006, Australia. 2023; Unpublished. [Google Scholar]
- Johnston, T.E.; Smith, B.T.; Mulcahey, M.J.; Betz, R.R.; Lauer, R.T. A randomized controlled trial on the effects of cycling with and without electrical stimulation on cardiorespiratory and vascular health in children with spinal cord injury. Arch. Phys. Med. Rehabil. 2009, 90, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Brurok, B.; Helgerud, J.; Karlsen, T.; Leivseth, G.; Hoff, J. Effect of aerobic high-intensity hybrid training on stroke volume and peak oxygen consumption in men with spinal cord injury. Am. J. Phys. Med. Rehabil. 2011, 90, 407–414. [Google Scholar] [CrossRef]
- Hasnan, N.; Engkasan, J.P.; Husain, R.; Davis, G.M. High-Intensity Virtual-reality Arm plus FES-leg Interval Training in Individuals with Spinal Cord Injury. Biomed. Tech. 2013, 58 (Suppl. S1), 000010151520134028. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Carter, W.; Ballance, B.; Gill, R.; Khan, R.; Goetz, L.; Lavis, T.; Sima, A.P.; Adler, R.A. Effects of two different paradigms of electrical stimulation exercise on cardio-metabolic risk factors after spinal cord injury. A randomized clinical trial. Front. Neurol. 2023, 14, 1254760. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Mather, K.J.; Cupp, H.R.; Gater, D.R. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med. Sci. Sports Exerc. 2012, 44, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.J.; Mossayebi, A.; Sigaroodi, S.; Apaflo, J.N.; Galvan, M.J.; Min, K.; Agullo, F.J.; Wagler, A.; Bajpeyi, S. Effects of neuromuscular electrical stimulation on glycemic control: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1222532. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.; Decker, M.J.; Hwang, J.Y.; Wang, B.; Kitchen, K.; Ding, Z.; Ivy, J. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J. Electromyogr. Kinesiol. 2009, 19, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Rosley, N.; Hasnan, N.; Hamzaid, N.A.; Davis, G.M.; Manaf, H. Effects of a combined progressive resistance training and functional electrical stimulation-evoked cycling exercise on lower limb muscle strength of individuals with incomplete spinal cord injury: A randomized controlled study. Turk. J. Phys. Med. Rehabil. 2022, 69, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dolbow, D.R.; Credeur, D.P.; Lemacks, J.L.; Stokic, D.S.; Pattanaik, S.; Corbin, G.N.; Courtner, A.S. Electrically induced cycling and nutritional counseling for counteracting obesity after spinal cord injury: A pilot study. J. Spinal Cord Med. 2021, 44, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.E.; Marino, R.J.; Oleson, C.V.; Schmidt-Read, M.; Benjamin, E.; Leiby, B.E.; Sendecki, J.; Modlesky, C.M. Musculoskeletal effects of 2 functional electrical stimulation cycling paradigms conducted at different cadences for people with spinal cord injury: A pilot study. Arch. Phys. Med. Rehabil. 2016, 97, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Khalil, R.E.; Gill, R.; Gater, D.R.; Lavis, T.D.; Cardozo, C.P.; Adler, R.A. Low-dose testosterone and evoked resistance exercise after spinal cord injury on cardio-metabolic risk factors: An open-label randomized clinical trial. J. Neurotrauma 2019, 36, 2631–2645. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Graham, Z.A.; Chen, Q.; Rivers, J.F.; Adler, R.A.; Lesnefsky, E.J.; Cardozo, C.P. Sixteen weeks oftestosterone with or without evoked resistance training on protein expression, fiber hypertrophy and mitochondrial health after spinal cord injury. J. Appl. Physiol. 2020, 128, 1487–1496. [Google Scholar] [CrossRef]
- Lai, R.E.; Gorgey, A.S. Low-dose testosterone replacement therapy and electrically evoked resistance training enhance muscle quality after spinal cord injury. Neural Regen. Res. 2021, 16, 1544–1545. [Google Scholar] [CrossRef]
- Bekhet, A.H.; Jahan, A.M.; Bochkezanian, V.; Musselman, K.E.; Elsareih, A.A.; Gorgey, A.S. Effects of Electrical Stimulation Training on Body Composition Parameters After Spinal Cord Injury: A Systematic Review. Arch. Phys. Med. Rehabil. 2022, 103, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Sutor, T.W.; Kura, J.; Mattingly, A.J.; Otzel, D.M.; Yarrow, J.F. The Effects of Exercise and Activity-Based Physical Therapy on Bone after Spinal Cord Injury. Int. J. Mol. Sci. 2022, 23, 608. [Google Scholar] [CrossRef]
- Holman, M.E.; Chang, G.; Ghatas, M.P.; Saha, P.K.; Zhang, X.; Khan, M.R.; Sima, A.P.; Adler, R.A.; Gorgey, A.S. Bone and non-contractile soft tissue changes following open kinetic chain resistance training and testosterone treatment in spinal cord injury: An exploratory study. Osteoporos. Int. 2021, 32, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Frotzler, A.; Coupaud, S.; Perret, C.; Kakebeeke, T.H.; Hunt, K.J.; Donaldson, N.d.N.; Eser, P. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone 2008, 43, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Dudley-Javoroski, S.; Petrie, M.; Suneja, M.; Littmann, A.E.; Iguchi, M.; DiMarco, A.F.; Kowalski, K.E.; Harris, R.L.W.; Putman, C.T.; et al. Musculoskeletal plasticity after acute spinal cord injury: Effects of long-term neuromuscular electrical stimulation training. J. Neurophysiol. 2006, 95, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Khalil, R.E.; Sutor, T.W.; Goldsmith, J.A.; Cifu, D.X. Employment of Neuromuscular Electrical Stimulation to Examine Muscle and Bone Qualities after Spinal Cord Injury. J. Clin. Med. 2022, 11, 6681. [Google Scholar] [CrossRef] [PubMed]
- Bersch, I.; Koch-Borner, S.; Fridén, J. Electrical stimulation-a mapping system for hand dysfunction in tetraplegia. Spinal Cord 2018, 56, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Bersch, I.; Koch-Borner, S.; Fridén, J. Motor Point Topography of Fundamental Grip Actuators in Tetraplegia: Implications in Nerve Transfer Surgery. J. Neurotrauma 2020, 37, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Lee, J.; Shin, H.I. The natural course of passive tenodesis grip in individuals with spinal cord injury with preserved wrist extension power but paralyzed fingers and thumbs. Spinal Cord 2018, 56, 900–906. [Google Scholar] [CrossRef]
- Johanson, M.E.; Murray, W.M. The unoperated hand: The role of passive forces in hand function after tetraplegia. Hand Clin. 2002, 18, 391–398. [Google Scholar] [CrossRef]
- Bersch, I.; Krebs, J.; Fridén, J. A Prediction Model for Various Treatment Pathways of Upper Extremity in Tetraplegia. Front. Rehabil. Sci. 2022, 3, 889577. [Google Scholar] [CrossRef] [PubMed]
- Koch-Borner, S.; Bersch, U.; Grether, S.; Fridén, J.; Schibli, S.; Bersch, I. Different thumb positions in the tetraplegic hand. Arch. Phys. Med. Rehabil. 2023, 105, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bersch, I.; Fridén, J. Electrical stimulation alters muscle morphological properties in denervated upper limb muscles. Ebiomedicine 2021, 74, 103737. [Google Scholar] [CrossRef]
- Zijdewind, I.; Thomas, C.K. Motor unit firing during and after voluntary contractions of human thenar muscles weakened by spinal cord injury. J. Neurophysiol. 2003, 89, 2065–2071. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolbow, D.R.; Bersch, I.; Gorgey, A.S.; Davis, G.M. The Clinical Management of Electrical Stimulation Therapies in the Rehabilitation of Individuals with Spinal Cord Injuries. J. Clin. Med. 2024, 13, 2995. https://doi.org/10.3390/jcm13102995
Dolbow DR, Bersch I, Gorgey AS, Davis GM. The Clinical Management of Electrical Stimulation Therapies in the Rehabilitation of Individuals with Spinal Cord Injuries. Journal of Clinical Medicine. 2024; 13(10):2995. https://doi.org/10.3390/jcm13102995
Chicago/Turabian StyleDolbow, David R., Ines Bersch, Ashraf S. Gorgey, and Glen M. Davis. 2024. "The Clinical Management of Electrical Stimulation Therapies in the Rehabilitation of Individuals with Spinal Cord Injuries" Journal of Clinical Medicine 13, no. 10: 2995. https://doi.org/10.3390/jcm13102995
APA StyleDolbow, D. R., Bersch, I., Gorgey, A. S., & Davis, G. M. (2024). The Clinical Management of Electrical Stimulation Therapies in the Rehabilitation of Individuals with Spinal Cord Injuries. Journal of Clinical Medicine, 13(10), 2995. https://doi.org/10.3390/jcm13102995








