Sarcopenia Prevalence among Hospitalized Patients with Severe Obesity: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Anthropometric Parameters
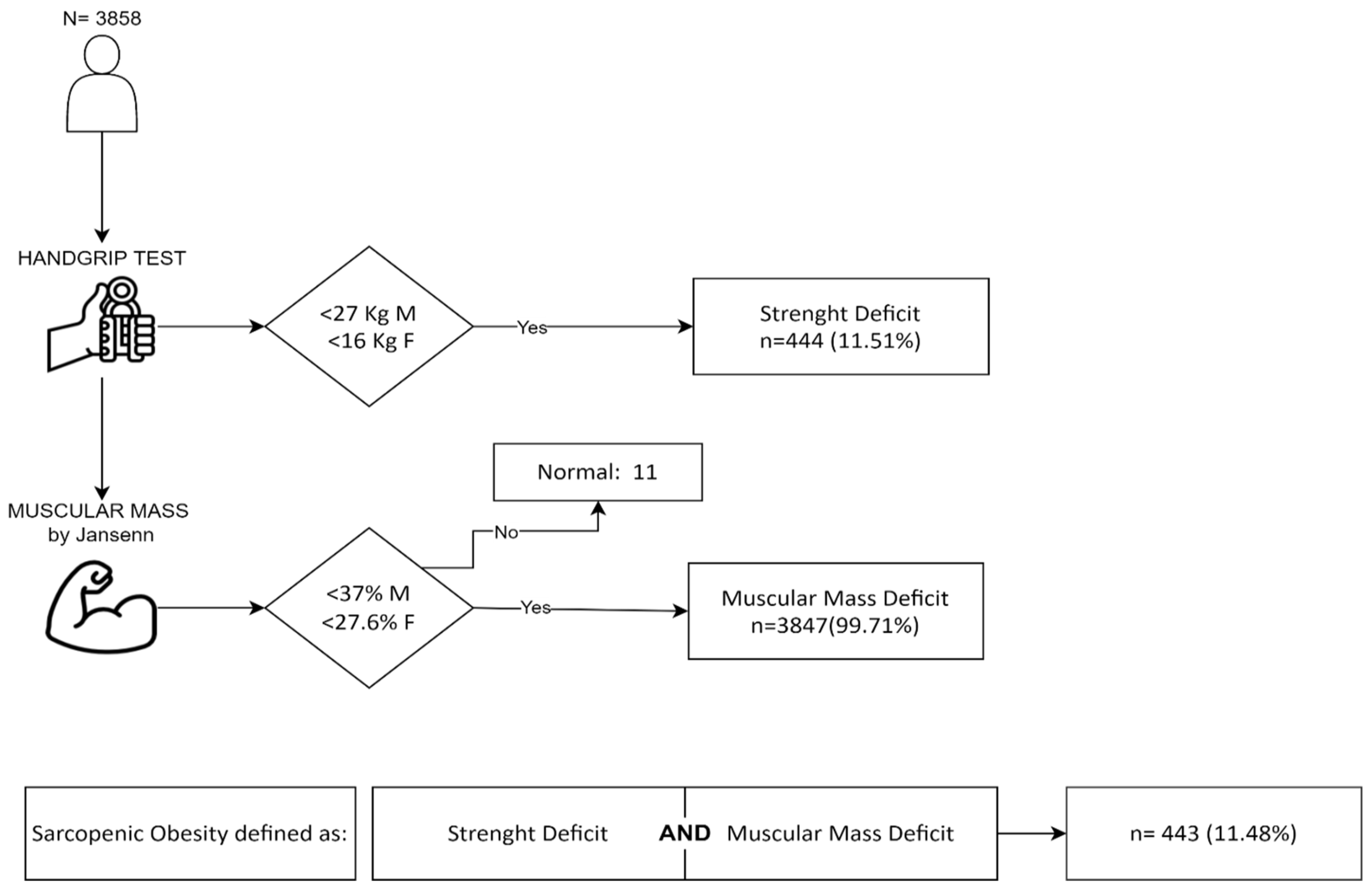
2.4. Body Composition
2.5. Skeletal Muscle Mass Index
2.6. Muscle Strength
2.7. Psychological General Well-Being Index (PGWBI) Questionnaire
2.8. Criteria for SO Definition
2.9. Staging of SO
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zhu, Y.; Teng, Y.; Long, Q.; Hao, Q.; Yu, X.; Yang, L.; Lv, Y.; Liu, J.; Lu, S. The analysis of osteosarcopenia as a risk factor for fractures, mortality, and falls. Osteoporos. Int. 2021, 32, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, C.L.; Dantas, W.S.; Kirwan, J.P. Sarcopenic obesity: Emerging mechanisms and therapeutic potential. Metabolism 2023, 146, 155639. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Chong, M.S.; Tay, L.; Yang, Y.X.; Leung, B.P.; Yeo, A.; Yew, S.; Tan, C.H.; Lim, W.S. Inter-muscular adipose tissue is associated with adipose tissue inflammation and poorer functional performance in central adiposity. Arch. Gerontol. Geriatr. 2019, 81, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Di Martino, M.; Catalano, C.; Lenzi, A.; Donini, L.M. The decline in muscle strength and muscle quality in relation to metabolic derangements in adult women with obesity. Clin. Nutr. 2019, 38, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Atkins, J.L. Muscle loss and obesity: The health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015, 74, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Hanatani, S.; Izumiya, Y.; Yamamoto, M.; Araki, S.; Fujisue, K.; Arima, Y.; Takashio, S.; Yamamoto, E.; Kaikita, K.; Matsushita, K.; et al. A simple method of sarcopenia detection can predict adverse cardiovascular events in patients with abdominal obesity. Int. J. Obes. 2021, 45, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bauer, J.M.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cruz-Jentoft, A.J.; Dicker, D.; Frühbeck, G.; Giustina, A.; et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin. Nutr. 2020, 39, 2368–2388. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin. Nutr. 2022, 41, 990–1000. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 331–335. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Haidar, S.G.; Kumar, D.; Bassi, R.S.; Deshmukh, S.C. Average versus maximum grip strength: Which is more consistent? J. Hand Surg. Br. 2004, 29, 82–84. [Google Scholar] [CrossRef]
- Abe, T.; Loenneke, J.P. Handgrip strength dominance is associated with difference in forearm muscle size. J. Phys. Ther. Sci. 2015, 27, 2147–2149. [Google Scholar] [CrossRef]
- Wenger, N.K.; Mattson, M.E.; Furberg, C.D.; Elinson, J. Assessment of quality of life in clinical trials of cardiovascular therapies. Am. J. Cardiol. 1984, 54, 908–913. [Google Scholar] [CrossRef]
- Grossi, E.; Compare, A. Psychological general well-being index (PGWB). In Quality of Life Encyclopedia; Michalos, A., Ed.; Springer: Dordrecht, The Netherland, 2012; pp. 5152–5156. [Google Scholar]
- Clopper, C.J.; Pearson, E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
- Shimokata, H.; Shimada, H.; Satake, S.; Endo, N.; Shibasaki, K.; Ogawa, S.; Arai, H. Chapter 2 Epidemiology of sarcopenia. Geriatr. Gerontol. Int. 2018, 18 (Suppl. S1), 13–22. [Google Scholar] [CrossRef]
- Baumgartner, R.N. Body composition in healthy aging. Ann. N. Y. Acad. Sci. 2000, 904, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Blyth, F.; Naganathan, V.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Hirani, V. Sarcopenia prevalence and functional outcomes in older men with obesity: Comparing the use of the EWGSOP2 sarcopenia versus ESPEN-EASO sarcopenic obesity consensus definitions. Clin. Nutr. 2023, 42, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Schluessel, S.; Huemer, M.T.; Peters, A.; Drey, M.; Thorand, B. Sarcopenic obesity using the ESPEN and EASO consensus statement criteria of 2022—Results from the German KORA-Age study. Obes. Res. Clin. Pract. 2023, 17, 349–352. [Google Scholar] [CrossRef]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Tallis, J.; Shelley, S.; Degens, H.; Hill, C. Age-Related Skeletal Muscle Dysfunction Is Aggravated by Obesity: An Investigation of Contractile Function, Implications and Treatment. Biomolecules 2021, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Wannamathee, S.G. Sarcopenic obesity in ageing: Cardiovascular outcomes and mortality. Br. J. Nutr. 2020, 124, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Chiles Shaffer, N.; Gonzalez-Freire, M.; Shardell, M.D.; Zoli, M.; Studenski, S.A.; Ferrucci, L. Early body composition, but not body mass, is associated with future accelerated decline in muscle quality. J. Cachexia Sarcopenia Muscle 2017, 8, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Correa-de-Araujo, R.; Harris-Love, M.O.; Miljkovic, I.; Fragala, M.S.; Anthony, B.W.; Manini, T.M. The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report. Front. Physiol. 2017, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; López-Soriano, J.; Almendro, V.; Busquets, S.; López-Soriano, F.J. Cross-talk between skeletal muscle and adipose tissue: A link with obesity? Med. Res. Rev. 2005, 25, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef]
- Wells, J.C. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Mendes, I.; Ong, B.; Prado, C.M.; Mocciaro, G.; Mazidi, M.; Lubrano, C.; Lenzi, A.; Donini, L.M.; Siervo, M. Sarcopenic obesity and insulin resistance: Application of novel body composition models. Nutrition 2020, 75–76, 110765. [Google Scholar] [CrossRef]
- Hadjispyrou, S.; Giannopoulos, A.; Philippou, A.; Theos, A. Mitochondrial Dysfunction and Sarcopenic Obesity: The Role of Exercise. J. Clin. Med. 2023, 12, 5628. [Google Scholar] [CrossRef]
- Sack, C.; Ferrari, N.; Friesen, D.; Haas, F.; Klaudius, M.; Schmidt, L.; Torbahn, G.; Wulff, H.; Joisten, C. Health Risks of Sarcopenic Obesity in Overweight Children and Adolescents: Data from the CHILT III Programme (Cologne). J. Clin. Med. 2022, 11, 277. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Wang, Y.; Li, H. Association between sarcopenia and osteoarthritis: A protocol for meta-analysis. PLoS ONE 2022, 17, e0272284. [Google Scholar] [CrossRef]
- Roh, E.; Choi, K.M. Health Consequences of Sarcopenic Obesity: A Narrative Review. Front. Endocrinol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Itani, L.; Kreidieh, D.; El Masri, D.; Tannir, H.; El Ghoch, M. The Impact of Sarcopenic Obesity on Health-Related Quality of Life of Treatment-Seeking Patients with Obesity. Curr. Diabetes Rev. 2020, 16, 635–640. [Google Scholar] [CrossRef]
- Kreidieh, D.; Itani, L.; El Masri, D.; Tannir, H.; El Ghoch, M. Association Between Reduced Daily Steps and Sarcopenic Obesity in Treatment-Seeking Adults with Obesity. Front. Endocrinol. 2020, 6, 23. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Nagano, F.; Matsumoto, A.; Shimazu, S.; Shiraishi, A.; Kido, Y.; Bise, T. The Applicability of the ESPEN and EASO-Defined Diagnostic Criteria for Sarcopenic Obesity in Japanese Patients after Stroke: Prevalence and Association with Outcomes. Nutrients 2022, 14, 4205. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Maeda, K.; Ueshima, J.; Inoue, T.; Murotani, K.; Ohno, T.; Nomoto, A.; Nagano, A.; Kayashita, J.; Mori, N.; et al. Prevalence of sarcopenic obesity based on newly proposed diagnostic criteria and functional outcomes in older adults undergoing rehabilitation. Mech. Ageing Dev. Actions 2022, 208, 111728. [Google Scholar] [CrossRef]
- Vieira, F.T.; Godziuk, K.; Lamarca, F.; Melendez-Araújo, M.S.; Lima, R.M.; Prado, C.M.; Baiocchi de Carvalho, K.M.; Dutra, E.S. Sarcopenic obesity diagnosis by different criteria mid-to long-term post-bariatric surgery. Clin. Nutr. 2022, 41, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Gortan Cappellari, G.; Semolic, A.; Zanetti, M.; Vinci, P.; Ius, M.; Guarnieri, G.; Busetto, L.; Donini, L.M.; Barazzoni, R. Sarcopenic obesity in free-living older adults detected by the ESPEN-EASO consensus diagnostic algorithm: Validation in an Italian cohort and predictive value of insulin resistance and altered plasma ghrelin profile. Metabolism 2023, 145, 155595. [Google Scholar] [CrossRef] [PubMed]

| n (%) | |
|---|---|
| DEMOGRAPHIC—ANTHROPOMETRIC | |
| SEX | |
| Women | 2348 (61%) |
| Men | 1510 (39%) |
| AGE class (Years) | |
| 18–50 | 1142 (30%) |
| 51–70 | 2090 (54%) |
| >70 | 626 (16%) |
| BMI class (kg/m2) | |
| 30–40 | 1595 (41%) |
| >40 | 2263 (59%) |
| COMORBIDITIES | |
| Endocrine disease | |
| T2DM | 1101 (29%) |
| Hypothyroidism | 126 (3%) |
| Pituitary gland disfunction | 5 (1%) |
| Adrenal diseases | 0 (0%) |
| Gonadal diseases | 16 (1%) |
| Dyslipidemia | 219 (6%) |
| Vitamin D deficit | 230 (6%) |
| Chronic heart diseases | |
| Chronic heart failure | 1287 (33%) |
| Varices and lymphedema | 55 (1%) |
| Lower limb ulcers | 0 (0%) |
| Chronic respiratory diseases | |
| OSAS | 1284 (33%) |
| COPD | 138 (4%) |
| Chronic neurologic and neurodegenerative diseases | |
| Peripheral neuropathy | 53 (1%) |
| Psychiatric disease | |
| Depression and psychiatric disease | 556 (14%) |
| Chronic liver disease | |
| Hepatic steatosis | 242 (6%) |
| Osteoarthritis | |
| Osteoarticular degenerative disease | 1573 (41%) |
| Chronic kidney diseases | |
| Kidney failure and stones | 93 (2%) |
| BPH | 0 (0%) |
| Chronic bowel diseases | |
| GERD | 5 (1%) |
| Gastroduodenal ulcers | 1 (0%) |
| Ventral hernia | 7 (1%) |
| Intestinal diverticula | 3 (0%) |
| PGWBI class * | |
| Good | 1258 (40.11%) |
| Normal | 640 (20.41%) |
| Poor | 1238 (39.48%) |
| n | SO | Prevalence (95% CI) | p-Value | |
|---|---|---|---|---|
| OVERALL | ||||
| 3858 | 443 | 11.48% (10.49–12.53%) | ||
| SEX | <0.0001 ӻ | |||
| Women | 2348 | 291 | 12.39% (11.09–13.79%) | |
| Men | 1510 | 152 | 10.07% (8.19–11.21%) | |
| AGE (Years) | <0.0001 ԏ | |||
| 18–50 | 1142 | 53 | 4.64% (3.50–6.03%) | |
| 51–70 | 2090 | 221 | 10.57% (9.29–11.97%) | |
| 70+ | 626 | 169 | 27.00% (23.55–30.66%) | |
| BMI (kg/m2) | 0.1710 ӻ | |||
| 30–40 | 1595 | 211 | 13.23% (11.60–14.99%) | |
| >40 | 2263 | 232 | 10.25% (9.03–11.58%) | |
| PGWBI * | <0.0001 ԏ | |||
| Good | 1258 | 79 | 6.28% (5.00–7.77%) | |
| Normal | 640 | 56 | 8.75% (6.68–11.21%) | |
| Poor | 1238 | 156 | 12.60% (10.80–14.58%) | |
| Sex | BMI (kg/m2) | Age (Years) | No. Total Patients/ Sarcopenic Patients | Prevalence (95% CI) | p-Value (Trend Test) | |
|---|---|---|---|---|---|---|
| WOMEN | 30–40 | 18–50 | 197/11 | 5.58% (2.82–9.77%) | <0.0001 | |
| 51–70 | 502/61 | 12.15% (9.42–15.33%) | ||||
| 70+ | 213/59 | 27.70% (21.80–34.23%) | ||||
| >40 | 18–50 | 431/19 | 4.41% (2.67–6.80%) | <0.0001 | ||
| 51–70 | 782/81 | 10.36% (8.31–12.71%) | ||||
| 70+ | 223/60 | 26.91% (21.20–33.23%) | ||||
| MEN | 30–40 | 18–50 | 157/9 | 5.73% (2.65–10.60%) | <0.0001 | |
| 51–70 | 405/36 | 8.89% (6.30–12.09%) | ||||
| 70+ | 121/35 | 28.93% (21.05–37.87%) | ||||
| >40 | 18–50 | 357/14 | 3.92% (2.16–6.49%) | <0.0001 | ||
| 51–70 | 401/43 | 10.72% (7.87–14.17%) | ||||
| 70+ | 69/15 | 21.74% (12.71–33.31%) | ||||
| n (%) | |
|---|---|
| Endocrine disease | |
| T2DM | 145 (34%) |
| Hypothyroidism | 11 (3%) |
| Pituitary gland disfunction | 2 (1%) |
| Adrenal diseases | 0 (0%) |
| Gonadal diseases | 0 (0%) |
| Dyslipidemia | 16 (4%) |
| Vitamin D deficit | 17 (4%) |
| Chronic heart diseases | |
| Chronic heart failure | 159 (38%) |
| Varices and lymphedema | 4 (1%) |
| Lower limb ulcers | 0 (0%) |
| Chronic respiratory diseases | |
| OSAS | 137 (32%) |
| COPD | 22 (5%) |
| Chronic neurologic and neurodegenerative diseases | |
| Peripheral neuropathy | 12 (3%) |
| Psychiatric disease | |
| Depression and psychiatric Disease | 58 (14%) |
| Chronic liver disease | |
| Hepatic steatosis | 20 (5%) |
| Osteoarthritis | |
| Osteoarticular degenerative disease | 241 (57%) |
| Chronic kidney diseases | |
| Kidney failure and stones | 16 (4%) |
| BPH | 0 (0%) |
| Chronic bowel diseases | |
| GERD | 0 (0%) |
| Gastroduodenal ulcers | 0 (0%) |
| Ventral hernia | 1 (1%) |
| Intestinal diverticula | 1 (1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cancello, R.; Brenna, E.; Soranna, D.; Zambon, A.; Villa, V.; Castelnuovo, G.; Donini, L.M.; Busetto, L.; Capodaglio, P.; Brunani, A. Sarcopenia Prevalence among Hospitalized Patients with Severe Obesity: An Observational Study. J. Clin. Med. 2024, 13, 2880. https://doi.org/10.3390/jcm13102880
Cancello R, Brenna E, Soranna D, Zambon A, Villa V, Castelnuovo G, Donini LM, Busetto L, Capodaglio P, Brunani A. Sarcopenia Prevalence among Hospitalized Patients with Severe Obesity: An Observational Study. Journal of Clinical Medicine. 2024; 13(10):2880. https://doi.org/10.3390/jcm13102880
Chicago/Turabian StyleCancello, Raffaella, Ettore Brenna, Davide Soranna, Antonella Zambon, Valentina Villa, Gianluca Castelnuovo, Lorenzo Maria Donini, Luca Busetto, Paolo Capodaglio, and Amelia Brunani. 2024. "Sarcopenia Prevalence among Hospitalized Patients with Severe Obesity: An Observational Study" Journal of Clinical Medicine 13, no. 10: 2880. https://doi.org/10.3390/jcm13102880
APA StyleCancello, R., Brenna, E., Soranna, D., Zambon, A., Villa, V., Castelnuovo, G., Donini, L. M., Busetto, L., Capodaglio, P., & Brunani, A. (2024). Sarcopenia Prevalence among Hospitalized Patients with Severe Obesity: An Observational Study. Journal of Clinical Medicine, 13(10), 2880. https://doi.org/10.3390/jcm13102880









