Reassessing Normal Voiding Standards: A Cross-Sectional Study Based on Medical Professionals’ Evaluations with Portable Uroflowmetry and IPSS
Abstract
1. Introduction
2. Materials and Methods
2.1. Type of Study, Setting, and Participants
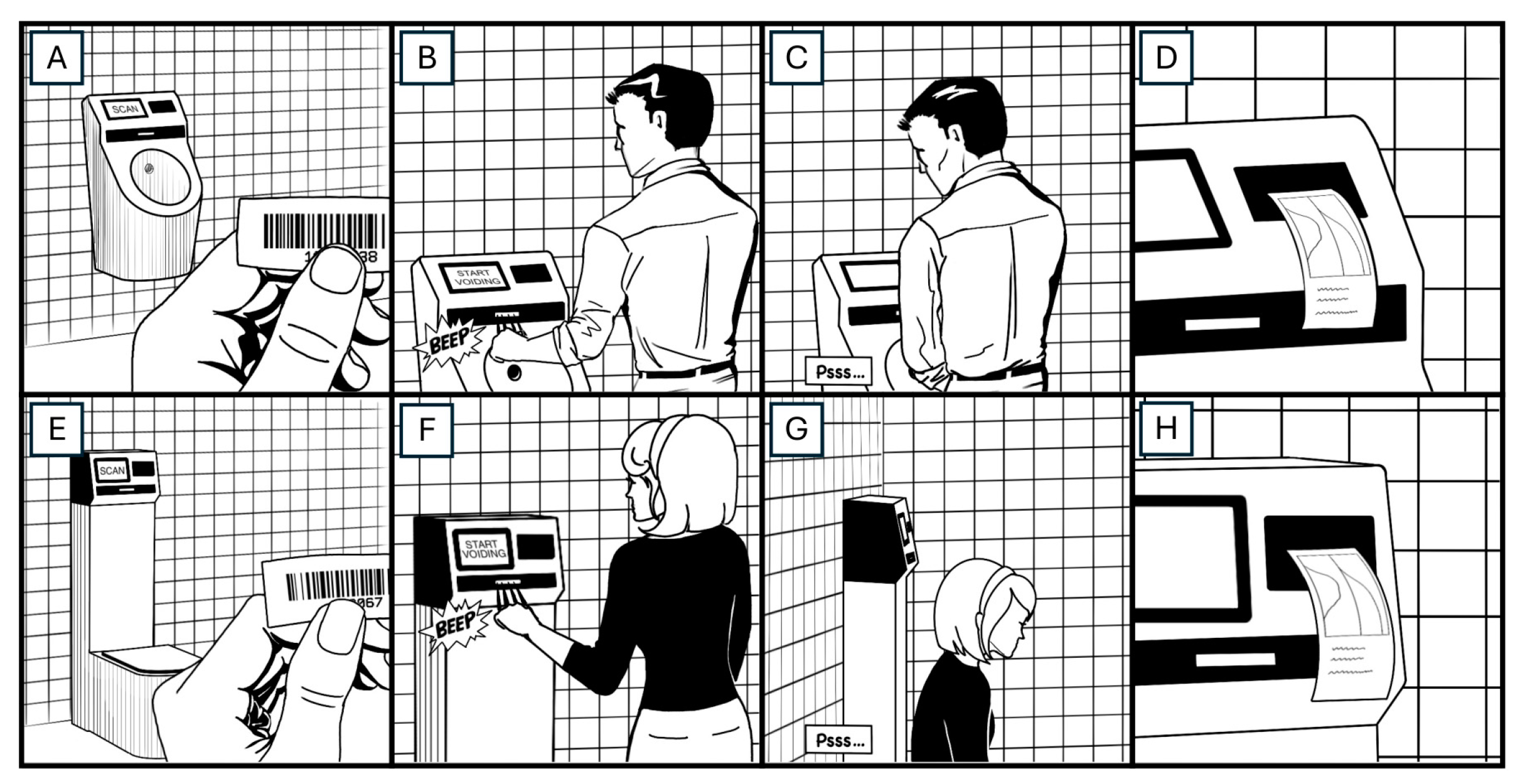
2.2. Data Collection
2.3. Data Analysis
3. Results
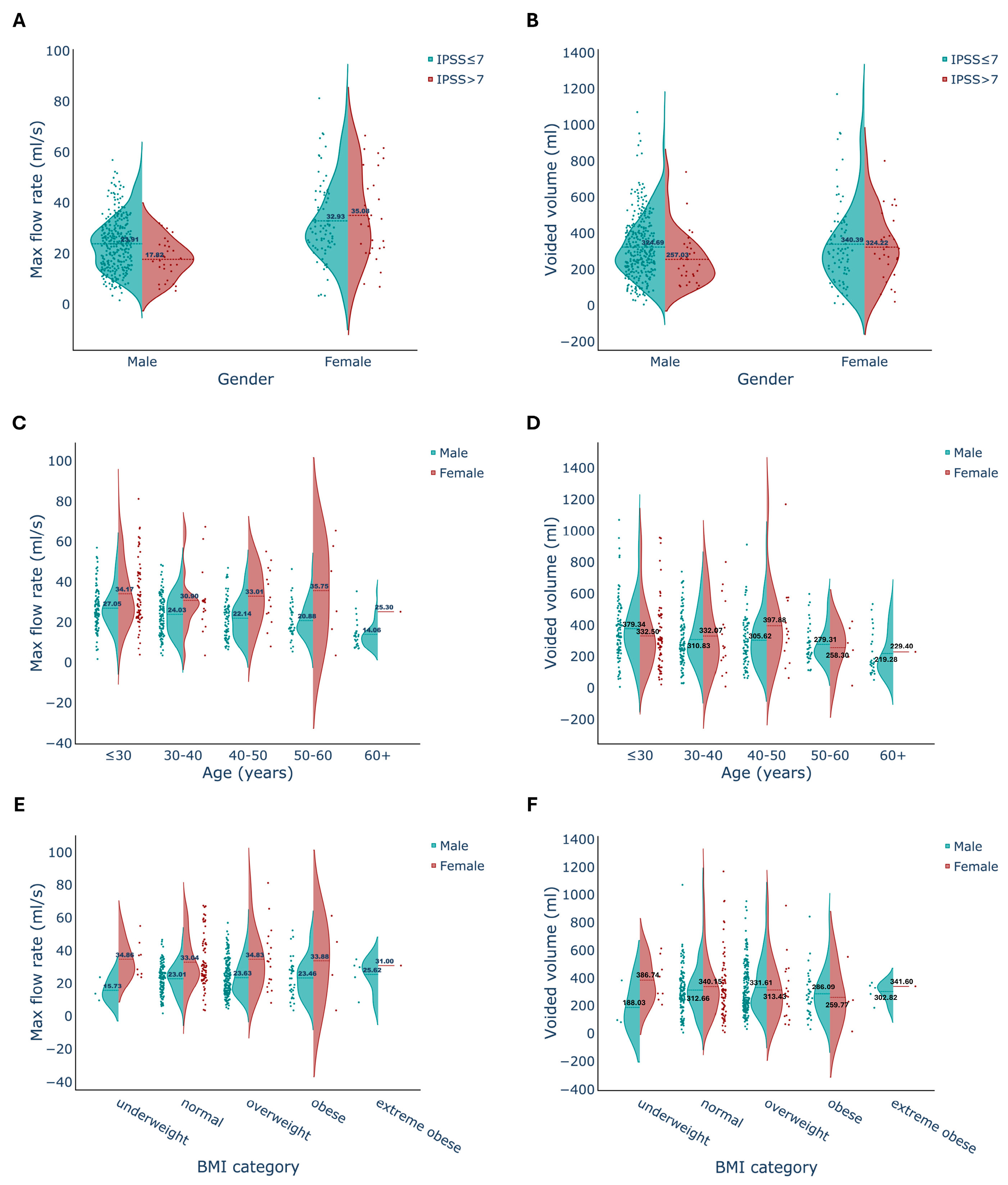
3.1. Voided Volume
3.2. Maximum Flow Rate
3.3. IPSS
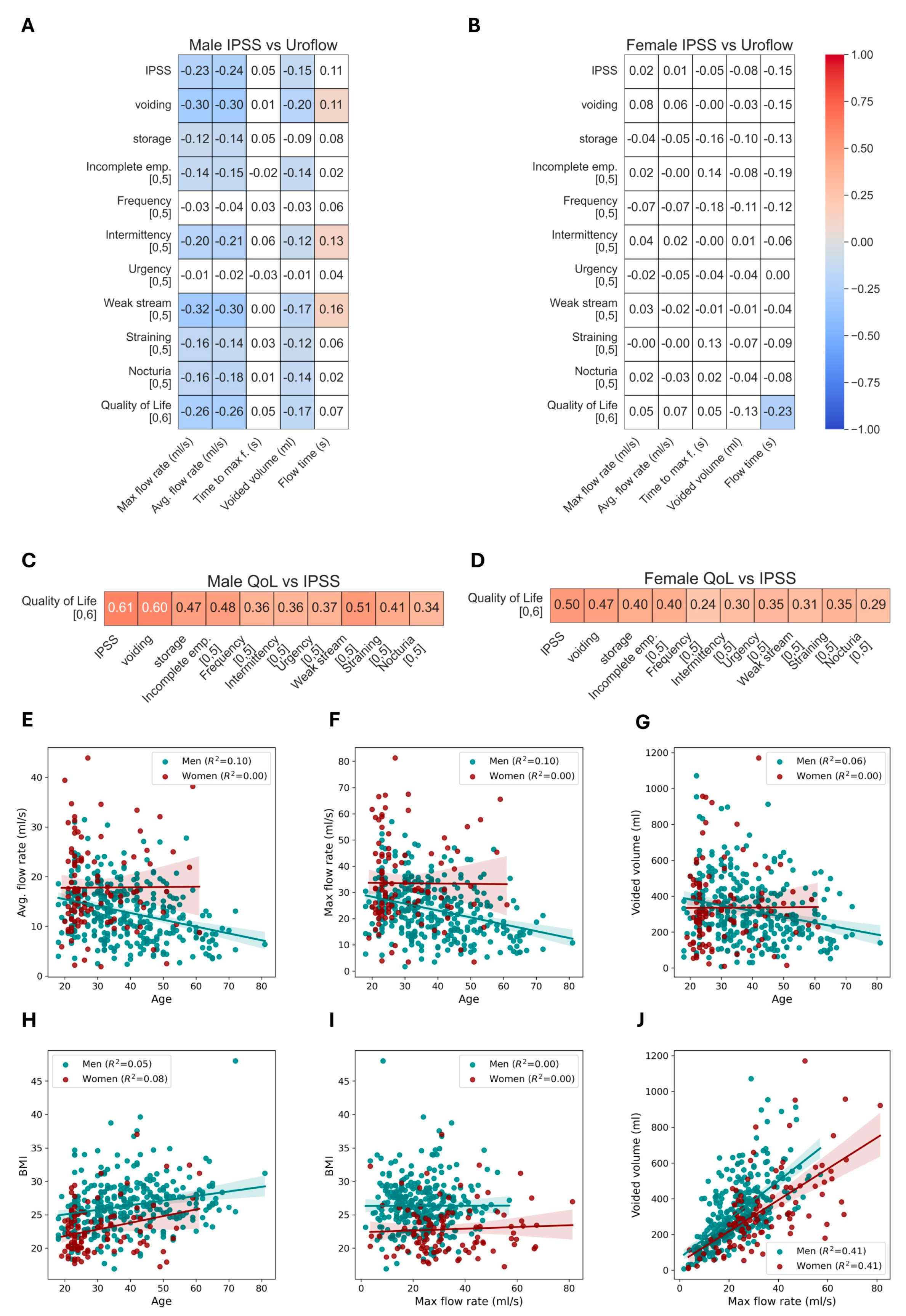
3.4. Correlation of Uroflow Parameters
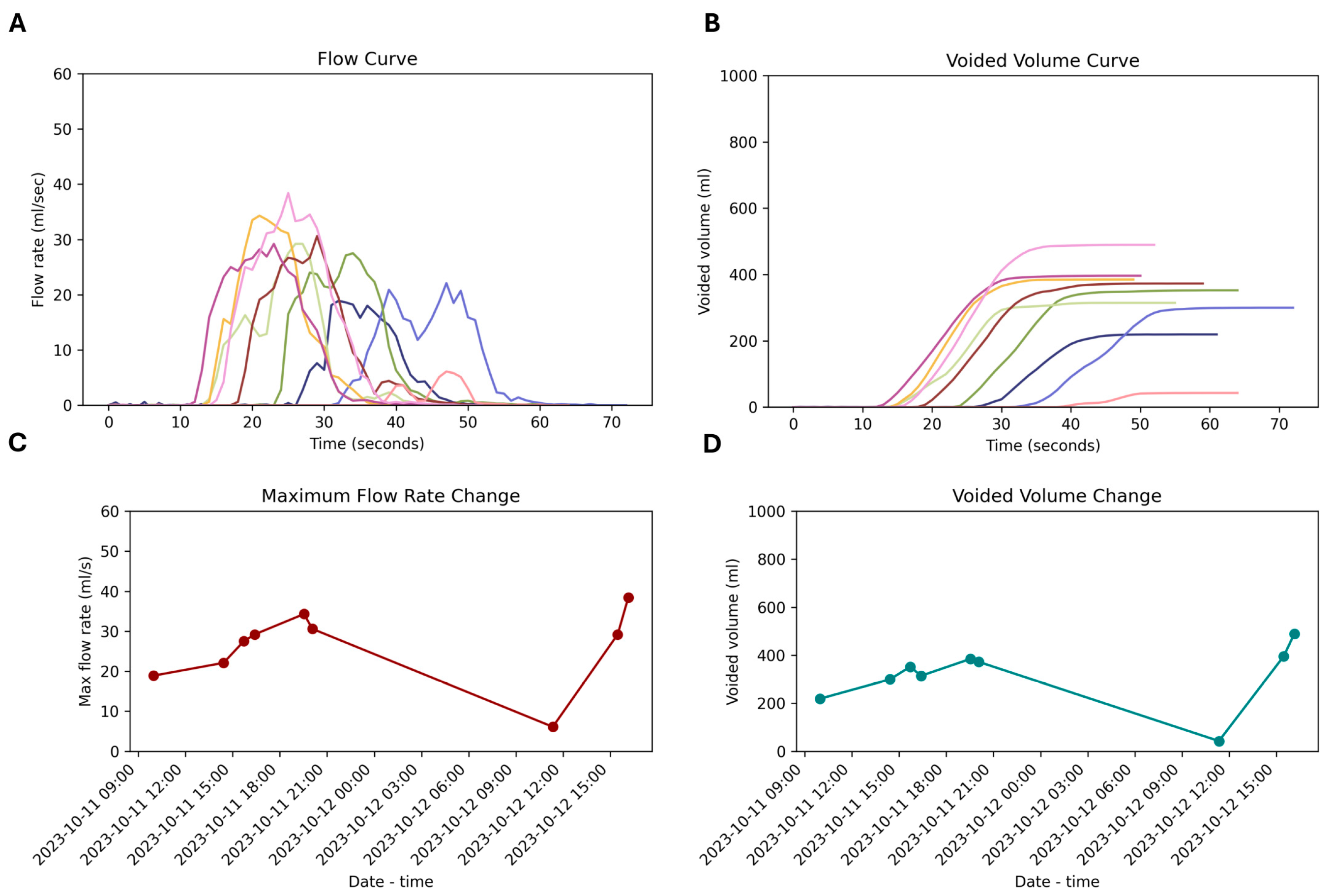
3.5. Repeated Measurement
4. Discussion
4.1. Physiological Voiding Patterns and Portable (Home) Flowmetry
4.2. Gender Differences
4.3. Multiple Voiding Visits
4.4. Relationships between Uroflow and Participants’ Characteristics
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groutz, A.; Gordon, D.; Lessing, J.B.; Wolman, I.; Jaffa, A.; David, M.P. Prevalence and Characteristics of Voiding Difficulties in Women: Are Subjective Symptoms Substantiated by Objective Urodynamic Data? Urology 1999, 54, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Sountoulides, P.; van Dijk, M.M.; Wijkstra, H.; de la Rosette, J.J.M.C.H.; Michel, M.C. Role of Voiding and Storage Symptoms for the Quality of Life before and after Treatment in Men with Voiding Dysfunction. World J. Urol. 2010, 28, 3–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, Y.-C.; Fan, Y.-H.; Lin, A.T.L.; Chen, K.-K. Do Female Patients with Predominant Voiding Symptoms Really Have Objective Voiding-Phase Dysfunction? Urol. Sci. 2017, 28, 152–155. [Google Scholar] [CrossRef]
- Liao, C.-H.; Kuo, H.-C. Use of the International Prostate Symptom Score Voiding-to-Storage Subscore Ratio in Assessing Lower Urinary Tract Symptoms. Tzu Chi. Med. J. 2014, 26, 61–63. [Google Scholar] [CrossRef]
- Choi, E.P.; Lam, C.L.; Chin, W.-Y. Validation of the International Prostate Symptom Score in Chinese Males and Females with Lower Urinary Tract Symptoms. Health Qual. Life Outcomes 2014, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S. Comparison of Self-Conducted and Assistant-Supervised Uroflowmetry Methods. Cureus 2022, 14, e22030. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; McNanley, A.; Perevich, M.; Glantz, J.C.; Buchsbaum, G. Uroflow Measurements in Healthy Female Volunteers. Female Pelvic Med. Reconstr. Surg. 2010, 16, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Floor Dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- de la Rosette, J.J.M.C.H.; Witjes, W.P.J.; Debruyne, F.M.J.; Kersten, P.L.; Wijkstra, H. Improved Reliability of Uroflowmetry Investigations: Results of a Portable Home-based Uroflowmetry Study. Br. J. Urol. 1996, 78, 385–390. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, J.C.; Lee, K.S.; Seo, J.T.; Kim, H.-J.; Yoo, T.K.; Lee, J.B.; Choo, M.-S.; Lee, J.G.; Lee, J.Y. Analysis of Female Voiding Dysfunction: A Prospective, Multi-Center Study. Int. Urol. Nephrol. 2013, 45, 989–994. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, Y.; Park, J.; Cho, S.Y.; Cho, M.C.; Jeong, H.; Son, H. Voided Volume < 150 ML on Initial Uroflowmetry in Men with Storage Symptoms: Is It an Unreliable Test Result or a Sign of Severe Storage Symptoms? PLoS ONE 2019, 14, e0207208. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.D.; Steinberg, J.R.; Rossignol, M.; Heaton, J.; Corcos, J. Normal Variation and Influence of Stress, Caffeine Intake, and Sexual Activity on Uroflowmetry Parameters of a Middle-aged Asymptomatic Cohort of Volunteer Male Urologists. Neurourol. Urodyn. 2002, 21, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kanodia, G.; Sokhal, A.K.; Singh, K.; Agrawal, M.; Sankhwar, S. Evaluation of Impact of Voiding Posture on Uroflowmetry Parameters in Men. World J. Mens. Health 2017, 35, 100. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Torimoto, K.; Matsushita, C.; Gotoh, D.; Yoshida, H.; Saka, T.; Hirao, Y.; Hirayama, A.; Fujimoto, K. A New Nomogram of Urinary Flow Rate and Volume Based on Multiple Measurements per Healthy Adult Japanese Men Using a Portable Uroflowmeter (P-Flowdiary®). BMC Urol. 2022, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Bladt, L.; Kashtiara, A.; Platteau, W.; De Wachter, S.; De Win, G. First-Year Experience of Managing Urology Patients with Home Uroflowmetry: Descriptive Retrospective Analysis. JMIR Form. Res. 2023, 7, e51019. [Google Scholar] [CrossRef] [PubMed]
- Øverland, G.B.; Øverland, G.B.; Vatten, L.; Vatten, L.; Rhodes, T.; Rhodes, T.; DeMuro, C.; DeMuro, C.; Jacobsen, G.; Jacobsen, G.; et al. Lower Urinary Tract Symptoms, Prostate Volume and Uroflow in Norwegian Community Men. Eur. Urol. 2001, 39, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, C.S.; Chalise, P.R.; Bhandari, B.B. Correlation of Prostate Volume with International Prostate Symptom Score and Quality of Life in Men with Benign Prostatic Hyperplasia. Nepal. Med. Coll. J. 2008, 10, 104–107. [Google Scholar]
- Scarpero, H.M.; Fiske, J.; Xue, X.; Nitti, V.W. American Urological Association Symptom Index for Lower Urinary Tract Symptoms in Women: Correlation with Degree of Bother and Impact on Quality of Life. Urology 2003, 61, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Witjes, W.P.J.; de la Rosette, J.J.M.C.H.; van den Berg-Segers, A.; Colongo, D.; Koch, G.; Zlotta, A.R.; Colau, A.; de Wildt, M.J.A.M.; Wijkstra, H. Computerised Assessment of Maximum Urinary Flow: An Efficient, Consistent and Valid Approach. Eur. Urol. 2002, 41, 206–213. [Google Scholar] [CrossRef]
- Cornu, J.N.; Gacci, M.; Hashim, H.; Herrmann, T.R.W.; Malde, S.; Netsch, C.; Rieken, M.; Sakalis, V.; Tutolo, M. EAU Guidelines on Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS), Incl. Benign Prostatic Obstruction (BPO). Available online: https://uroweb.org/guidelines/management-of-non-neurogenic-male-luts (accessed on 8 January 2024).
- Barry, M.J.; Fowler, F.J.; O’Leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T.K. The American Urological Association Symptom Index for Benign Prostatic Hyperplasia. J. Urol. 1992, 148, 1549–1557. [Google Scholar] [CrossRef]
- Gan, Z.S.; Zderic, S.A. Current State and Future Considerations for Home Uroflowmetry. Nat. Rev. Urol. 2023, 20, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Reynard, J.M.; Yang, Q.; Donovan, J.L.; Peters, T.J.; Schafer, W.; de la Rosette, J.J.; Dabhoiwala, N.F.; Osawa, D.; Lim, A.T.; Abrams, P. The ICS-‘BPH’ Study: Uroflowmetry, Lower Urinary Tract Symptoms and Bladder Outlet Obstruction. Br. J. Urol. 1998, 82, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-M.; Lin, H.-H.; Kuo, H.-C. International Prostate Symptom Score for Assessing Lower Urinary Tract Dysfunction in Women. Int. Urogynecol. J. 2013, 24, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.; Skelton, D.A.; Hagen, S.; Booth, J. Age and Gender Stratified Normative Values for the International Prostate Symptom Score for Adults Aged 60 Years and Over. Neurourol. Urodyn. 2018, 37, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Nojiri, Y.; Osuga, Y.; Tange, C. Psychometric Analysis of International Prostate Symptom Score for Female Lower Urinary Tract Symptoms. Urology 2009, 73, 1199–1202. [Google Scholar] [CrossRef]
- Maserejian, N.N.; Chen, S.; Chiu, G.R.; Wager, C.G.; Kupelian, V.; Araujo, A.B.; McKinlay, J.B. Incidence of Lower Urinary Tract Symptoms in a Population-Based Study of Men and Women. Urology 2013, 82, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.; Robertson, C.; Mazzetta, C.; Keech, M.; Hobbs, F.D.R.; Fourcade, R.; Kiemeney, L.; Lee, C. The Prevalence of Lower Urinary Tract Symptoms in Men and Women in Four Centres. The UrEpik Study. BJU Int. 2003, 92, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Suebnukanwattana, T.; Lohsiriwat, S.; Chaikomin, R.; Tantiwongse, A.; Soontrapa, S. Uroflowmetry in Normal Thai Subjects. J. Med. Assoc. Thai 2003, 86, 353–360. [Google Scholar] [PubMed]
- Zambon, J.P.; Batezini, N.S.d.S.; Karam, A.J., Jr.; Conceicao, R.D.O.; de Carvalho, J.A.M.; Almeida, F.G. Uroflowmetry in a Large Population of Brazilian Men Submitted to a Health Check Up Program and Its Correlation with IPSS and Prostate Size. Int. Braz. J. Urol. 2013, 39, 841–846. [Google Scholar] [CrossRef][Green Version]
- Kumar, V.; Dhabalia, J.; Nelivigi, G.; Punia, M.; Suryavanshi, M. Age, Gender, and Voided Volume Dependency of Peak Urinary Flow Rate and Uroflowmetry Nomogram in the Indian Population. Indian J. Urol. 2009, 25, 461. [Google Scholar] [CrossRef]
- Pernkopf, D.; Plas, E.; Lang, T.; Daha, K.; Kubin, K.; Treu, T.; Pflüger, H. Uroflow Nomogram for Male Adolescents. J. Urol. 2005, 174, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Wyman, J.F.; Zhou, J.; LaCoursiere, D.Y.; Markland, A.D.; Mueller, E.R.; Simon, L.; Stapleton, A.; Stoll, C.R.T.; Chu, H.; Sutcliffe, S. Normative Noninvasive Bladder Function Measurements in Healthy Women: A Systematic Review and Meta-analysis. Neurourol. Urodyn. 2020, 39, 507–522. [Google Scholar] [CrossRef]
- van Haarst, E.P.; Heldeweg, E.A.; Newling, D.W.W.; Schlatmann, T.J.M. A Cross-Sectional Study of the International Prostate Symptom Scores Related to Age and Gender in Dutch Adults Reporting No Voiding Complaints. Eur. Urol. 2005, 47, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Lunacek, L.; Gärtner, M.; Krhut, J.; Mika, D.; Sykora, R.; Zvara, P. Evaluation of Intra-Individual Test–Re-Test Variability of Uroflowmetry in Healthy Women and Women Suffering from Stress, Urge, and Mixed Urinary Incontinence. Int. Urogynecol. J. 2018, 29, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Madersbacher, S.; Pycha, A.; Schatzl, G.; Mian, C.; Klingler, C.H.; Marberger, M. The Aging Lower Urinary Tract: A Comparative Urodynamic Study of Men and Women. Urology 1998, 51, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Haylen, B.T.; Ashby, D.; Sutherst, J.R.; Frazer, M.I.; West, C.R. Maximum and Average Urine Flow Rates in Normal Male and Female Populations—The Liverpool Nomograms. Br. J. Urol. 1989, 64, 30–38. [Google Scholar] [CrossRef]
- Fantl, J.A.; Smith, P.J.; Schneider, V.; Hurt, W.G.; Dunn, L.J. Fluid Weight Uroflowmetry in Women. Am. J. Obs. Gynecol. 1983, 145, 1017–1023. [Google Scholar] [CrossRef]
- Sørensen, S.; Jønler, M.; Knudsen, U.B.; Djurhuus, J.C. The Influence of a Urethral Catheter and Age on Recorded Urinary Flow Rates in Healthy Women. Scand. J. Urol. Nephrol. 1989, 23, 261–266. [Google Scholar] [CrossRef]
- Barapatre, Y.; Agarwal, M.M.; Singh, S.K.; Sharma, S.K.; Mavuduru, R.; Mete, U.K.; Kumar, S.; Mandal, A.K. Uroflowmetry in Healthy Women: Development and Validation of Flow–Volume and Corrected Flow–Age Nomograms. Neurourol. Urodyn. 2009, 28, 1003–1009. [Google Scholar] [CrossRef]
- Moon, T.D.; Hagen, L.; Heisey, D.M. Urinary Symptomatology in Younger Men. Urology 1997, 50, 700–703. [Google Scholar] [CrossRef]
- Ezz El Din, K.; Kiemeney, L.A.L.M.; De Wildt, M.J.A.M.; Debruyne, F.M.J.; de la Rosette, J.J.M.C.H. Correlation between Uroflowmetry, Prostate Volume, Postvoid Residue, and Lower Urinary Tract Symptoms as Measured by the International Prostate Symptom Score. Urology 1996, 48, 393–397. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Whole Cohort (n = 431) | Female (n = 103) | Male (n = 328) | p Values |
|---|---|---|---|---|
| Age (years) | 36.87 ± 12.77 | 30.20 ± 10.33 | 38.96 ± 12.76 | <0.01 |
| BMI (kg/m2) | 25.51 ± 4.04 | 22.82 ± 3.70 | 26.35 ± 3.77 | <0.01 |
| IPSS Total Score | 2.0 (5.0) | 4.0 (6.5) | 2.0 (4.0) | <0.01 |
| Incomplete Emptying | 0.0 (0.0) | 0.0 (1.0) | 0.0 (0.0) | <0.01 |
| Frequency | 0.0 (1.0) | 1.0 (2.0) | 0.0 (1.0) | <0.01 |
| Intermittency | 0.0 (0.0) | 0.0 (1.0) | 0.0 (0.0) | <0.01 |
| Urgency | 0.0 (0.0) | 0.0 (1.0) | 0.0 (0.0) | <0.01 |
| Weak Stream | 0.0 (1.0) | 0.0 (1.0) | 0.0 (0.25) | 0.23 |
| Straining | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.17 |
| Nocturia | 0.0 (1.0) | 1.0 (1.0) | 0.0 (1.0) | 0.03 |
| QoL | 0.0 (1.0) | 1.0 (2.0) | 0.0 (1.0) | <0.01 |
| Qmax (mL/s) | 25.78 ± 12.70 | 33.51 ± 16.05 | 23.35 ± 10.34 | <0.01 |
| Qave (mL/s) | 14.05 ± 6.78 | 17.81 ± 8.62 | 12.87 ± 5.61 | <0.01 |
| Voided Volume (mL) | 322.68 ± 184.66 | 336.00 ± 219.85 | 318.50 ± 172.31 | 0.40 |
| Time to Qmax (s) | 8.88 ± 4.83 | 6.78 ± 3.93 | 9.54 ± 4.90 | <0.01 |
| Flow Time (s) | 23.79 ± 10.95 | 19.17 ± 10.36 | 25.24 ± 10.74 | <0.01 |
| Parameters | Female (n = 103) | Male (n = 328) | ||||
|---|---|---|---|---|---|---|
| Vvoided < 150 (n = 21) | Vvoided ≥ 150 (n = 82) | p Value | Vvoided < 150 (n = 48) | Vvoided ≥ 150 (n = 280) | p Value | |
| Age (years) | 28.33 ± 9.14 | 30.68 ± 10.61 | 0.35 | 43.62 ± 14.67 | 38.16 ± 12.26 | <0.01 |
| BMI (kg/m2) | 22.56 ± 3.74 | 22.89 ± 3.71 | 0.72 | 25.88 ± 3.99 | 26.43 ± 3.73 | 0.35 |
| IPSS Total Score | 4.0 (4.0) | 4.0 (7.0) | 0.66 | 2.0 (4.0) | 1.0 (4.0) | 0.03 |
| Incomplete Emptying | 0.0 (1.0) | 0.0 (1.0) | 0.57 | 0.0 (0.25) | 0.0 (0.0) | 0.12 |
| Frequency | 1.0 (1.0) | 1.0 (2.0) | 0.16 | 0.0 (1.0) | 0.0 (1.0) | 0.96 |
| Intermittency | 0.0 (1.0) | 0.0 (1.0) | 0.82 | 0.0 (1.0) | 0.0 (0.0) | 0.04 |
| Urgency | 0.0 (1.0) | 0.0 (1.0) | 0.56 | 0.0 (0.0) | 0.0 (0.0) | 0.55 |
| Weak Stream | 0.0 (1.0) | 0.0 (1.0) | 0.78 | 0.0 (1.0) | 0.0 (0.0) | 0.05 |
| Straining | 0.0 (0.0) | 0.0 (0.0) | 0.47 | 0.0 (0.25) | 0.0 (0.0) | 0.06 |
| Nocturia | 1.0 (1.0) | 1.0 (1.0) | 0.74 | 1.0 (1.0) | 0.0 (1.0) | 0.02 |
| QoL | 1.0 (2.0) | 1.0 (2.0) | 0.82 | 1.0 (1.25) | 0.0 (1.0) | 0.01 |
| Qmax (mL/s) | 17.35 ± 9.15 | 37.65 ± 14.78 | <0.01 | 10.42 ± 4.27 | 25.57 ± 9.41 | <0.01 |
| Qavg (mL/s) | 8.68 ± 3.96 | 20.15 ± 7.91 | <0.01 | 5.69 ± 2.07 | 14.10 ± 5.07 | <0.01 |
| Voided Volume (mL) | 89.88 ± 41.08 | 399.02 ± 201.81 | <0.01 | 95.55 ± 32.50 | 356.72 ± 156.85 | <0.01 |
| Time to Qmax (s) | 4.71 ± 1.74 | 7.30 ± 4.16 | <0.01 | 6.96 ± 3.80 | 9.98 ± 4.93 | <0.01 |
| Flow Time (s) | 11.62 ± 9.51 | 21.10 ± 9.71 | <0.01 | 17.98 ± 7.91 | 26.49 ± 10.67 | <0.01 |
| Parameters | Female (n = 103) | Male (n = 431) | ||||
|---|---|---|---|---|---|---|
| IPSS ≤ 7 (n = 75) | IPSS > 7 (n = 28) | p Value | IPSS ≤ 7 (n = 298) | IPSS > 7 (n = 30) | p Value | |
| Age (years) | 30.08 ± 10.04 | 30.54 ± 11.23 | 0.84 | 38.25 ± 12.23 | 46.03 ± 15.76 | <0.01 |
| BMI (kg/m2) | 22.72 ± 3.72 | 23.10 ± 3.68 | 0.64 | 26.17 ± 3.74 | 28.10 ± 3.64 | <0.01 |
| IPSS Total Score | 2.0 (3.5) | 10.5 (4.25) | <0.01 | 1.0 (3.0) | 10.5 (6.0) | <0.01 |
| Incomplete Emptying | 0.0 (0.0) | 1.0 (2.0) | <0.01 | 0.0 (0.0) | 2.0 (2.75) | <0.01 |
| Frequency | 0.0 (1.0) | 2.0 (2.25) | <0.01 | 0.0 (1.0) | 2.0 (2.0) | <0.01 |
| Intermittency | 0.0 (1.0) | 2.0 (2.0) | <0.01 | 0.0 (0.0) | 1.0 (1.0) | <0.01 |
| Urgency | 0.0 (0.0) | 1.0 (3.0) | <0.01 | 0.0 (0.0) | 2.0 (1.0) | <0.01 |
| Weak Stream | 0.0 (0.0) | 1.0 (3.0) | <0.01 | 0.0 (0.0) | 2.0 (2.75) | <0.01 |
| Straining | 0.0 (0.0) | 1.0 (1.0) | <0.01 | 0.0 (0.0) | 1.0 (1.75) | <0.01 |
| Nocturia | 0.0 (1.0) | 1.0 (2.0) | <0.01 | 0.0 (1.0) | 1.0 (1.0) | <0.01 |
| QoL | 1.0 (1.0) | 3.0 (4.0) | <0.01 | 0.0 (1.0) | 3.0 (1.0) | <0.01 |
| Qmax (mL/s) | 32.93 ± 15.58 | 35.08 ± 17.45 | 0.55 | 23.91 ± 10.42 | 17.82 ± 7.65 | <0.01 |
| Qavg (mL/s) | 17.41 ± 7.88 | 18.87 ± 10.44 | 0.45 | 13.18 ± 5.63 | 9.78 ± 4.34 | <0.01 |
| Voided Volume (mL) | 340.39 ± 232.46 | 324.22 ± 185.23 | 0.74 | 324.69 ± 173.87 | 257.03 ± 144.48 | 0.04 |
| Time to Qmax (s) | 6.53 ± 3.78 | 7.43 ± 4.32 | 0.31 | 9.58 ± 5.02 | 9.13 ± 3.55 | 0.64 |
| Flow Time (s) | 19.29 ± 9.71 | 18.82 ± 12.12 | 0.84 | 25.04 ± 10.87 | 27.27 ± 9.22 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almas, F.; Dasdelen, M.F.; Seyhan, Z.; Sargolzaeimoghaddam, M.; Sarg, A.; Unlu, O.; Dasdelen, Z.B.; Horuz, R.; Albayrak, S.; Kocak, M.; et al. Reassessing Normal Voiding Standards: A Cross-Sectional Study Based on Medical Professionals’ Evaluations with Portable Uroflowmetry and IPSS. J. Clin. Med. 2024, 13, 2857. https://doi.org/10.3390/jcm13102857
Almas F, Dasdelen MF, Seyhan Z, Sargolzaeimoghaddam M, Sarg A, Unlu O, Dasdelen ZB, Horuz R, Albayrak S, Kocak M, et al. Reassessing Normal Voiding Standards: A Cross-Sectional Study Based on Medical Professionals’ Evaluations with Portable Uroflowmetry and IPSS. Journal of Clinical Medicine. 2024; 13(10):2857. https://doi.org/10.3390/jcm13102857
Chicago/Turabian StyleAlmas, Furkan, Muhammed Furkan Dasdelen, Zuleyha Seyhan, Maral Sargolzaeimoghaddam, Arya Sarg, Omer Unlu, Zehra Betul Dasdelen, Rahim Horuz, Selami Albayrak, Mehmet Kocak, and et al. 2024. "Reassessing Normal Voiding Standards: A Cross-Sectional Study Based on Medical Professionals’ Evaluations with Portable Uroflowmetry and IPSS" Journal of Clinical Medicine 13, no. 10: 2857. https://doi.org/10.3390/jcm13102857
APA StyleAlmas, F., Dasdelen, M. F., Seyhan, Z., Sargolzaeimoghaddam, M., Sarg, A., Unlu, O., Dasdelen, Z. B., Horuz, R., Albayrak, S., Kocak, M., Laguna, P., & de la Rosette, J. (2024). Reassessing Normal Voiding Standards: A Cross-Sectional Study Based on Medical Professionals’ Evaluations with Portable Uroflowmetry and IPSS. Journal of Clinical Medicine, 13(10), 2857. https://doi.org/10.3390/jcm13102857








