Abstract
Background: Lipedema is a subcutaneous adipose tissue disorder characterized by increased pathological adipocytes mainly in the extremities. Vitamin D is stored in adipocytes, and serum levels inversely correlate with BMI. As adipocytes are removed during liposuction, lipedema patients might be prone to further substantial vitamin D loss while their levels are already decreased. Therefore, we examined the effect of liposuction on perioperative serum 25-hydroxyvitamin D levels. Methods: In patients undergoing lipedema liposuction, blood samples were obtained pre- and postoperatively. Statistical analyses were performed to correlate the volume of lipoaspirate, patients’ BMI and number of sessions to vitamin D levels. Results: Overall, 213 patients were analyzed. Mean liposuction volume was 6615.33 ± 3884.25 mL, mean BMI was 32.18 ± 7.26 kg/m2. mean preoperative vitamin D levels were 30.1 ± 14.45 ng/mL (borderline deficient according to the endocrine society) and mean postoperative vitamin D levels were 21.91 ± 9.18 ng/mL (deficient). A significant decrease in serum vitamin D was seen in our patients (p < 0.001) of mean 7.83 ng/mL. The amount of vitamin D loss was not associated with BMI or aspiration volume in our patients (p > 0.05). Interestingly, vitamin D dynamics showed a steady drop regardless of volume aspirated or preoperative levels. Conclusions: Many lipedema patients have low vitamin D levels preoperatively. Liposuction significantly reduced these levels additionally, regardless of aspirated volume or BMI. However, vitamin D loss was constant and predictable; thus, patients at risk are easily identified. Overall, lipedema patients undergoing liposuction are prone to vitamin D deficiency, and the long-term effects in this population are currently unknown.
1. Introduction
Lipedema is a subcutaneous adipose tissue disorder almost solely affecting women [1,2]. Patients mainly suffer from painful localized fat deposition in the extremities with consecutive restrictions in daily life [2,3,4]. Due to its complex etiology and variable clinical manifestations, it presents as a multifaceted challenge for modern surgery. A vast number of comorbidities are associated with lipedema, such as hypertension, depression or increased BMI [5,6]. Vitamin D is stored in fat tissue, and due to its inverse correlation with BMI status and body fat, vitamin D serum deficiency can frequently be seen in lipedema patients [5,7,8,9,10,11,12,13,14,15,16]. It is the most common deficiency in obese patients worldwide with a prevalence of 80–90% [9,17,18,19,20,21,22,23,24,25].
Besides their painful nature, lipedematous adipocytes are resistant to diets and bariatric surgery [6,26,27]. Despite growing recognition, effective treatment modalities for lipedema remain limited. Therefore, liposuction has emerged as the only suitable procedure for managing lipedema-related symptoms, offering symptomatic relief to reduce patients’ burden and enhance their quality of life [25,26].
To our knowledge, this is the first study investigating the relationship of liposuction and vitamin D serum levels in lipedema patients. Hence, in this study we seek to critically examine the implications of vitamin D deficiency in lipedema patients after liposuction, elucidating the potential challenges and proposing strategies for mitigating adverse outcomes. We aimed to analyze perioperative vitamin D alternations in lipedema patients undergoing liposuction.
2. Materials and Methods
2.1. Study Design and Patient Analysis
In this study we analyzed pre- and postoperative vitamin D serum levels in lipedema patients undergoing liposuction at the Clinical Department for Plastic, Aesthetic and Reconstructive Surgery at the University Hospital St. Poelten, between 1 January 2018 and 31 December 2022. The study was conducted as a retrospective single center study. Ethical approval was obtained from the local institutional review board at the Karl Landsteiner University of Health Sciences Krems (reference number: ECS 1041/2021). Analyzed factors included the patients’ age at surgery, BMI, volume of lipoaspiration, pre- and postoperative serum vitamin D levels, localization of treated area (upper and lower extremities) and liposuction sessions (one, two or three sessions).
2.2. Operative Procedure
At our department, liposuction is performed under general anesthesia using tumescent technique. Patients are examined and marked preoperatively while standing to assess areas to be treated. All patients receive intravenous antibiotic shielding with either 2.2 g of amoxicillin/clavulanic acid combination (Curam®, Sandoz GmbH, 6250 Kundl, Austria) or 600 mg of clindamycin (Dalacin®, Fareva Amboise Zone Industrielle, Routes des Industries 29, 37530 Pocé-sur-Cisse, France) in case of penicillin allergy. Antibiotic administration is given 30 min before surgical incision and is continued for one week postoperatively. Patients receive a modified Klein’s solution with 1.000 mL Ringer’s lactate (Ringer lactate®, Fresenius Kabi, Rue du Rempart 6, 27400 Louviers, France) containing 1 mL of 1:1.000 epinephrin (Suprarenin® Sanofi-Aventis GmbH, 65926 Frankfurt am Main, Germany). The solution is infiltrated with specialized infiltration cannulas through small stab incisions at strategically placed locations using a number 11 blade, which can easily be camouflaged postoperatively by the patient’s clothing (e.g., the groin). After an indwelling time of approximately 15 min for the tumescent solution to set, vibration-assisted liposuction (VAL) is performed using Moeller’s liposuction device (Moeller Vibrasat Pro, Moeller medical® GmbH, Wasserkuppenstraße 29-31, 36043 Fulda, Germany) with 3 and/or 4 mm multiport cannulas (multiport rapid extraction cannula, Moeller Medical® GmbH, Wasserkuppenstraße 29-31, 36043 Fulda, Germany) (Figure 1). Incisions are not sutured and are solely covered with plasters after antiseptic irrigation with Octenisept® (Schülke & Mayr GmbH, Robert-Koch-Straße 2, 22851, Norderstedt, Germany) and Skinsept® (Ecolab Germany GmbH, Ecolab-Allee 1, 40789 Monheim am Rhein, Germany). Compression garments are installed immediately postoperatively in the operating room. Compression garments are worn day and night for three months postoperatively. Patients receive antithrombotic shielding using low molecular heparin for 10 to 30 days postoperatively.
Figure 1.
Illustration demonstrating the depletion of vitamin D during liposuction.
2.3. Blood Sampling
Blood samples were collected preoperatively at a maximum of one week prior to surgery and analyzed for serum 25-hydroxyvitamin D levels at our clinical institute of laboratory medicine. The vacutainers used for vitamin D sampling were BD Vacutainer® with stabilizing gel (Fischer Scientific GmbH, Im Heiligen Feld 17, 58239 Schwerte, Germany). Vitamin D components were separated using Elecsys Vitamin D total III Cobas® (Roche Diagnostics GmbH, Sandhofer Straße 116, 68305 Mannheim, Germany). All samples were retrieved and processed using the same instruments. Sample results were digitally stored at the hospital’s data working space adhering to Austrian regulations for data protection. Postoperative sample collection was performed on the first postoperative day. Serum vitamin D levels below 30 ng/mL, according to the Endocrine Society were indicated as deficiency [28].
2.4. Statistics and Data Management
The endpoint of our analyses was to assess the alteration of vitamin D serum levels after liposuction and high-volume liposuction in lipedema patients. All data were reported anonymously. Data protection management complied with Austrian legislation. Data collection and processing were performed with Microsoft Excel (Software Version 2021, Microsoft Corp., One Microsoft Way, Redmond, 98052 Washington, DC, USA), and statistical analyses were performed using IBM SPSS Statistics version 26 (©IBM, Armonk, NY, USA). Nominal data were described using absolute frequencies and percentages. For metric data, mean and standard deviation were indicated. To correlate the volume of lipoaspirate and patients’ BMI to vitamin D alterations, correlation analyses using Spearman’s rho test were performed. Further, paired t-test analyses were conducted. Two-sided p ≤ 0.05 was regarded as statistically significant. To analyze the decrease in vitamin D levels regarding liposuction sessions, analysis of variance (ANOVA) was used.
3. Results
3.1. Demographics
In total, 213 liposuctions in 100 patients suffering from lipedema were identified during the study period. Thereof, 163 liposuctions in 61 patients were excluded due to missing data (Figure 2).
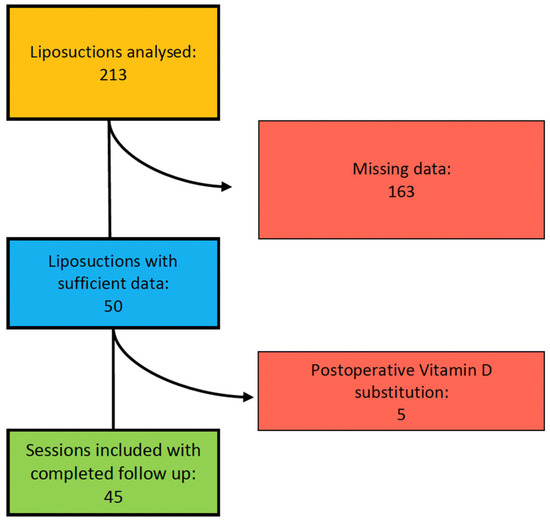
Figure 2.
Organigram of patient selection for study inclusion.
Additionally, five liposuctions in three patients were excluded because of self-supplied postoperative vitamin D substitution. Consequently, 45 liposuctions in 36 patients met our criteria and were included in our study. We analyzed 35 liposuctions on lower extremities and 10 liposuctions on upper extremities (Figure 3). All patients included were Caucasian women and did not expose themselves excessively to the sun or substitute vitamin D independently according to the anamnesis.
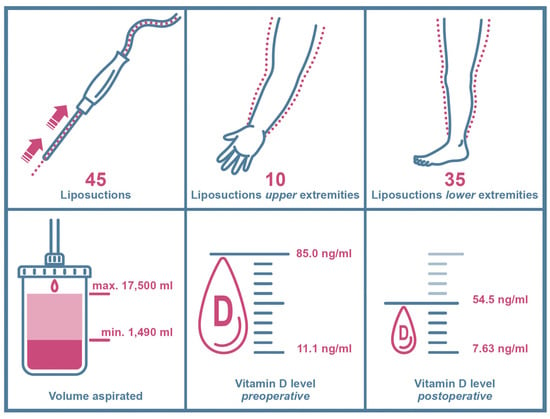
Figure 3.
Key data chart of included study patients and clinical findings. Arrows and dotted lines in the upper left window show the suction path (arrows) of lipoaspirate (dotted lines) during liposuction.
Mean patient age was 38.11 ± 13.74 years overall, ranging from 19 years as the youngest to 71 years as the oldest at time of surgery (Table 1). Mean BMI was 32.18 ± 7.26 kg/m2, varying from 21.7 kg/m2 to 53.1 kg/m2 (Table 1). Mean volume aspirated was 6615.33 ± 3884.253 mL, with a minimum of 1490 mL and maximum of 17,500 mL in one session (Table 1). Patients were further divided into two groups as higher volumes of liposuction mainly occur in the lower extremities: patients undergoing liposuction on upper extremities and patients undergoing liposuction on lower extremities.

Table 1.
Baseline characteristics of patients. Significantly lower vitamin D serum levels can be observed postoperatively.
3.2. Liposuction of Upper Extremities
Patients’ mean age in this group was 39.0 ± 15.74 years, ranging from 20 years to 57 years. Mean BMI in the upper extremity group was 29.21 ± 3.96 kg/m2, ranging from 24.2 kg/m2 to 36.4 kg/m2. Mean volume aspirated was 3845 mL ± 3884.25 mL with a minimum of 1800 mL and a maximum of 7600 mL in one session (Table 1).
3.3. Liposuction of Lower Extremities
In the lower extremity group, patients’ mean age was 37.86 ± 13.36, ranging from 19 to 71 years. Mean BMI was 33.03 ± 7.79 kg/m2, ranging from 21.7 kg/m2 to 53.1 kg/m2. Mean volume aspirated was 7406.86 ± 3997.92 mL with a minimum of 1490 mL and a maximum of 17,500 mL in one session (Table 1).
3.4. Vitamin D Serum Levels
In total, mean preoperative vitamin D levels were 30.1 ± 14.45 ng/mL, ranging from 11.1 ng/mL to 85.0 ng/mL (Table 1, Figure 4).
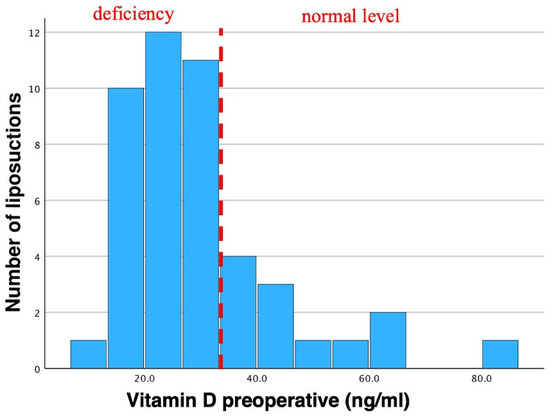
Figure 4.
Histogram of preoperative vitamin D. Mean vitamin D levels were 30.1 ng/mL in total. Std. was 14.45 ng/mL (N = 46). Preoperative vitamin D insufficiency (according to the endocrine society) can be observed as most bars are shifted to the left. This vitamin D insufficiency is often seen in lipedema patients. Dotted line indicates the threshold of vitamin D deficiency to non-deficiency in preoperative patients.
Mean postoperative vitamin D levels were 21.91 ± 9.18 ng/mL, ranging from 7.63 ng/mL to 54.5 ng/mL (Table 1, Figure 5).
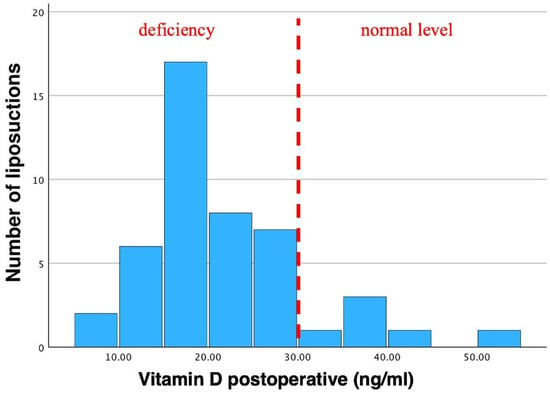
Figure 5.
Histogram of postoperative vitamin D levels. Mean vitamin D was 21.91 ng/mL. Std. was 9.18 ng/mL (N = 46). A vitamin D insufficiency can clearly be seen in postoperative values as the bars are shifted to the left. Pre-existing vitamin D insufficiencies are further aggravated through liposuction. Dotted line indicates the threshold of vitamin D deficiency to non-deficiency in postoperative patients.
In total, 29 patients showed preoperative vitamin D levels below 30 ng/mL. Postoperatively, 40 patients showed vitamin D levels below 30 ng/mL. None of our patients with preoperative vitamin D levels below 30 ng/mL showed any clinical sign of deficiencies. Vitamin D was not substituted in our cohort, either pre- or postoperatively.
3.5. Vitamin D Serum Levels of Liposuction of Upper Extremities
3.6. Vitamin D Serum Levels of Liposuction of Lower Extremities
3.7. Correlation Analysis
We correlated patients’ BMI with the amount of ml aspirated during liposuction, expecting a higher BMI drop at higher liposuction volumes. Using Spearman’s rho test for rank correlation, our analyses showed no significant correlation, either in absolute numbers (p = 0.006) or in relative numbers (p = 1.97) (Table 2), demonstrating that the absolute amount of volume reduced does not interfere with the patients’ BMI.

Table 2.
Spearman’s rho test for rank correlation demonstrating no significant correlation between the volume of fat removed and the decrease in patients’ BMI (p-values > 0.05). Regardless of the volume aspirated, the difference in pre- and postoperative BMI did not show significant changes. This finding is displayed in absolute and relative numbers within this table.
Investigating the patients’ vitamin D serum levels pre- and postoperatively, we found a statistically significant decrease regarding vitamin D levels using a paired t-test (p < 0.001, Table 3). These findings were both significant overall and between the different groups (p < 0.001, Table 3). Since our data set turned out not to be normally distributed (outliers included in our data set), we additionally conducted the according non-parametric tests. Nonetheless, our data were still significant, additionally supporting our statistical findings (p < 0.001; labeled in red, Table 3).

Table 3.
t-Test analysis showing the significant correlation between the measured vitamin D serum levels pre- and postoperatively (p < 0.001).
This demonstrates that high-volume liposuction has a significant impact on postoperative vitamin D level changes. The abovementioned findings were also significant when analyzing areas treated separately (upper extremities and lower extremities). Hence, the decrease in vitamin D after liposuction was significant, no matter of liposuction location (p < 0.001).
The abovementioned findings were also significant when analyzing areas treated separately (upper extremities and lower extremities, Table 4). Hence, the decrease in vitamin D after liposuction was significant, no matter the area treated (p < 0.001, Table 4). Again, p-values of non-perimetric tests for non-normal distribution were also significant (p = 0.005 in upper extremities and p < 0.001 in lower extremities; labeled in red, Table 4).

Table 4.
t-Test analysis on account of vitamin D decrease after liposuction in upper and lower extremities, demonstrating the statistical significance p < 0.001 in both groups (arms and legs). p-Values were still significant after non-perimetric testing (p < 0.001 and p = 0.005; labeled in red).
Interestingly, after performing ANOVA (analysis of variance) for correlation of vitamin D level changes and the volume aspirated, we did not find any significant correlation (p = 0.906 in absolute numbers, and p = 0.451 in relative numbers, Table 5). This finding was also seen in non-perimetric testing (p = 0.481 in absolute numbers, and p = 0.128 in relative numbers; labeled in red, Table 5). These findings were consistent throughout the session of liposuction.

Table 5.
Analysis of variance (ANOVA) of volume aspirated during liposuction and decrease in serum vitamin D levels. Here, no significant correlation can be observed (p = 0.906 in absolute numbers, and p = 0.451 in relative numbers), More likely, our ANOVA analysis shows a non-correlation, concluding that no matter the amount of volume aspirated, vitamin D levels do not drop concordantly. Rather, a stable decrease in vitamin D can be seen regardless of volume of lipoaspirate.
The findings demonstrate that no matter the volume removed during liposuction, vitamin D levels did not drop concordantly. Our analyses rather showed a non-correlation between the decrease in vitamin D after liposuction and the volume aspirated, hence demonstrating a stable drop in vitamin D between a mean of 6.86 ng/mL and 8.81 ng/mL (mean 7.83 ng/mL) no matter the volume of lipoaspirate.
4. Discussion
Many studies have been conducted to analyze serum levels of vitamin D after diets or bariatric surgery [10,11,12,29,30,31,32,33], yet none have investigated the alteration in vitamin D levels after liposuction in lipedema patients. To our knowledge, this is the first study investigating the correlation of vitamin D serum levels after liposuction.
Adipose tissue plays a significant role in energy supply and distribution and is essential in storing fat-soluble vitamins, such as vitamin D. Its bioactivity includes the reduction in inflammatory processes, neuromuscular regulation as well as the absorption of calcium, an essential mineral in osteosynthesis [29,34]. Vitamin D deficiency can lead to diminished immune responses, muscle weakness, osteoporosis and increased fracture rates [19,22,23,34,35,36,37]. Approximately 1 billion people (developing and developed countries) suffer from vitamin D deficiency, consequently making it a global public health issue [20,21,37,38]. It is the most common deficiency in obese patients worldwide [9,17,18,19,20,21,22,23,24,25]. Vitamin D is normally synthesized through the skin, yet obese patients have significantly lower levels in their blood stream compared to non-obese patients, despite having increased body surfaces [8,19,39]. This results from the inverse correlation of vitamin D and the patients’ BMI, as more adipocytes store more vitamin D [5,7,8,9,10,11,12,13,14,15], thus, leading to serum deficiency. Since most lipedema patients have elevated BMI due to pathologically engorged adipocyte, this cohort often shows vitamin D deficiency as well. These patients not only display low vitamin D serum levels due to its inverse correlation to BMI but also because engorged and inflamed adipocytes in lipedema traps vitamin D [14,40,41,42]. This bidirectional relationship between vitamin D deficiency and elevated BMI with low serum vitamin D levels is also evident in our study.
Several studies have demonstrated that weight loss by lifestyle changes or bariatric surgeries increases vitamin D levels [8,10,11,29,43,44]. Nevertheless, this effect was not detected in our study after liposuction so far. Contrarily, our results showed a significant decrease in postoperative vitamin D levels after treatment (Figure 5).
Since liposuction is the gold standard in treating lipedema, patients already suffering from vitamin D deficiency are at risk of further vitamin D loss. The lack of vitamin D has already been linked to entailing chronic cellular stress, which can be seen in lipedema patients [40,42,45]. By further diminishing vitamin D levels after liposuction, patients experiencing lipedema further maintain oxidative stress, resulting in sustained lipedema symptoms [14,42,45]. Thus, lipedema symptoms might not ameliorate after treatment, potentially aggravating their symptoms. Although the postoperative 25-hydroxyvitamin D decrease did not drop concordantly with the volume aspirated, its decrease was significant in our findings. Therefore, lipedema patients ought to be screened for vitamin D deficiency and, if needed, it should be substituted to prevent further sequelae, such as osteopenia or osteoporosis.
To personalize liposuction for lipedema patients, preoperative assessment of vitamin D levels in concordance with the estimated amount of lipoaspirate is needed. Thereby, vitamin D levels can be improved before surgery and ensure patients face the operation in an optimized manner. Parenthetically, high-volume liposuction needs to be separated into several sessions to obviate excessive vitamin D loss postoperatively. Vitamin D levels in addition are to be monitored precisely between sessions to prevent patients from experiencing vitamin D deficiency in between treatment sessions. Interdisciplinary collaboration involving endocrinologists and nutritionists is essential for implementing patient-tailored strategies. Perioperative optimization of nutrition management or the administration of supplements to individually address the needs of lipedema patients is therefore favored to guarantee long-term patient safety.
5. Conclusions
Although liposuction is a relatively safe procedure, its aftereffects are not to be neglected. To protect this patient cohort from further long-term sequelae and to sufficiently relieve patients’ burden, perioperative improvement of treatment modalities is necessary. By addressing vitamin D deficiency comprehensively, healthcare providers can enhance the efficacy of liposuction as a therapeutic tool for managing lipedema. To reduce postoperative vitamin D loss in lipedema patients, high-volume liposuction ought to be stratified and personalized to each patient individually for optimized vitamin D preservation. Therefore, lipedema patients might not suffer further comorbidities related to their underlying disease after treatment. Despite the significant findings in our research, our study faces a few limitations. Postoperative vitamin D levels should be monitored over a longer period. Also, the storage behavior and characteristics of lipedema adipocytes ought to be investigated through controlled histological analyses. Additional cross-section studies are needed for further detection of this underacknowledged threat.
Author Contributions
Conceptualization, T.F., C.K., F.J.J., C.G., H.S., J.N., P.W., M.W., K.D.B. and K.F.S.; methodology, T.F., C.K., F.J.J., C.G., H.S., J.N., P.W., M.W., K.D.B. and K.F.S.; validation, T.F., C.K., F.J.J., C.G., H.S., J.N., P.W., M.W., K.D.B. and K.F.S.; formal analysis, T.F., C.K., F.J.J., C.G., H.S., J.N., P.W., M.W., K.D.B. and K.F.S.; investigation, T.F., C.K., F.J.J., C.G., H.S., J.N., P.W., M.W., K.D.B. and K.F.S.; resources, T.F., C.K., F.J.J., C.G., H.S., J.N., P.W., M.W., K.D.B. and K.F.S.; data curation, T.F., C.K., F.J.J., C.G., H.S., J.N., P.W., M.W., K.D.B. and K.F.S.; writing—original draft preparation, T.F., C.K., F.J.J., C.G., H.S., J.N., P.W., M.W., K.D.B. and K.F.S.; writing—review and editing, T.F., C.K., F.J.J., C.G., H.S., J.N., P.W., M.W., K.D.B. and K.F.S.; visualization, T.F., C.K., F.J.J., C.G., H.S., J.N., P.W., M.W., K.D.B. and K.F.S.; supervision, K.D.B. and K.F.S.; project administration, T.F.; funding acquisition, T.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding for open access publication by the Karl Landsteiner University of Health Sciences, Dr. Karl-Dorrek-Straße 30, 3500, Krems, Austria.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Karl Landsteiner University of Health Sciences Krems (protocol code: 1041/2021, 2 November 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective character of this study.
Data Availability Statement
All the data analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors want to show appreciation for the contribution of NÖ Landesgesundheitsagentur, the legal entity of University Hospitals in Lower Austria, for providing the organizational framework to conduct this research. The authors also would like to acknowledge support from the Open Access Publishing Fund of Karl Landsteiner University of Health Sciences, Krems, Austria. This work was supported by Forschungsimpulse [project ID: SF_56], a program of Karl Landsteiner University of Health Sciences, funded by the Federal Government of Lower Austria. We also thank Aron Cserveny for providing the graphics. Open Access Funding by Karl Landsteiner University of Health Sciences, Krems, Austria.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Okhovat, J.P.; Alavi, A. Lipedema: A Review of the Literature. Int. J. Low. Extrem. Wounds 2015, 14, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U. Lipedema-An update. Dermatol. Ther. 2019, 32, e12805. [Google Scholar] [CrossRef] [PubMed]
- Katzer, K.; Hill, J.L.; McIver, K.B.; Foster, M.T. Lipedema and the Potential Role of Estrogen in Excessive Adipose Tissue Accumulation. Int. J. Mol. Sci. 2021, 22, 11720. [Google Scholar] [CrossRef] [PubMed]
- Michelini, S.; Chiurazzi, P.; Marino, V.; Dell’Orco, D.; Manara, E.; Baglivo, M.; Fiorentino, A.; Maltese, P.E.; Pinelli, M.; Herbst, K.L.; et al. Aldo-Keto Reductase 1C1 (AKR1C1) as the First Mutated Gene in a Family with Nonsyndromic Primary Lipedema. Int. J. Mol. Sci. 2020, 21, 6264. [Google Scholar] [CrossRef] [PubMed]
- Al-Wardat, M.; Alwardat, N.; Lou De Santis, G.; Zomparelli, S.; Gualtieri, P.; Bigioni, G.; Romano, L.; Di Renzo, L. The association between serum vitamin D and mood disorders in a cohort of lipedema patients. Horm. Mol. Biol. Clin. Investig. 2021, 42, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.T.; von Lukowicz, D.; Lossagk, K.; Aitzetmueller, M.; Moog, P.; Cerny, M.; Erne, H.; Schmauss, D.; Duscher, D.; Machens, H.G. New Insights on Lipedema: The Enigmatic Disease of the Peripheral Fat. Plast. Reconstr. Surg. 2019, 144, 1475–1484. [Google Scholar] [CrossRef]
- Vranic, L.; Mikolasevic, I.; Milic, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Kobylinska, M.; Antosik, K.; Decyk, A.; Kurowska, K. Malnutrition in Obesity: Is It Possible? Obes. Facts 2022, 15, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Gangloff, A.; Bergeron, J.; Pelletier-Beaumont, E.; Nazare, J.A.; Smith, J.; Borel, A.L.; Lemieux, I.; Tremblay, A.; Poirier, P.; Almeras, N.; et al. Effect of adipose tissue volume loss on circulating 25-hydroxyvitamin D levels: Results from a 1-year lifestyle intervention in viscerally obese men. Int. J. Obes. 2015, 39, 1638–1643. [Google Scholar] [CrossRef]
- Pramyothin, P.; Biancuzzo, R.M.; Lu, Z.; Hess, D.T.; Apovian, C.M.; Holick, M.F. Vitamin D in adipose tissue and serum 25-hydroxyvitamin D after roux-en-Y gastric bypass. Obesity 2011, 19, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Mallard, S.R.; Howe, A.S.; Houghton, L.A. Vitamin D status and weight loss: A systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials. Am. J. Clin. Nutr. 2016, 104, 1151–1159. [Google Scholar] [CrossRef]
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D Storage in Adipose Tissue of Obese and Normal Weight Women. J. Bone Miner. Res. 2017, 32, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.F. Vitamin D and Obesity/Adiposity-A Brief Overview of Recent Studies. Nutrients 2022, 14, 2049. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L.; Kahn, L.A.; Iker, E.; Ehrlich, C.; Wright, T.; McHutchison, L.; Schwartz, J.; Sleigh, M.; Donahue, P.M.; Lisson, K.H.; et al. Standard of care for lipedema in the United States. Phlebology 2021, 36, 779–796. [Google Scholar] [CrossRef]
- Via, M. The malnutrition of obesity: Micronutrient deficiencies that promote diabetes. ISRN Endocrinol. 2012, 2012, 103472. [Google Scholar] [CrossRef] [PubMed]
- Guardiola-Marquez, C.E.; Santos-Ramirez, M.T.; Segura-Jimenez, M.E.; Figueroa-Montes, M.L.; Jacobo-Velazquez, D.A. Fighting Obesity-Related Micronutrient Deficiencies through Biofortification of Agri-Food Crops with Sustainable Fertilization Practices. Plants 2022, 11, 3477. [Google Scholar] [CrossRef]
- Tobias, D.K.; Luttmann-Gibson, H.; Mora, S.; Danik, J.; Bubes, V.; Copeland, T.; LeBoff, M.S.; Cook, N.R.; Lee, I.M.; Buring, J.E.; et al. Association of Body Weight with Response to Vitamin D Supplementation and Metabolism. JAMA Netw. Open 2023, 6, e2250681. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar]
- Gordon, C.M.; DePeter, K.C.; Feldman, H.A.; Grace, E.; Emans, S.J. Prevalence of vitamin D deficiency among healthy adolescents. Arch. Pediatr. Adolesc. Med. 2004, 158, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J. Vitamin D: Emerging roles in infection and immunity. Expert. Rev. Anti Infect. Ther. 2010, 8, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Ghods, M.; Georgiou, I.; Schmidt, J.; Kruppa, P. Disease progression and comorbidities in lipedema patients: A 10-year retrospective analysis. Dermatol. Ther. 2020, 33, e14534. [Google Scholar] [CrossRef] [PubMed]
- Dudek, J.E.; Bialaszek, W.; Ostaszewski, P. Quality of life in women with lipoedema: A contextual behavioral approach. Qual. Life Res. 2016, 25, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Fife, C.E.; Maus, E.A.; Carter, M.J. Lipedema: A frequently misdiagnosed and misunderstood fatty deposition syndrome. Adv. Skin. Wound Care 2010, 23, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, bnae009. [Google Scholar] [CrossRef]
- Buscemi, S.; Buscemi, C.; Corleo, D.; De Pergola, G.; Caldarella, R.; Meli, F.; Randazzo, C.; Milazzo, S.; Barile, A.M.; Rosafio, G.; et al. Obesity and Circulating Levels of Vitamin D before and after Weight Loss Induced by a Very Low-Calorie Ketogenic Diet. Nutrients 2021, 13, 1829. [Google Scholar] [CrossRef] [PubMed]
- Lespessailles, E.; Toumi, H. Vitamin D alteration associated with obesity and bariatric surgery. Exp. Biol. Med. 2017, 242, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Goldner, W.S.; Stoner, J.A.; Lyden, E.; Thompson, J.; Taylor, K.; Larson, L.; Erickson, J.; McBride, C. Finding the optimal dose of vitamin D following Roux-en-Y gastric bypass: A prospective, randomized pilot clinical trial. Obes. Surg. 2009, 19, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Carlin, A.M.; Rao, D.S.; Yager, K.M.; Parikh, N.J.; Kapke, A. Treatment of vitamin D depletion after Roux-en-Y gastric bypass: A randomized prospective clinical trial. Surg. Obes. Relat. Dis. 2009, 5, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.T.; Nakhoul, N.; Akl, E.A.; Mantzoros, C.S.; El Hajj Fuleihan, G.A. Guidelines on vitamin D replacement in bariatric surgery: Identification and systematic appraisal. Metabolism 2016, 65, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, I.; Goldberg, G.R.; Prentice, A. Abundant sunshine and vitamin D deficiency. Br. J. Nutr. 2008, 99, 1171–1173. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Bolland, M.J.; Grey, A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-analysis. Lancet 2014, 383, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; Hosking, D.; Lippuner, K.; Norquist, J.M.; Wehren, L.; Maalouf, G.; Ragi-Eis, S.; Chandler, J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: An international epidemiological investigation. J. Intern. Med. 2006, 260, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.J.; Edelman, M.; Uwaifo, G.I.; Freedman, R.J.; Semega-Janneh, M.; Reynolds, J.; Yanovski, J.A. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J. Clin. Endocrinol. Metab. 2004, 89, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Poojari, A.; Dev, K.; Rabiee, A. Lipedema: Insights into Morphology, Pathophysiology, and Challenges. Biomedicines 2022, 10, 3081. [Google Scholar] [CrossRef] [PubMed]
- Verde, L.; Camajani, E.; Annunziata, G.; Sojat, A.; Marina, L.V.; Colao, A.; Caprio, M.; Muscogiuri, G.; Barrea, L. Ketogenic Diet: A Nutritional Therapeutic Tool for Lipedema? Curr. Obes. Rep. 2023, 12, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Della Nera, G.; Sabatino, L.; Gaggini, M.; Gorini, F.; Vassalle, C. Vitamin D Determinants, Status, and Antioxidant/Anti-inflammatory-Related Effects in Cardiovascular Risk and Disease: Not the Last Word in the Controversy. Antioxidants 2023, 12, 948. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Han, S.N. The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation. J. Lipid Atheroscler. 2021, 10, 130–144. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).