Management of Neonatal Hepatic Hemangiomas: A Single-Center Experience Focused on Challenging Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Characteristics
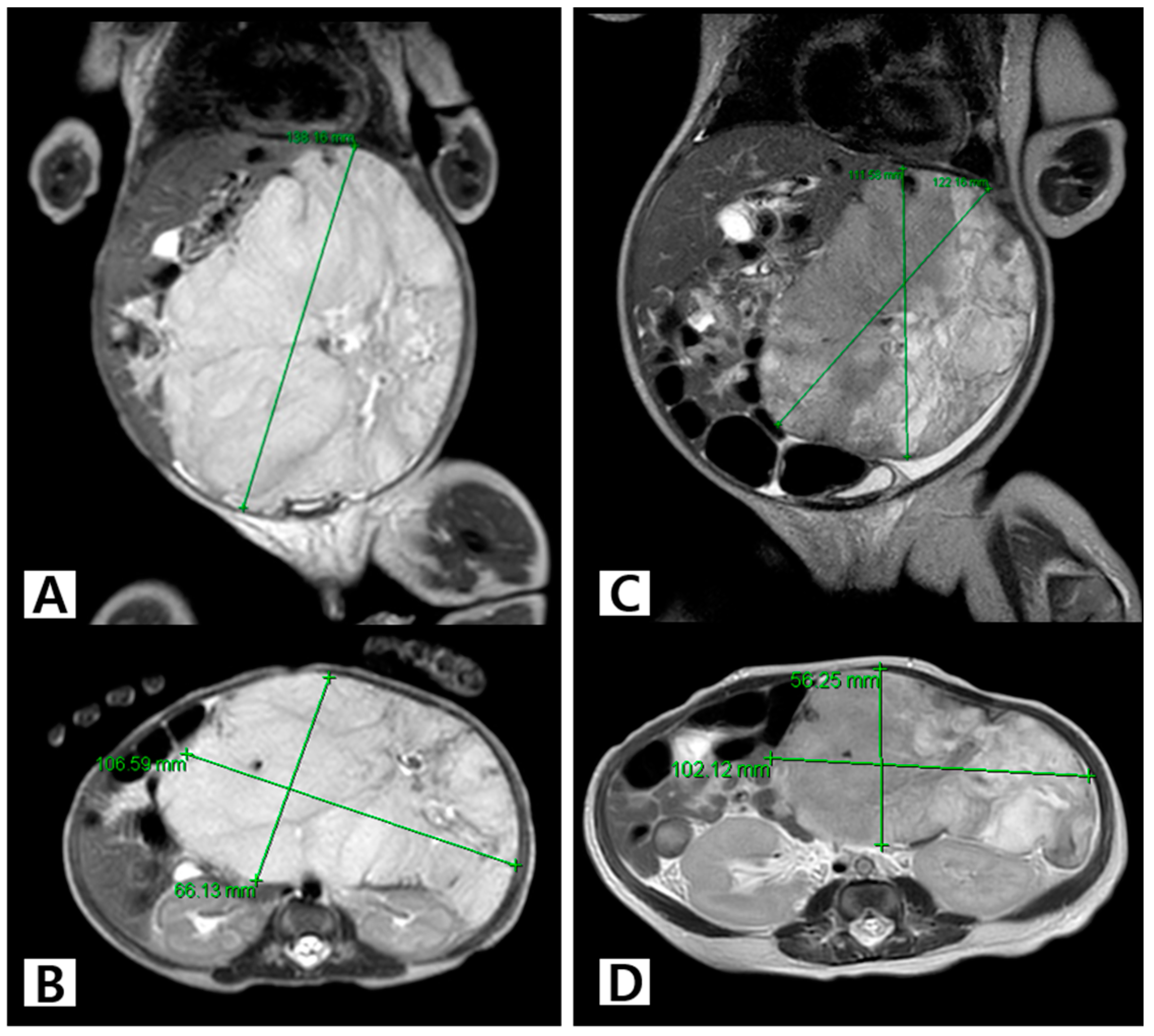
2.2. Diagnosis and Measurement of HH
2.3. Measurement of Half-Life of Serum Alpha-Fetoprotein
2.4. Treatment Indications, Options, and Outcomes
2.5. Study Outcomes and Statistical Analysis
3. Results
3.1. Demographic Characteristics and Clinical Outcomes of the Study Population
3.2. Individual Clinical Courses and Outcomes of the Treatment Group
3.3. Trends in HH Size in the First 2 Years of Life
3.4. Trends in Serum AFP Levels and Half-Life in the First 2 Years of Life
3.5. Treatment and Outcomes of Challenging Cases
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makin, E.; Davenport, M. Fetal and neonatal liver tumours. Early Hum. Dev. 2010, 86, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Al, I.O.; Demirağ, B.; Erdem, M.; Genç, S.; Karapinar, T.H. A Retrospective Analysis of Clinical Characteristics, Treatment Modalities and Outcome of the Patients with Infantile Hepatic Hemangiomas: Single-center Experience from Turkey. J. Pediatr. Hematol. Oncol. 2023, 45, e259–e265. [Google Scholar]
- Zavras, N.; Dimopoulou, A.; Machairas, N.; Paspala, A.; Vaos, G. Infantile hepatic hemangioma: Current state of the art, controversies, and perspectives. Eur. J. Pediatr. 2020, 179, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Triana, P.; Rodríguez-Laguna, L.; Giacaman, A.; Salinas-Sanz, J.A.; Martín-Santiago, A.; López-Santamaría, M.; Palacios, E.; Beato, M.J.; Martinez-González, V.; López-Gutierrez, J.C. Congenital hepatic hemangiomas: Clinical, histologic, and genetic correlation. J. Pediatr. Surg. 2020, 55, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, I.; Phung, T.L.; Adams, D.M.; Trenor, C.C.; Blei, F.; Fishman, D.S.; Hammill, A.; Masand, P.M.; Fishman, S.J. Guidance Document for Hepatic Hemangioma (Infantile and Congenital) Evaluation and Monitoring. J. Pediatr. 2018, 203, 294–300.e2. [Google Scholar] [CrossRef]
- North, P.E.; Waner, M.; Mizeracki, A.; Mihm, M.C. GLUT1: A newly discovered immunohistochemical marker for juvenile hemangiomas. Hum. Pathol. 2000, 31, 11–22. [Google Scholar] [CrossRef]
- Christison-Lagay, E.R.; Burrows, P.E.; Alomari, A.; Dubois, J.; Kozakewich, H.P.; Lane, T.S.; Paltiel, H.J.; Klement, G.; Mulliken, J.B.; Fishman, S.J. Hepatic hemangiomas: Subtype classification and development of a clinical practice algorithm and registry. J. Pediatr. Surg. 2007, 42, 62–67; discussion 67–68. [Google Scholar] [CrossRef]
- Nam, S.H.; Cho, M.J.; Kim, D.Y.; Kim, S.C. Half-life of alpha-fetoprotein in neonatal sacrococcygeal teratoma. J. Pediatr. Surg. 2018, 53, 2470–2474. [Google Scholar] [CrossRef]
- Pott Bärtsch, E.M.; Paek, B.W.; Yoshizawa, J.; Goldstein, R.B.; Ferrell, L.D.; Coakley, F.V.; Harrison, M.R.; Albanese, C.T. Giant fetal hepatic hemangioma. Case report and literature review. Fetal Diagn. Ther. 2003, 18, 59–64. [Google Scholar] [CrossRef]
- Sur, A.; Manraj, H.; Lavoie, P.M.; Lim, K.; Courtemanche, D.; Brooks, P.; Albersheim, S. Multiple Successful Angioembolizations for Refractory Cardiac Failure in a Preterm with Rapidly Involuting Congenital Hemangioma. Am. J. Perinatol. Rep. 2016, 6, e99–e103. [Google Scholar] [CrossRef]
- Morimura, Y.; Fujimori, K.; Ishida, T.; Ito, A.; Nomura, Y.; Sato, A. Fetal hepatic hemangioma representing non-reassuring pattern in fetal heart rate monitoring. J. Obstet. Gynaecol. Res. 2003, 29, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Franchi-Abella, S.; Foetale, S.-G.; Gorincour, G.; Avni, F.; Guibaud, L.; Chevret, L.; Pariente, D. Hepatic haemangioma—Prenatal imaging findings, complications and perinatal outcome in a case series. Pediatr. Radiol. 2012, 42, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Geng, X.-P.; Chen, K.-S.; He, Q.-M.; Li, X.-F.; Yang, B.-Y.; Fang, Q. Huge fetal hepatic Hemangioma: Prenatal diagnosis on ultrasound and prognosis. BMC Pregnancy Childbirth 2018, 18, 2. [Google Scholar]
- Finegold, M.J.; Egler, R.A.; Goss, J.A.; Guillerman, R.P.; Karpen, S.J.; Krishnamurthy, R.; O’Mahony, C.A. Liver tumors: Pediatric population. Liver Transplant. 2008, 14, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Itinteang, T.; Chibnall, A.M.; Marsh, R.; Dunne, J.C.; de Jong, S.; Davis, P.F.; Leadbitter, P.; Tan, S.T. Elevated Serum Levels of Alpha-Fetoprotein in Patients with Infantile Hemangioma Are Not Derived from within the Tumor. Front. Surg. 2016, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-J.; Lee, Y.S.; Song, Y.S.; Park, C.K.; Shim, S.I.; Kang, C.S.; Lee, K.-Y. Infantile hemangioendothelioma with elevated serum α fetoprotein: Report of 2 cases with immunohistochemical analysis. Hum. Pathol. 2010, 41, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Book, L.; Sudar, K. Serum alpha fetoprotein (AFP) levels in normal infants. Pediatr. Res. 1981, 15, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Maibach, R.; Roebuck, D.; Brugieres, L.; Capra, M.; Brock, P.; Dall’igna, P.; Otte, J.-B.; De Camargo, B.; Zsiros, J.; Zimmermann, A.; et al. Prognostic stratification for children with hepatoblastoma: The SIOPEL experience. Eur. J. Cancer 2012, 48, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Blohm, M.E.G.; Vesterling-Hörner, D.; Calaminus, G.; Göbel, U. Alpha1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr. Hematol. Oncol. 1998, 15, 135–142. [Google Scholar] [CrossRef]
- Krivec, J.L.; Lah, N.; Glušič, M.; Velikonja, O.; Paro-Panjan, D. Treatment of Symptomatic Focal Hepatic Hemangioma with Propranolol in Neonates: Is It Efficient? Pediatr. Gastroenterol. Hepatol. Nutr. 2023, 26, 70–77. [Google Scholar] [CrossRef]
- Storch, C.; Hoeger, P. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br. J. Dermatol. 2010, 163, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Hermans, D.J.J.; van Beynum, I.M.; Kool, L.J.S.; van de Kerkhof, P.C.M.; Wijnen, M.H.W.A.; van der Vleuten, C.J.M. Propranolol, a very promising treatment for ulceration in infantile hemangiomas: A study of 20 cases with matched historical controls. J. Am. Acad. Dermatol. 2011, 64, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cai, P.; Zhu, J.; Chen, J.; Wu, B.; Gu, Z.; Huang, S.; Wang, J. Neonatal giant hepatic hemangioma: A case report. Medicine 2018, 97, e12863. [Google Scholar] [CrossRef] [PubMed]
- Vergine, G.; Marsciani, A.; Pedini, A.; Brocchi, S.; Marsciani, M.; Desiderio, E.; Bertelli, S.; Vecchi, V. Efficacy of propranolol treatment in thyroid dysfunction associated with severe infantile hepatic hemangioma. Horm. Res. Paediatr. 2012, 78, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Hammill, A.M.; Wentzel, M.; Gupta, A.; Nelson, S.; Lucky, A.; Elluru, R.; Dasgupta, R.; Azizkhan, R.G.; Adams, D.M. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr. Blood Cancer 2011, 57, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Queisser, A.; Seront, E.; Boon, L.M.; Vikkula, M. Genetic Basis and Therapies for Vascular Anomalies. Circ. Res. 2021, 129, 155–173. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Z.; Tan, L.; Luo, J. Giant Hepatic Hemangioma Regressed Significantly without Surgical Management: A Case Report and Literature Review. Front. Med. 2021, 8, 712324. [Google Scholar] [CrossRef] [PubMed]
- Lekwuttikarn, R.; Josephs, S.; Teng, J.M. Successful Medical Management of Life-threatening Hepatic Hemangioma in Neonates. Pediatrics 2019, 144, e20191339. [Google Scholar] [CrossRef] [PubMed]
- Gnarra, M.; Behr, G.; Kitajewski, A.; Wu, J.K.; Anupindi, S.A.; Shawber, C.J.; Zavras, N.; Schizas, D.; Salakos, C.; Economopoulos, K.P. History of the infantile hepatic hemangioma: From imaging to generating a differential diagnosis. World J. Clin. Pediatr. 2016, 5, 273–280. [Google Scholar] [CrossRef]
- Macdonald, A.; Durkin, N.; Deganello, A.; Sellars, M.E.; Makin, E.; Davenport, M. Historical and Contemporary Management of Infantile Hepatic Hemangioma: A 30-year Single-center Experience. Ann. Surg. 2022, 275, e250–e255. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, M.; Chen, J.-Q.; Ma, F.; Wang, H.-H.; Lv, Y. Tumor size is not a criterion for resection during the management of giant hemangioma of the liver. Eur. J. Gastroenterol. Hepatol. 2015, 27, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Shen, Z.-C.; Fang, X.-S.; Wang, X.-M. Enucleation versus hepatectomy for hepatic hemangiomas: A meta-analysis. Front. Surg. 2022, 9, 960768. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, T.; Kumagai, M.; Nosaka, S.; Nakazawa, A.; Takimoto, T.; Hoshino, K. Critical infantile hepatic hemangioma: Results of a nationwide survey by the Japanese Infantile Hepatic Hemangioma Study Group. J. Pediatr. Surg. 2011, 46, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Tan, H.; Liu, L.; Xu, L.; Sun, Y.; Si, S.; Huang, J.; Zhou, W. Characteristics and operative treatment of extremely giant liver hemangioma >20 cm. Surgery 2017, 161, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, R.; Zhao, S.; Zhu, H.; Zhang, W.; Kong, X.; Li, P.; Ma, D.; Gao, J.; Sun, W. Safety and effectiveness of laparoscopic intratumoral resection facilitated by coagulation of giant hepatic hemangioma: A matched case–control study and literature review. Surg. Endosc. 2022, 36, 5149–5159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, F.; Ding, J.; Wei, Y.; Li, B. Surgical outcomes and quality of life between laparoscopic and open approach for hepatic hemangioma: A propensity score matching analysis. Medicine 2019, 98, e14485. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.-S.; Chen, Z.-X.; Zhao, Y.-J.; Gu, H.; Geng, X.-P.; Liu, F.-B. Outcomes of surgery for giant hepatic hemangioma. BMC Surg. 2021, 21, 186. [Google Scholar] [CrossRef]
- Kaplan, B.; Qazi, Y.; Wellen, J.R. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant. Rev. 2014, 28, 126–133. [Google Scholar] [CrossRef]





| Total (n = 87) | Treatment Group (n = 12) | Observation Group (n = 75) | p | |
|---|---|---|---|---|
| Male, n (%) | 43 (49.4) | 6 (50.0) | 37 (49.3) | 0.966 |
| Gestational age, weeks | 36.4 (32.0–38.2) | 35.4 (32.6–39.0) | 36.7 (31.8–38.2) | 0.873 |
| Birth weight, kg | 2.59 (1.72–3.26) | 3.27 (1.87–3.48) | 2.58 (1.70–3.15) | 0.193 |
| Prenatal diagnosis of HH, n (%) | 13 (14.9) | 4 (33.3) | 9 (12.0) | 0.076 |
| Age at diagnosis of HH, days | 61.0 (21.0–126.0) | 15.5 (1.0–37.8) | 75 (27–137) | 0.002 |
| Diagnosis method, n (%) | 0.001 | |||
| Only US | 45 (51.7) | 1 (8.3) | 44 (58.7) | |
| US + MRI or CT | 42 (48.3) | 11 (91.7) | 31 (41.3) | |
| Appearance of HH, n (%) | 0.027 | |||
| Focal | 61 (70.1) | 7 (58.3) | 54 (72) | |
| Multifocal | 24 (27.6) | 3 (25.0) | 21 (28) | |
| Diffuse | 2 (2.3) | 2 (16.7) | 0 (0) | |
| Heart failure, n (%) | 5 (5.7) | 3 (25.0) | 2 (2.7) | 0.018 |
| HH size, cm | ||||
| Size at initial diagnosis | 1.0 (0.4–10.3) | 2.2 (0.5–10.3) | 1.0 (0.4–4.0) | 0.035 |
| Maximum size | 1.1 (0.4–13.2) | 2.1 (0.7–13.2) | 1.1 (0.4–4.0) | 0.007 |
| Size at last follow-up | 0 (0–7.2) | 0 (0–7.2) | 0 (0–0.3.6) | 0.780 |
| Serum AFP level, initial, ng/mL | 939.7 (3.9–120,000) | 76,818.7 (231.2–120,000) | 627.2 (3.93–120,000) | 0.001 |
| Serum AFP level, last, ng/mL | 10.4 (1.3–76,818) | 98.4 (4.3–76,818.7) | 8.7 (1.3–44,439) | 0.026 |
| Treatment outcomes, n (%) | 1.000 | |||
| Complete resolution | 53 (60.9) | 8 (66.7) | 45 (60) | |
| Partial resolution | 24 (27.6) | 3 (25.0) | 21 (28) | |
| No resolution | 10 (11.5) | 1 (8.3) | 9 (12.0) | |
| Follow-up duration, days | 581 (224–946) | 631.5 (152.0–912.3) | 570.0 (224.0–963.0) | 0.622 |
| No. | Sex | Prenatal Diagnosis | Age at Diagnosis (days) | GA (wks) | BW (gm) | Associated Anomaly | Appearance | Initial/ Maximum Size (cm) * | Symptoms | Indication of Treatment ** | Medical Treatment (Days after Birth) | Embolization (Days after Birth) | Operation (Days after Birth) | Outcomes /Time to Complete Resolution (days) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Yes | 1 | 34 | 1990 | None | Focal | 5.46/5.46 | CHF | 1 | PL (4–5) +INFa (4–41) | Yes (2) | Yes, Right hemihepat-ectomy (41) | Complete/41 (at op day) | Postoperative cholestasis, delayed surgical wound healing |
| 2 | F | No | 38 | 26 | 960 | ASD | Multifocal | 0.5/0.8 | Cutaneous hemangiomas | 4 | BB (147–326) | No | No | Complete/844 | None |
| 3 | F | No | 10 | 40 | 3800 | ASD | Diffuse | 2/2.1 | KMS, CHF, hypothyroidism | 3 | BB (11–18) +PL (11–18) | No | No | No | Pulmonary hemorrhage, death |
| 4 | F | No | 29 | 40 | 3530 | Biliary atresia | Focal | 0.8/0.9 | N | 2 | BB (63–157) | No | No | Complete/115 | None |
| 5 | F | No | 6 | 34 | 1830 | ASD | Focal | 1.5/2.1 | N | 2 | BB (45–150) | No | No | Partial | None |
| 6 | M | No | 37 | 38 | 2500 | None | Multifocal | 2/2 | Cutaneous hemangiomas | 4 | BB (40–194) | No | No | Complete/666 | None |
| 7 | M | Yes | 0 | 37 | 3270 | ASD | Focal | 4.8/5.8 | Feeding difficulty | 2 | BB (11–379) | No | No | Partial | None |
| 8 | M | No | 21 | 32 | 1530 | ASD | Multifocal | 0.46/0.7 | Cutaneous hemangiomas | 4 | BB (36–295) | No | No | Complete/180 | None |
| 9 | M | No | 136 | 37 | 3260 | None | Focal | 8.8/8.8 | Asymptomatic | 2 | BB (148–221) | No | No | Partial | None |
| 10 | F | Yes | 1 | 39 | 3320 | None | Focal | 5.2/7.4 | Abdominal distension, feeding difficulty | 1 | BB (3–200) +PL (28–82) +SRL (37–200) | No | No | Partial | None |
| 11 | M | Yes | 0 | 35 | 3300 | VSD, ASD, syringomyelia | Focal | 13.8/14 | Respiratory distress, abdominal distension, jaundice, CHF, hypothyroidism | 1 | BB (6–60) +PL (13–57) +SRL (30–37) | Yes (15) | Yes, Left lateral sectionectomy of liver (61) | Complete/61 (at op day) | Septic shock after SRL, delayed surgical wound healing |
| 12 | F | No | 68 | 35 | 2270 | Beckwith–Wiedemann syndrome, ASD, cleft palate, ileal atresia | Diffuse | 0.82/1.54 | N | 2 | BB (93–233) | No | No | Partial | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Jeon, H.; Han, J.; Song, I.-K.; Baek, S.H.; Shim, S.; Eun, H.; Park, M.S.; Jang, H.; Shin, J.E.; et al. Management of Neonatal Hepatic Hemangiomas: A Single-Center Experience Focused on Challenging Cases. J. Clin. Med. 2024, 13, 2839. https://doi.org/10.3390/jcm13102839
Lee S, Jeon H, Han J, Song I-K, Baek SH, Shim S, Eun H, Park MS, Jang H, Shin JE, et al. Management of Neonatal Hepatic Hemangiomas: A Single-Center Experience Focused on Challenging Cases. Journal of Clinical Medicine. 2024; 13(10):2839. https://doi.org/10.3390/jcm13102839
Chicago/Turabian StyleLee, Sumin, Hojong Jeon, Jungho Han, In-Kyu Song, Seung Hwan Baek, Sungbo Shim, Hoseon Eun, Min Soo Park, Hyeonguk Jang, Jeong Eun Shin, and et al. 2024. "Management of Neonatal Hepatic Hemangiomas: A Single-Center Experience Focused on Challenging Cases" Journal of Clinical Medicine 13, no. 10: 2839. https://doi.org/10.3390/jcm13102839
APA StyleLee, S., Jeon, H., Han, J., Song, I.-K., Baek, S. H., Shim, S., Eun, H., Park, M. S., Jang, H., Shin, J. E., & Ihn, K. (2024). Management of Neonatal Hepatic Hemangiomas: A Single-Center Experience Focused on Challenging Cases. Journal of Clinical Medicine, 13(10), 2839. https://doi.org/10.3390/jcm13102839






