Advancing Age May Decrease Mitochondrial Activity in Cumulus Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
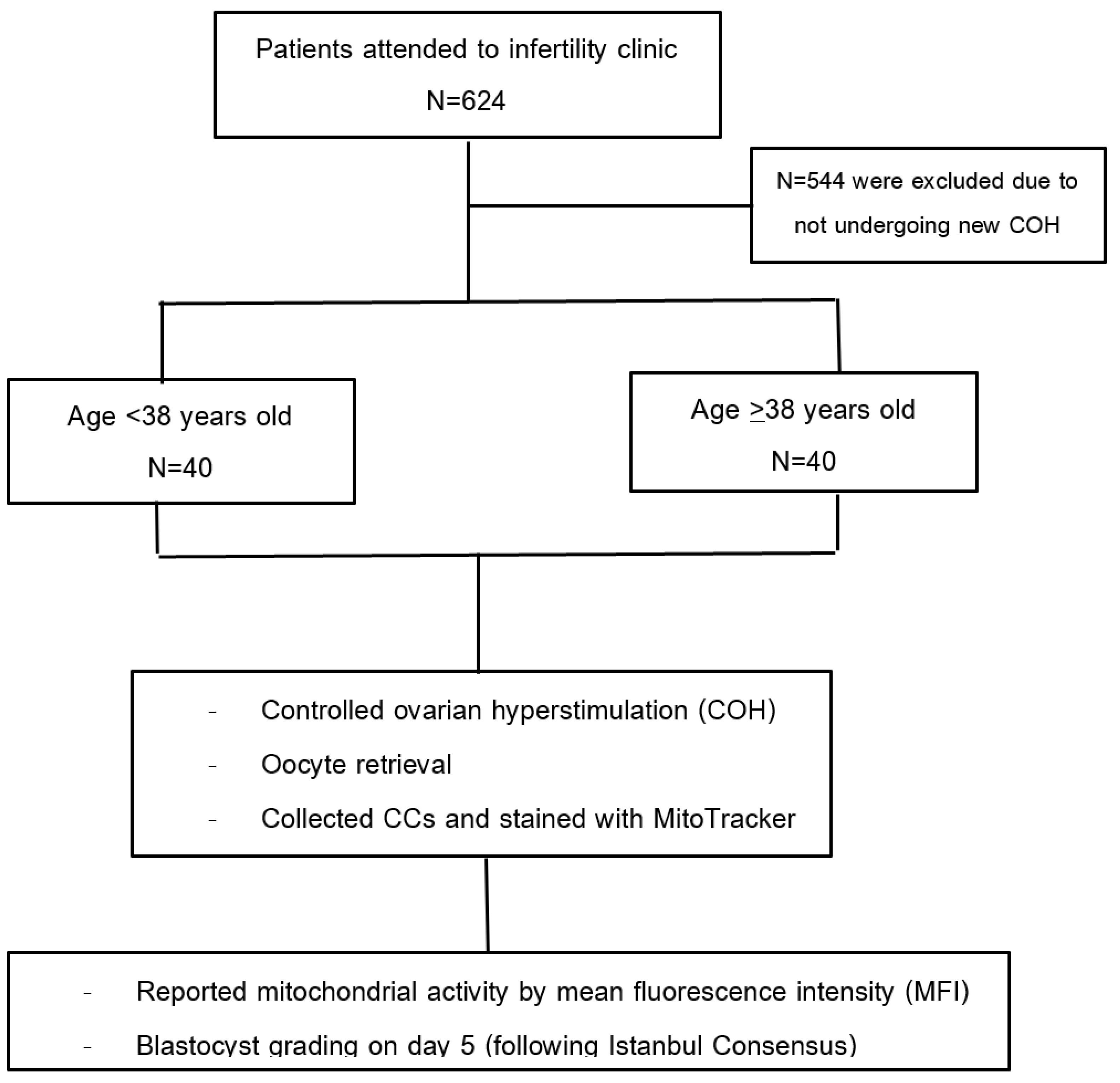
2.2. Study Population
2.3. Intervention
2.4. Ovarian Stimulation Protocol
2.5. CCs Collection Methods
2.6. Quantified Mitochondrial Activity Using MitoTracker Staining
2.7. ICSI/Embryo Culture/Embryo Grading
2.8. Statistical Analysis
3. Results
3.1. Participant Flow
3.2. Baseline Characteristics
3.3. Factors Affect Mitochondrial Mean Fluorescence Intensity (MFI)
3.4. Factors Associated with Good Blastocyst Quality
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zia, A.; Pourbagher-Shahri, A.M.; Farkhondeh, T.; Samarghandian, S. Molecular and cellular pathways contributing to brain aging. Behav. Brain Funct. 2021, 17, 6. [Google Scholar] [CrossRef]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef]
- Bentov, Y.; Casper, R.F. The aging oocyte--can mitochondrial function be improved? Fertil. Steril. 2013, 99, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.B.; Yang, Q.; Allran, K.; Taft, L.F.; Sherman, S.L. Women with a reduced ovarian complement may have an increased risk for a child with Down syndrome. Am. J. Hum. Genet. 2000, 66, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Kosiol, M.; Mayr, M.; Schulz, R.; Rohrbach, S. Mitochondria and ageing: Role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle 2017, 8, 349–369. [Google Scholar] [CrossRef]
- May-Panloup, P.; Chretien, M.F.; Malthiery, Y.; Reynier, P. Mitochondrial DNA in the oocyte and the developing embryo. Curr. Top. Dev. Biol. 2007, 77, 51–83. [Google Scholar] [CrossRef]
- Inoue, K.; Nakada, K.; Ogura, A.; Isobe, K.; Goto, Y.; Nonaka, I.; Hayashi, J.I. Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat. Genet. 2000, 26, 176–181. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef]
- Dalton, C.M.; Szabadkai, G.; Carroll, J. Measurement of ATP in single oocytes: Impact of maturation and cumulus cells on levels and consumption. J. Cell. Physiol. 2014, 229, 353–361. [Google Scholar] [CrossRef]
- Richani, D.; Dunning, K.R.; Thompson, J.G.; Gilchrist, R.B. Metabolic co-dependence of the oocyte and cumulus cells: Essential role in determining oocyte developmental competence. Hum. Reprod. Update 2021, 27, 27–47. [Google Scholar] [CrossRef]
- Richards, J.S.; Pangas, S.A. The ovary: Basic biology and clinical implications. J. Clin. Investig. 2010, 120, 963–972. [Google Scholar] [CrossRef]
- Orisaka, M.; Tajima, K.; Tsang, B.K.; Kotsuji, F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J. Ovarian Res. 2009, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Al-Edani, T.; Assou, S.; Ferrières, A.; Bringer Deutsch, S.; Gala, A.; Lecellier, C.H.; Aït-Ahmed, O.; Hamamah, S. Female aging alters expression of human cumulus cells genes that are essential for oocyte quality. Biomed. Res. Int. 2014, 2014, 964614. [Google Scholar] [CrossRef]
- Boucret, L.; Chao de la Barca, J.M.; Morinière, C.; Desquiret, V.; Ferré-L’Hôtellier, V.; Descamps, P.; Marcaillou, C.; Reynier, P.; Procaccio, V.; May-Panloup, P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum. Reprod. 2015, 30, 1653–1664. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Q.; Li, H.; Xiang, W.; Zhang, L. Cell-free mitochondrial DNA increases granulosa cell apoptosis and reduces aged oocyte blastocyst development in the mouse. Reprod. Toxicol. 2020, 98, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Ogino, M.; Tsubamoto, H.; Sakata, K.; Oohama, N.; Hayakawa, H.; Kojima, T.; Shigeta, M.; Shibahara, H. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J. Assist. Reprod. Genet. 2016, 33, 367–371. [Google Scholar] [CrossRef]
- Desquiret-Dumas, V.; Clément, A.; Seegers, V.; Boucret, L.; Ferré-L’Hotellier, V.; Bouet, P.E.; Descamps, P.; Procaccio, V.; Reynier, P.; May-Panloup, P. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum. Reprod. 2017, 32, 607–614. [Google Scholar] [CrossRef]
- Taugourdeau, A.; Desquiret-Dumas, V.; Hamel, J.F.; Chupin, S.; Boucret, L.; Ferré-L’Hotellier, V.; Bouet, P.E.; Descamps, P.; Procaccio, V.; Reynier, P.; et al. The mitochondrial DNA content of cumulus cells may help predict embryo implantation. J. Assist. Reprod. Genet. 2019, 36, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moro, Á.; Lamas-Toranzo, I.; González-Brusi, L.; Pérez-Gómez, A.; Padilla-Ruiz, E.; García-Blanco, J.; Bermejo-Álvarez, P. mtDNA content in cumulus cells does not predict development to blastocyst or implantation. Hum. Reprod. Open. 2022, 2022, hoac029. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.J.; Patounakis, G.; Juneau, C.R.; Neal, S.A.; Scott, R.T.; Seli, E. Diminished ovarian reserve and poor response to stimulation in patients <38 years old: A quantitative but not qualitative reduction in performance. Hum. Reprod. 2018, 33, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Amicarelli, F.; Carbone, M.C.; Monteleone, P.; Caserta, D.; Marci, R.; Artini, P.G.; Piomboni, P.; Focarelli, R. Cellular and molecular aspects of ovarian follicle ageing. Hum. Reprod. Update 2008, 14, 131–142. [Google Scholar] [CrossRef]
- May-Panloup, P.; Boucret, L.; Chao de la Barca, J.M.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Xu, J.; Cao, X.; Zhang, D.; Liu, M.; Liu, S.; Dong, X.; Shi, H. Mitochondrial dysfunction in cumulus cells is related to decreased reproductive capacity in advanced-age women. Fertil. Steril. 2022, 118, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Seifer, D.B.; DeJesus, V.; Hubbard, K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil. Steril. 2002, 78, 1046–1048. [Google Scholar] [CrossRef]
- The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [CrossRef] [PubMed]
- Camaioni, A.; Ucci, M.A.; Campagnolo, L.; De Felici, M.; Klinger, F.G. The process of ovarian aging: It is not just about oocytes and granulosa cells. J. Assist. Reprod. Genet. 2022, 39, 783–792. [Google Scholar] [CrossRef]
- Wilding, M.; Dale, B.; Marino, M.; di Matteo, L.; Alviggi, C.; Pisaturo, M.L.; Lombardi, L.; De Placido, G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum. Reprod. 2001, 16, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.S.; Chang, H.Y.; Kao, S.H.; Kao, C.H.; Wu, Y.C.; Yeh, S.; Tzeng, C.R.; Chang, C. Abnormal mitochondrial function and impaired granulosa cell differentiation in androgen receptor knockout mice. Int. J. Mol. Sci. 2015, 16, 9831–9849. [Google Scholar] [CrossRef]
- Jiang, Z.; Shi, C.; Han, H.; Wang, Y.; Liang, R.; Chen, X.; Shen, H. Mitochondria-related changes and metabolic dysfunction in low prognosis patients under the POSEIDON classification. Hum. Reprod. 2021, 36, 2904–2915. [Google Scholar] [CrossRef]
- Gorshinova, V.K.; Tsvirkun, D.V.; Sukhanova, I.A.; Tarasova, N.V.; Volodina, M.A.; Marey, M.V.; Smolnikova, V.U.; Vysokikh, M.Y.; Sukhikh, G.T. Cumulus cell mitochondrial activity in relation to body mass index in women undergoing assisted reproductive therapy. BBA Clin. 2017, 7, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, M.; Xu, S.; Wang, Z.; Liu, T.; Zhou, J.; Zhang, D.; Dong, X.; Pan, B.; Wang, B.; et al. Follicular fluid steroid and gonadotropic hormone levels and mitochondrial function from exosomes predict embryonic development. Front. Endocrinol. 2022, 13, 1025523. [Google Scholar] [CrossRef] [PubMed]
- Podolak, A.; Woclawek-Potocka, I.; Lukaszuk, K. The Role of Mitochondria in Human Fertility and Early Embryo Development: What Can We Learn for Clinical Application of Assessing and Improving Mitochondrial DNA? Cells 2022, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, J.; Li, C.; Liao, H.; Qin, Y.; Huang, G. Mitochondrial DNA copy number of cumulus cells is not linked to embryo implantation in good prognosis IVF patients. Reprod. Biomed. Online 2021, 42, 901–908. [Google Scholar] [CrossRef]
- Kumar, K.; Venturas, M.; Needleman, D.J.; Racowsky, C.; Wells, D. Extensive analysis of mitochondrial DNA quantity and sequence variation in human cumulus cells and assisted reproduction outcomes. Hum. Reprod. 2021, 37, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Yu, E.J.; Park, J.K.; Kim, T.H.; Eum, J.H.; Paek, S.K.; Hwang, J.Y.; Lyu, S.W.; Kim, J.Y.; Lee, W.S.; et al. The Ratio of Mitochondrial DNA to Genomic DNA Copy Number in Cumulus Cell May Serve as a Biomarker of Embryo Quality in IVF Cycles. Reprod. Sci. 2021, 28, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Van Opstal, J.; Fieuws, S.; Spiessens, C.; Soubry, A. Male age interferes with embryo growth in IVF treatment. Hum. Reprod. 2021, 36, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.; Finkler, E.; Barritt, J.; Bar-Chama, N.; Sandler, B.; Copperman, A.B.; Grunfeld, L. Paternal age and assisted reproductive technology outcome in ovum recipients. Fertil. Steril. 2009, 92, 1772–1775. [Google Scholar] [CrossRef]
- Dain, L.; Auslander, R.; Dirnfeld, M. The effect of paternal age on assisted reproduction outcome. Fertil. Steril. 2011, 95, 1–8. [Google Scholar] [CrossRef]


| Young Age Group (Age < 38) (n = 40) | Advancing Age (Age ≥ 38) (n = 40) | p-Value | |
|---|---|---|---|
| Age; years (Mean ± SD) | 32.92 ± 2.54 | 40.92 ± 2.28 | <0.001 * |
| BMI (kg/m2) | 23.03 ± 3.77 | 23.65 ± 3.65 | 0.456 |
Infertility (%)
| 26 (65.00) 14 (35.00) | 25 (62.50) 15 (37.50) | 0.816 |
| Duration of infertility; years (Mean ± SD) | 5.75 ± 3.40 | 7 ± 3.91 | 0.131 |
| Male age; years (Mean ± SD) | 37.35 ± 5.42 | 42.27 ± 5.03 | <0.001 * |
Cause of infertility (%)
| 0 10 (25.00) 8 (20.00) 6 (15.00) 16 (40.00) | 4 (10.00) 5 (12.50) 12 (30.00) 6 (15.00) 13 (32.50) | 0.157 |
Ovarian reserve (%)
| 33 (82.50) 7 (17.50) | 15 (37.50) 25 (62.50) | <0.001 * |
| AFC | 9.15 ±4.35 | 4.55 ± 2.20 | <0.001 * |
Stimulation protocol (%)
| 34 (85.00) 6 (15.00) | 29 (72.50) 11 (27.50) | 0.172 |
| Total Gn dosages, IU (Mean ± SD) | 2540.12± 619.59 | 3137 ± 736.99 | <0.001 * |
| Total day of stimulation (Mean ± SD) | 10.22 ± 0.92 | 10.20 ± 0.91 | 0.903 |
Type of ovarian triggering drug
| 3 (7.50) 7 (17.50) 30 (75.00) | 8 (20.00) 3 (7.50) 29 (72.50) | 0.163 |
| Young Age (Age < 38) (n = 40) | Advancing Age (Age ≥ 38) (n = 40) | Mean Difference | p-Value (95% CI) | |
|---|---|---|---|---|
| MFI of CCs (Mean ± SD) | 228.39 ± 121.38 | 162.68 ± 79.87 | 65.71 | 0.005 * (19.97, 111.45) |
| Number of oocytes retrieved (Mean ± SD) | 16.3 ± 9.70 | 8 ± 5.04 | 8.3 | <0.001 * (4.85, 11.74) |
| MII oocytes (%) | 80.44 ± 13.45 | 81.83 ± 16.39 | −1.38 | 0.679 (−8.06, 5.28) |
| Number of 2PN (Mean ± SD) | 9.6 ± 5.93 | 4.77 ± 3.07 | 4.82 | <0.001 * (2.72, 6.929) |
| Fertilization rate (%) | 75.37 ± 15.19 | 76.59 ± 22.85 | −1.21 | 0.780 (−9.85, 7.42) |
| Cleavage rate (%) | 98.27 ± 6.90 | 96.40 ± 13.55 | 1.87 | 0.438 (−2.91, 6.66) |
| Blastocyst rate (%) | 56.99 ± 23.26 | 62.55 ± 29.93 | −5.56 | 0.356 (−17.49, 6.37) |
| Good-quality blastocyst rate (%) | 23.56 ± 23.71 | 19.22 ± 28.65 | 4.33 | 0.463 (−7.37, 16.04) |
| Frozen embryo transfer (%) | 30 (75.00) | 28 (70.00) | 0.40 a | 0.090 (0.14, 1.15) |
| Chemical pregnancy (%) | 17 (56.67) | 10 (34.48) | 0.51 a | 0.162 (0.20, 1.30) |
| Factors | MFI | |
|---|---|---|
| Univariate Analysis p-Value (95%CI) | Multivariate Analysis p-Value (95%CI) | |
| Age ≥ 38 years | 0.005 * (−111.45, −19.97) | 0.014 (−121.00, −14.30) * |
| Obesity (BMI ≥ 25 kg/m2) | 0.850 (−49.40, 59.84) | 0.712 (−42.64, 62.01) |
| Polycystic ovarian syndrome (PCOS) | 0.591 (−160.56, 92.04) | 0.198 (−219.82, 46.34) |
| Endometrioma | 0.467 (−115.89, 53.68) | 0.524 (−114.87, 59.01) |
| Previous ovarian surgery (unilateral LOC) | 0.113 (−384.47, 41.30) | 0.055 (−408.69, 4.19) |
| Total Gn dosages | 0.006 * (−0.08, −0.01) | 0.198 (−0.01, 0.01) |
Type of ovarian triggering drug
| Ref 0.006 * (36.46, 210.06) 0.867 (−70.74, 59.74) | Ref 0.133 (−21.44, 158.22) 0.171 (−120.50, 21.77) |
| Number of oocytes retrieved | 0.897 (−2.58, 2.95) | 0.557 (−4.19, 7.71) |
| Number of 2PN | 0.991 (−4.60, 4.55) | 0.353 (−14.41, 5.22) |
| Good Quality (n = 83) | Fair or Poor Quality (n = 246) | Univariate Analysis OR (p-Value; 95%CI) | Multivariate Analysis OR (p-Value; 95%CI) | Mixed Multivariate Analysis OR (p-Value; 95%CI) | |
|---|---|---|---|---|---|
| Age ≥ 38 | 31 (37.35) | 87 (35.37) | 1.08 (0.745; 0.65–1.82) | 1.30 (0.487; 0.61, 2.75) | 1.30 (0.535; 0.56, 3.04) |
| BMI | 23.99 ± 3.91 | 23.83 ± 3.74 | 1.01 (0735; 0.94–1.07) | 1.01 (0.671; 0.94, 1.09) | 1.01’ (0.835, 1.10) |
| Total Gn dosage | 2735.24 ± 704.58 | 2708.17 ± 684.86 | 1.00 (0.757; 0.99–1.00) | 1.00 (0.832; 0.99, 1.00) | 1.00 (0.740; 0.99, 1.00) |
| Protocol | |||||
| 64 (77.11) | 210 (85.37) | Ref | Ref | Ref |
| 19 (22.89) | 36 (14.63) | 1.73 (0.084; 0.92–3.22) | 2.14 (0.038; 1.04, 4.42) * | 2.20 (0.060; 0.96, 5.02) |
Trigger drugs
| 5 (6.02) 13 (15.66) 65 (78.31) | 17 (6.91) 27 (10.98) 202 (82.11) | Ref 1.63 (0.420; 0.49–5.41) 1.09 (0.865; 0.38–3.08) | Ref 2.84 (0.155; 0.67, 12.03) 1.08 (0.887; 034–3.38) | Ref 3.46 (0.137; 0.67, 17.76) 1.10 (0.881; 0.31, 3.84) |
| Male age | 38.26 ± 5.13 | 39.95 ± 5.96 | 0.94 (0.023; 0.90, 0.99) * | 0.91 (0.003; 0.86, 0.96) * | 0.91 (0.008; 0.85, 0.97) * |
| Abnormal SA | 25 (30.12) | 102 (41.46) | 0.60 (0.068; 0.35–1.03) | 0.56 (0.047; 0.32, 0.99) * | 0.56 (0.087; 0.29, 1.08) |
| Number of oocytes retrieved | 16.19± 9.46 | 17.64 ± 11.82 | 0.98 (0.311; 0.96,−1.01) | 0.99 (0.821; 0.96, 1.02) | 0.99 (0.956; 0.95, 1.04) |
| Percent MII oocytes | 80.91 ± 12.30 | 80.39 ± 13.84 | 1.00 (0.763; 0.98, 1.02) | 1.00 (0.760; 0.99, 1.00) | 0.99 (0.793; 0.97, 1.02) |
| MFI | 195.47 ± 115.03 | 192.19 ± 97.88 | 1.00 (0.800; 0.99, 1.00) | 1.00 (0.189; 0.9, 1.001) | 0.99 (0.222; 0.99, 1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jitngamsujarit, S.; Salang, L.; Saengboonmee, C.; Sorin, S.; Thithuan, K.; Pongsritasana, T.; Sukkasame, S. Advancing Age May Decrease Mitochondrial Activity in Cumulus Cells. J. Clin. Med. 2024, 13, 2800. https://doi.org/10.3390/jcm13102800
Jitngamsujarit S, Salang L, Saengboonmee C, Sorin S, Thithuan K, Pongsritasana T, Sukkasame S. Advancing Age May Decrease Mitochondrial Activity in Cumulus Cells. Journal of Clinical Medicine. 2024; 13(10):2800. https://doi.org/10.3390/jcm13102800
Chicago/Turabian StyleJitngamsujarit, Suwichaya, Lingling Salang, Charupong Saengboonmee, Supannika Sorin, Kanyarat Thithuan, Thanida Pongsritasana, and Sineenart Sukkasame. 2024. "Advancing Age May Decrease Mitochondrial Activity in Cumulus Cells" Journal of Clinical Medicine 13, no. 10: 2800. https://doi.org/10.3390/jcm13102800
APA StyleJitngamsujarit, S., Salang, L., Saengboonmee, C., Sorin, S., Thithuan, K., Pongsritasana, T., & Sukkasame, S. (2024). Advancing Age May Decrease Mitochondrial Activity in Cumulus Cells. Journal of Clinical Medicine, 13(10), 2800. https://doi.org/10.3390/jcm13102800






