Lidocaine–Liposomes—A Promising Frontier for Transdermal Pain Management
Abstract
1. Introduction
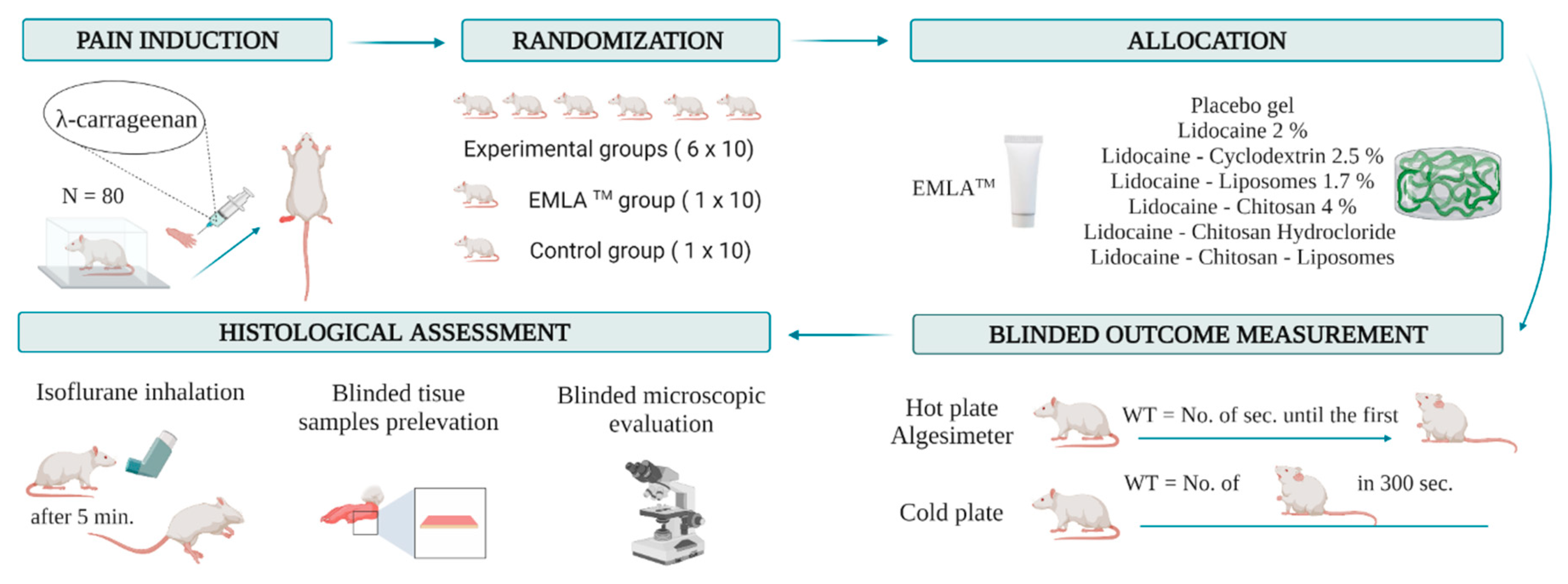
2. Materials and Methods
2.1. Formulation’s Preparation
- Quantitative determination of phospholipids
- b.
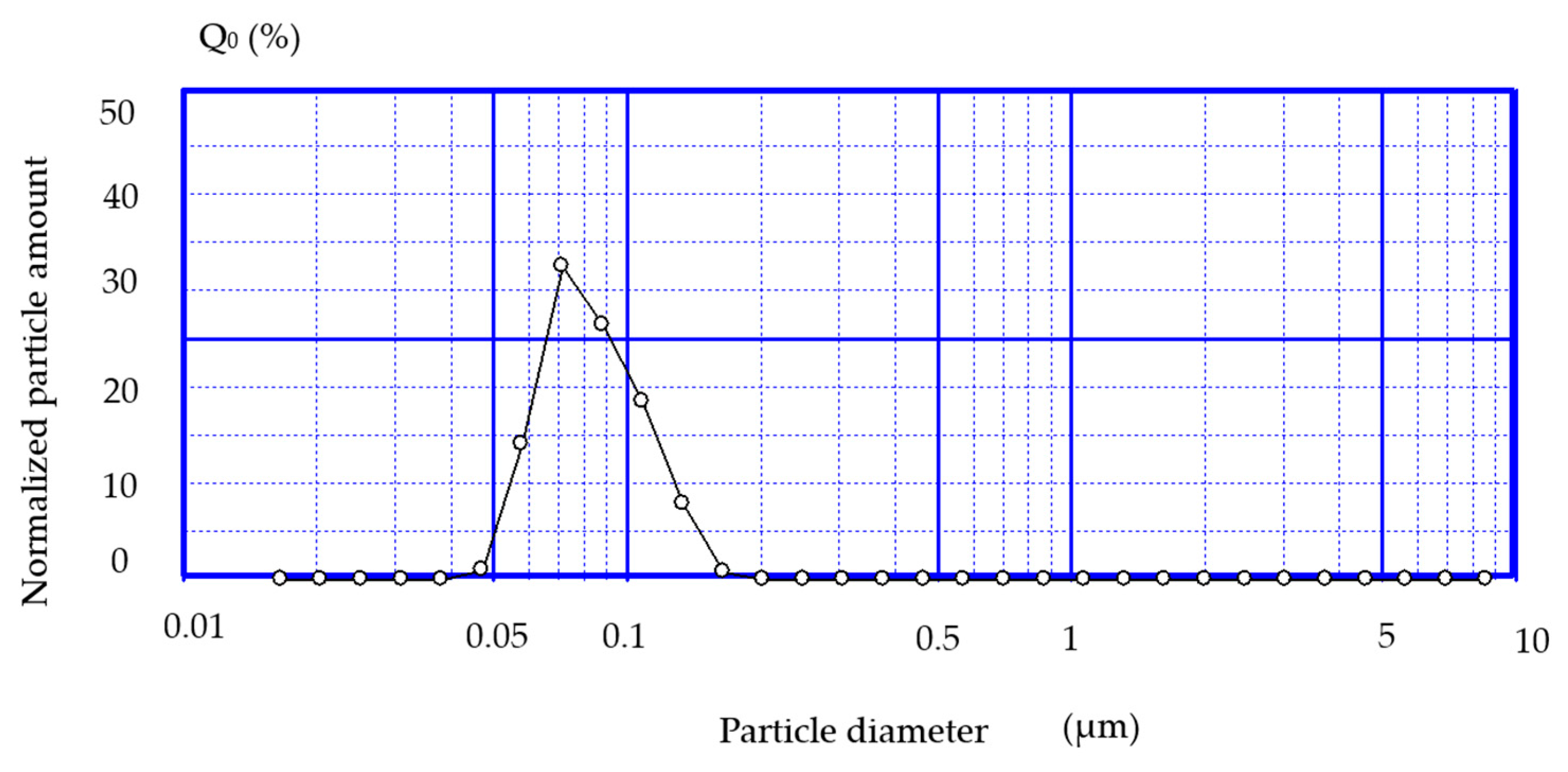
- Determining the size of liposomes
- c.
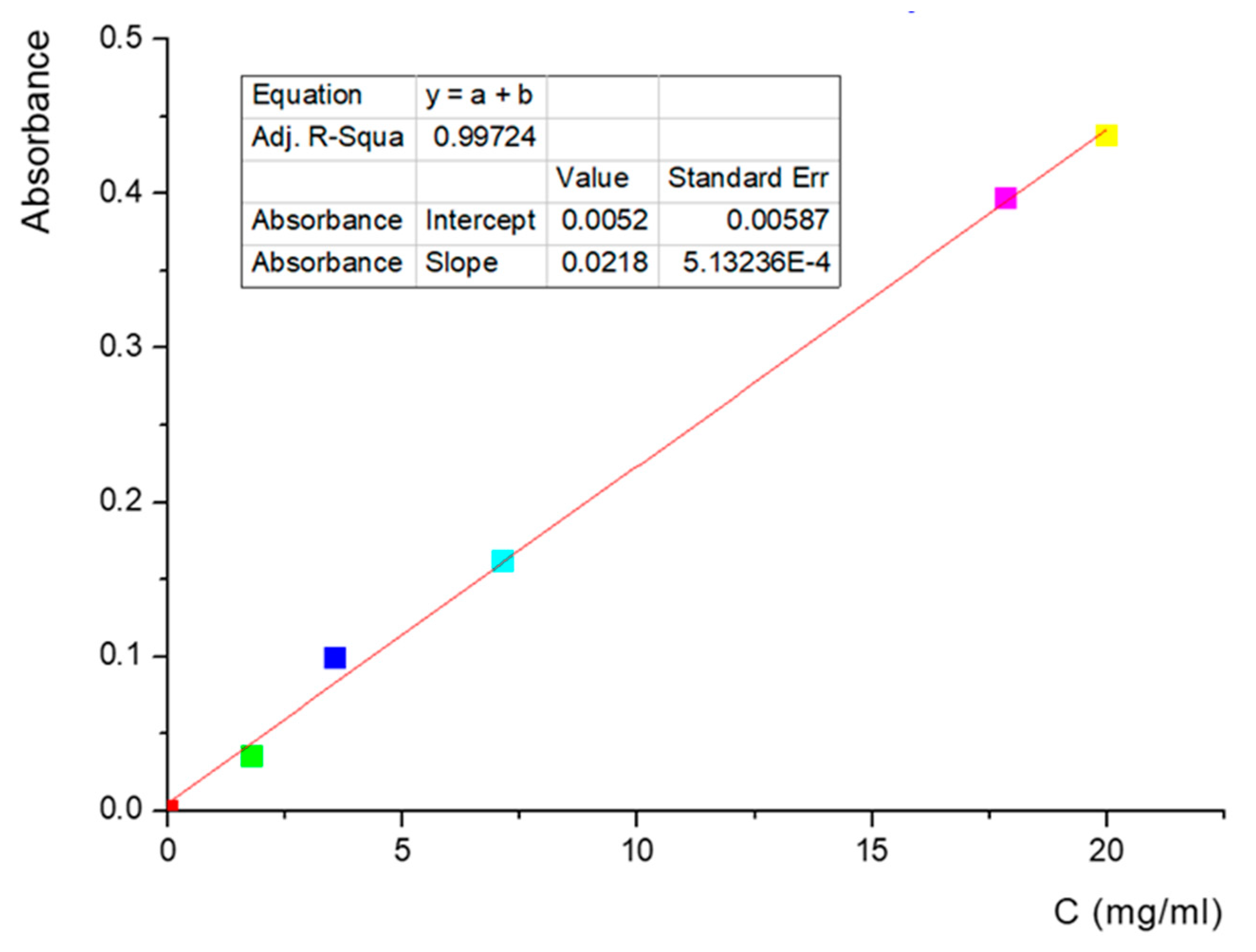
- Determination of lidocaine in liposomes
2.2. Experimental Animals
2.3. Ethical Approval
2.4. Carrageenan-Induced Inflammation
2.5. Group Allocation and Blindness
- Group C (control, hydroxyethylcellulose-based lubricant);
- Group E (EMLATM);
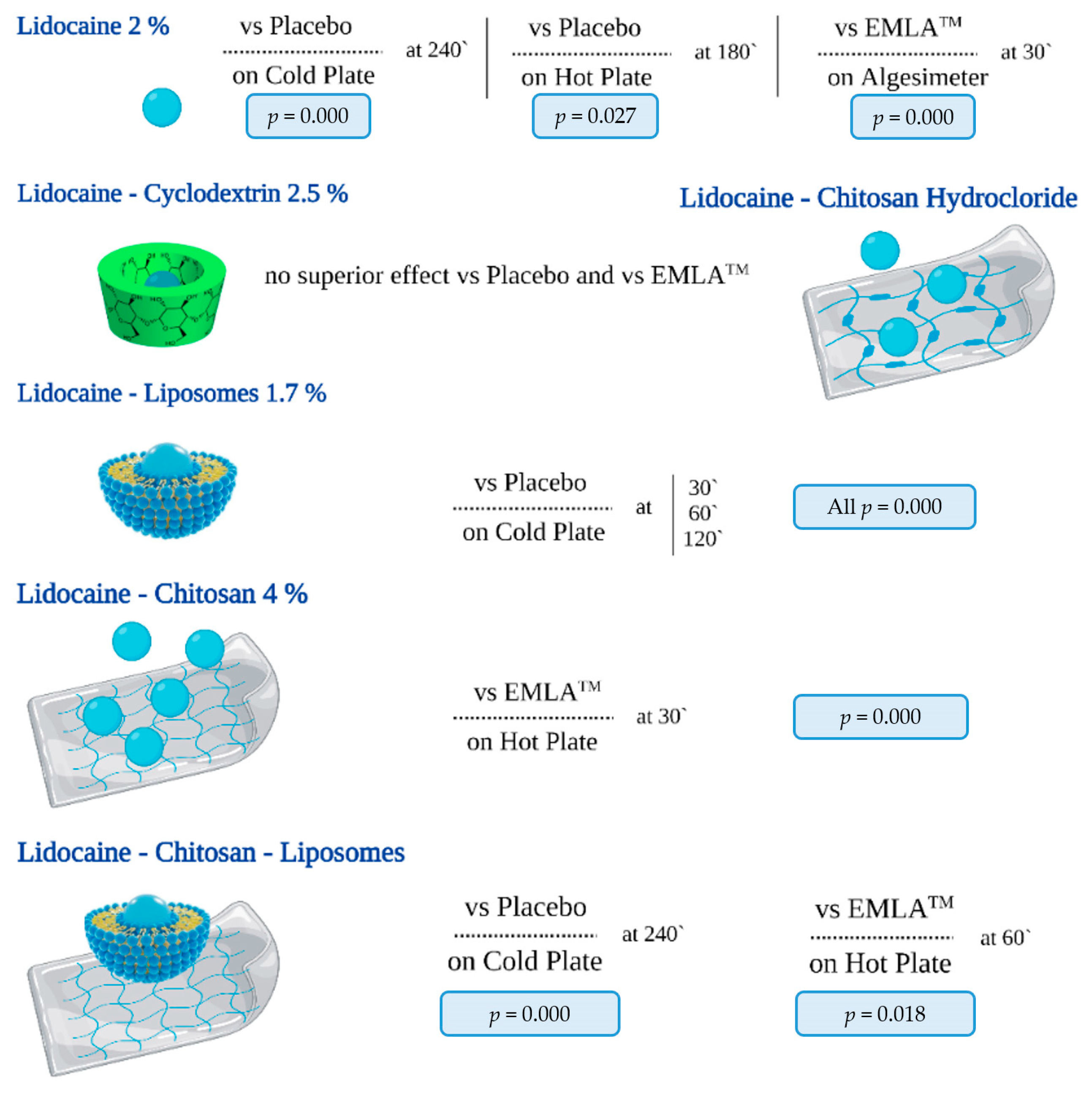
- Group L (lidocaine 2%);
- Group L-CD (lidocaine + cyclodextrin 2.5%);
- Group L-LP (lidocaine + liposomes 1.7%);
- Group L-CS (lidocaine + chitosan 4%);
- Group L-CSh (lidocaine + chitosan hydrochloride);
- Group L-CS-LP (lidocaine + chitosan + liposomes).
2.6. Behavioral Test
2.7. Euthanasia of the Experimental Animals
2.8. Histopathological Assessment
2.9. Statistical Analysis
3. Results
3.1. Primary Outcomes
3.1.1. Hot Plate
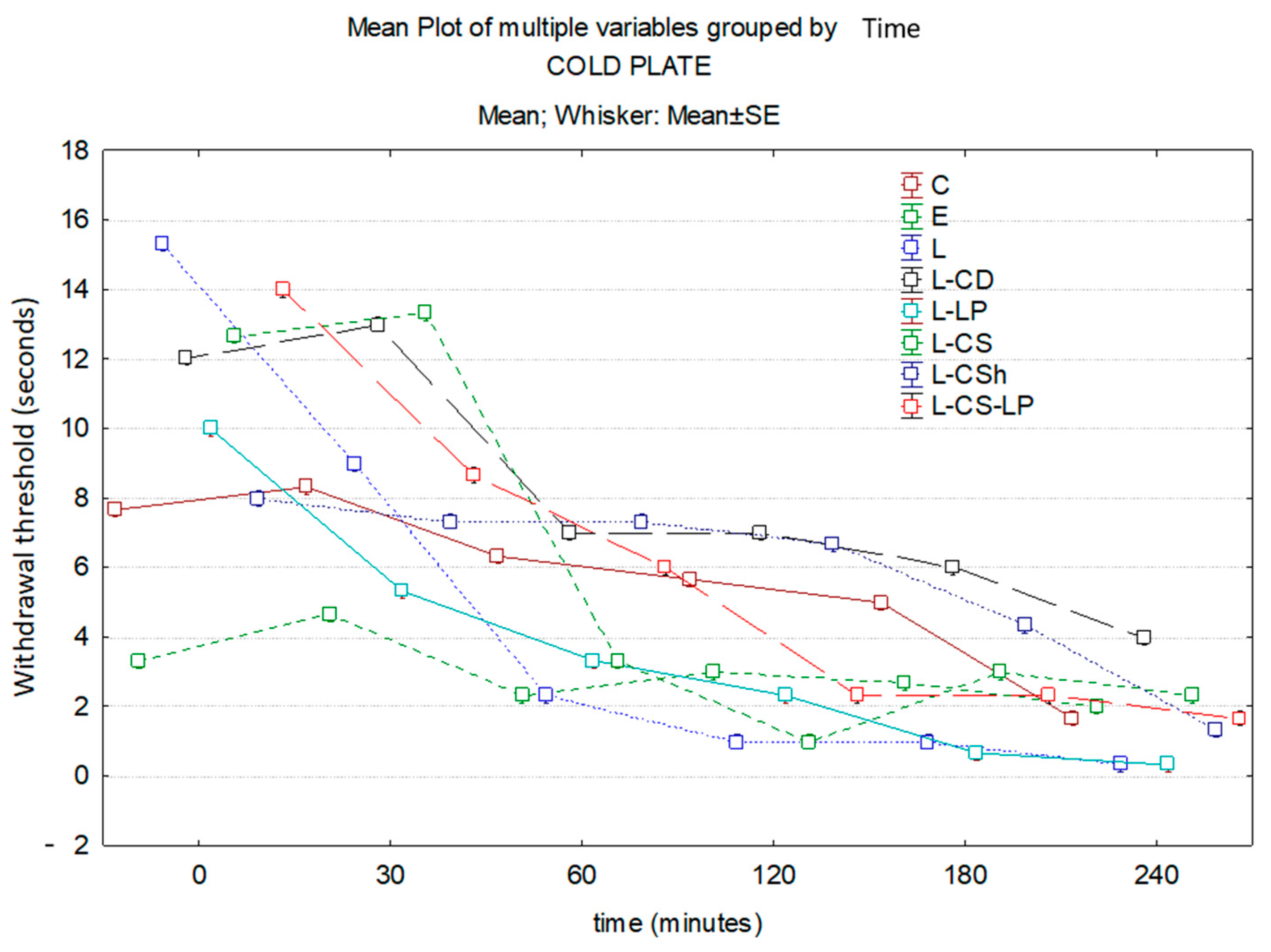
3.1.2. Cold Plate
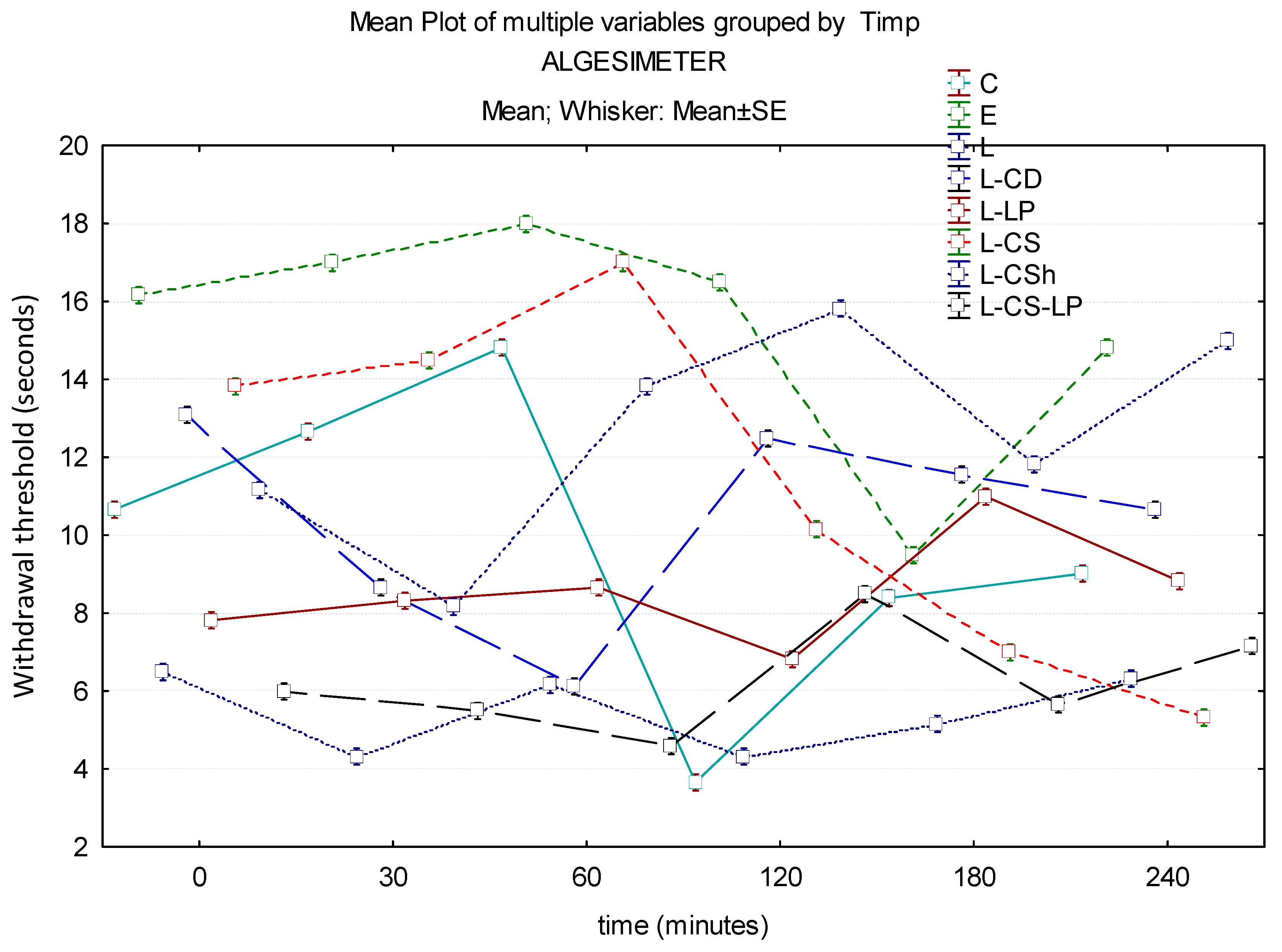
3.1.3. Algesimeter
3.2. Secondary Outcomes
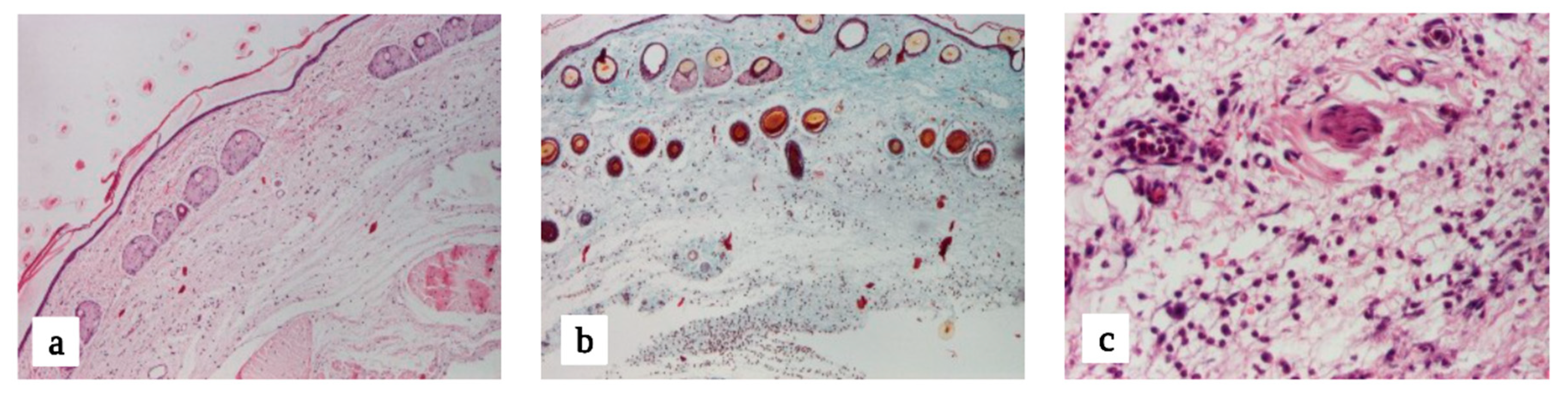
3.2.1. Macroscopic Evaluation
3.2.2. Microscopic Evaluation
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMLA | Eutectic Mixture of Local Anesthetics |
| WT | Withdrawal threshold |
| C | Control |
| L | Lidocaine 2% |
| L-CD | Lidocaine–cyclodextrin 2.5% |
| L-LP | Lidocaine–liposome 1.7% |
| L-CS | Lidocaine–chitosan 4% |
| L-CSh | Lidocaine–chitosan hydrochloride |
| L-CS-LP | Lidocaine–chitosan–liposome |
| HP | Hot plate |
| CP | Cold plate |
References
- Enright, A.; Goucke, R. The Global Burden of Pain: The Tip of the Iceberg? Anesth. Analg. 2016, 123, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Dinakar, P.; Stillman, A.M. Pathogenesis of Pain. Semin. Pediatr. Neurol. 2016, 23, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Nahm, F.S.; Han, W.K.; Lee, P.B.; Jo, J. Topical agents: A thoughtful choice for multimodal analgesia. Korean J. Anesthesiol. 2020, 73, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, X.; Mu, Y.; Li, Q.; Liu, J. A review of the mechanism of the central analgesic effect of lidocaine. Medicine 2020, 99, e19898. [Google Scholar] [CrossRef] [PubMed]
- Marcos Michel-Levy, J. Pharmacokinetics and Pharmacodynamics of Local Anesthetics; InTechOpen: London, UK, 2020; pp. 1–16. [Google Scholar]
- DeCou, J.M.; Abrams, R.S.; Hammond, J.H.; Lowder, L.R.; Gauderer, M.W. Iontophoresis: A needle-free, electrical system of local anesthesia de-livery for pediatric surgical office procedures. J. Pediatr. Surg. 1999, 34, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Kost, J. Low-frequency sonophoresis: A review. Adv. Drug Deliv. Rev. 2004, 56, 589–601. [Google Scholar] [CrossRef]
- Lener, E.V.; Bucalo, B.D.; Kist, D.A.; Moy, R.L. Topical anesthetic agents in dermatologic surgery. Dermatol. Surg. 1997, 23, 673–683. [Google Scholar] [CrossRef]
- Schmidt, J.; Metselaar, J.M.; Wauben, M.H.M.; Toyka, K.V.; Storm, G.; Gold, R. Drug-targetting by long-circulating liposomal glucocorticoids in-creases therapeutic efficacy in a model of multiple sclerosis. Brain 2003, 126, 1895–1904. [Google Scholar] [CrossRef]
- Negi, P.; Singh, B.; Sharma, G.; Beg, S.; Raza, K.; Katare, O.P. Phospholipid mi-croemulsion-based hydrogel for enhanced topical delivery of lidocaine and prilocaine: QbD-based development and evaluation. Drug Deliv. 2016, 23, 951–967. [Google Scholar] [CrossRef]
- Sun, N.; Zhu, Y.; Yuan, L.; Lang, B. Nano-liposomes of entrapment lidocaine hy-drochloride on in vitro permeability of narcotic. Pak. J. Pharm. Sci. 2015, 28, 325–328. [Google Scholar]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.I.; Kwon, I.-B.; Kim, M.H.; Lim, J.i. Simple fabrication of silver hybridized porous chitosan-based patch for transdermal drug-delivery system. Mater. Lett. 2013, 95, 48–51. [Google Scholar] [CrossRef]
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid electrospun chitosan-phospholipids nanofibers for transdermal drug delivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Wong, T.W. Microwave as skin permeation enhancer for transdermal drug delivery of chitosan-5-fluorouracil nanoparticles. Carbohydr. Polym. 2017, 157, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef] [PubMed]
- Sacha, M.; Faucon, L.; Hamon, E.; Ly, I.; Haltner-Ukomadu, E. Ex vivo transdermal absorption of a liposome formulation of diclofenac. Biomed. Pharmacother. 2019, 111, 785–790. [Google Scholar] [CrossRef]
- Lee, E.H.; Lim, S.J.; Lee, M.K. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr. Polym. 2019, 224, 115143. [Google Scholar] [CrossRef]
- Maestrelli, F.; González-Rodríguez, M.L.; Rabasco, A.M.; Mura, P. Effect of preparation technique on the properties of liposomes encapsulating ketoprofen-cyclodextrin complexes aimed for transdermal delivery. Int. J. Pharm. 2006, 312, 53–60. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Adrienne, T.B. Dermatological Drugs, Topical Agents, and Cosmetics; Elsevier: Amsterdam, The Netherlands, 2015; pp. 175–184. [Google Scholar] [CrossRef]
- McGrath, J.C.; Drummond, G.B.; McLachlan, E.M.; Kilkenny, C.; Wainwright, C.L. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Blaj, D.-A.; Balan-Porcarasu, M.; Petre, B.A.; Harabagiu, V.; Peptu, C. MALDI mass spectrometry monitoring of cyclodextrin-oligolactide derivatives synthesis. Polymer 2021, 233, 124186. [Google Scholar] [CrossRef]
- Pacharinsak, C.; Sharp, P.; Zintzsch, A.; Fuochi, S. Recognition of Pain, Distress, and Suffering. In Practical Handbook on the 3Rs in the Context of the Directive 2010/63/EU, 1st ed.; Dal Negro, G., Sabbioni, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 181–205. ISBN 978-012-821-180-9. [Google Scholar]
- Tamba, B.I.; Leon, M.M.; Petreus, T. Common trace elements alleviate pain in an experimental mouse model. J. Neurosci. Res. 2013, 91, 554–561. [Google Scholar] [CrossRef] [PubMed]
- van der Wal, S.; Cornelissen, L.; Behet, M.; Vaneker, M.; Steegers, M.; Vissers, K. Behavior of neuropathic pain in mice following chronic constriction injury comparing silk and catgut ligatures. Springerplus 2015, 4, 225. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Jaba, I.M.; Tamba, B.; Bohotin, C.; Neamtu, A.; Patras, X.; Simionescu, B.C.; Mungiu, O.C. Antinociceptive effect of morphiceptin loaded poly(butylcyanoacrylate) nanoparticles. Rev. Roum. Chim. 2010, 55, 923–931. [Google Scholar]
- Naduvilath, T.J.; John, R.K.; Dandona, L. Sample size for ophthalmology studies. Indian J. Ophthalmol. 2000, 48, 245–250. [Google Scholar]
- Bucalo, B.D.; Mirikitani, E.J.; Moy, R.L. Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol. Surg. 1998, 24, 537–541. [Google Scholar] [CrossRef]
- Milani, A.S.; Zand, V.; Abdollahi, A.A.; Froughreyhani, M.; Zakeri-Milani, P.; Jafarabadi, M.A. Effect of Topical Anesthesia with Lidocaine-prilocaine (EMLA) Cream and Local Pressure on Pain during Infiltration Injection for Maxillary Canines: A Randomized Double-blind clinical trial. JCDP 2016, 17, 592–596. [Google Scholar] [CrossRef]
- Bjerring, P.; Andersen, P.H.; Arendt-Nielsen, L. Vascular Response of Human Skin after Analgesia with Emla Cream. Br. J. Anaesth. 1989, 63, 655–660. [Google Scholar] [CrossRef]
- Wiles, M.D.; Dickson, E.; Moppett, I.K. Transient hyperaemic response to assess vascular reactivity of skin: Effect of topical anaesthesia. Br. J. Anaesth. 2008, 101, 320–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wong, B.J. Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R651–R656. [Google Scholar] [CrossRef] [PubMed]
- Strain, M.M.; Vineyard, M.A.; Roberto, M.E.; Brumley, M.R. Effectiveness of topical anesthetics on reducing tactile sensitivity in the paws of newborn rats. Dev. Psychobiol. 2014, 56, 126–132. [Google Scholar] [CrossRef]
- Jyväkorpi, M. A comparison of topical Emla cream with Bonain’s solution for anesthesia of the tympanic membrane during tympanocentesis. Eur. Arch. Otorhinolaryngol. 1996, 253, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Wiffen, P.J.; Kalso, E.A.; Bell, R.F.; Aldington, D.; Phillips, T.; Gaskell, H.; Moore, R.A. Topical analgesics for acute and chronic pain in adults—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2017, 5, CD008609. [Google Scholar] [CrossRef] [PubMed]
- Voute, M.; Morel, V.; Pickering, G. Topical Lidocaine for Chronic Pain Treatment. Drug Des. Devel Ther. 2021, 15, 4091–4103. [Google Scholar] [CrossRef]
- Sarah, S.N.; Rania, M.H.; Samar, M. Optimizing novel penetration enhancing hybridized vesicles for augmenting the in-vivo effect of an anti-glaucoma drug. Drug Deliv. 2017, 24, 99–108. [Google Scholar] [CrossRef]
- Hend, A.; Rania, M.H.; Rihab, O.; Imran, S.; Nahed, D.M. Exploiting gelatin nanocarriers in the pulmonary delivery of methotrexate for lung cancer therapy. Eur. J. Pharm. Sci. 2019, 133, 115–126. [Google Scholar] [CrossRef]
- Mick, G.; Correa-Illanes, G. Topical pain management with the 5% lidocaine medicated plaster--a review. Curr. Med. Res. Opin. 2012, 28, 937–951. [Google Scholar] [CrossRef]
- Nalamachu, S.; Crockett, R.S.; Gammaitoni, A.R.; Gould, E.M. A comparison of the lidocaine patch 5% vs naproxen 500 mg twice daily for the relief of pain associated with carpal tunnel syndrome: A 6-week, randomized, parallel-group study. Med. Gen. Med. 2006, 8, 33. [Google Scholar]
- Burch, F.; Codding, C.; Patel, N.; Sheldon, E. Lidocaine patch 5% improves pain, stiffness, and physical function in osteoarthritis pain patients. A prospective, multicenter, open-label effectiveness trial. Osteoarthr. Cartil. 2004, 12, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, A.; Nicholson, B.; Hans, G.; Brasseur, L. Outcome predictors for treatment success with 5% lidocaine medicated plaster in low back pain with neuropathic components and neuropathic pain after surgical and nonsurgical trauma. J. Pain. Res. 2011, 4, 25–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Peers, S.; Alcouffe, P.; Montembault, A.; Ladavière, C. Embedment of liposomes into chitosan physical hydrogel for the delayed release of antibiotics or anaesthetics, and its first ESEM characterization. Carbohydr. Polym. 2020, 229, 115532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, W.; Li, Q.; Li, C.; Wang, H.; Li, Y.; Cao, Y.; Chang, J.; Zhang, L. Preparation and evaluation of lidocaine hydrochloride-loaded TAT-conjugated polymeric liposomes for transdermal delivery. Int. J. Pharm. 2013, 441, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Han, S.; Qu, Z.; Zhao, J.; Chen, Y.; Chen, Z.; Duan, J.; Pan, Y.; Tang, X. Penetration enhancement of lidocaine hydrochlorid by a novel chitosan coated elastic liposome for transdermal drug delivery. J. Biomed. Nanotechnol. 2011, 7, 704–713. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nair, S.S.; Nair, A.S. Fabrication of a bioadhesive transdermal device from chitosan and hyaluronic acid for the controlled release of lidocaine. Carbohydr. Polym. 2016, 152, 687–698. [Google Scholar] [CrossRef]
- Kristl, J.; Šmid-Korbar, J.; Štruc, E.; Schara, M.; Rupprecht, H. Hydrocolloids and gels of chitosan as drug carriers. Int. J. Pharm. 1993, 99, 13–19. [Google Scholar] [CrossRef]
- Taveira, S.F.; Nomizo, A.; Lopez, R.F. Effect of the iontophoresis of a chitosan gel on doxorubicin skin penetration and cytotoxicity. J. Control Release 2009, 134, 35–40. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Xie, X.; Li, X. Development of photo-magnetic drug delivery system by facile-designed dual stimuli-responsive modified biopolymeric chitosan capped nano-vesicle to improve efficiency in the anesthetic effect and its biological investigations. J. Photochem. Photobiol. B 2020, 202, 111716. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Chi, H.; Wang, S. Local anesthetic lidocaine delivery system: Chitosan and hyaluronic acid-modified layer-by-layer lipid nanoparticles. Drug Deliv. 2016, 23, 3529–3537. [Google Scholar] [CrossRef] [PubMed]
- Mezei, M.; Gulasekharam, V. Liposomes--a selective drug delivery system for the topical route of administration. Lotion dosage form. Life Sci. 1980, 26, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, M.; Zhao, J.; Zhang, X.; Huang, Z.; Zang, Y.; Ding, Y.; Zhang, J.; Ding, Z. Transdermal Delivery of Lidocaine-Loaded Elastic Nano-Liposomes with Microneedle Array Pretreatment. Biomedicines 2021, 9, 592. [Google Scholar] [CrossRef]
- Henriksen, I.; Våagen, S.R.; Sande, S.A.; Smistad, G.; Karlsen, J. Interactions between liposomes and chitosan II: Effect of selected parameters on aggregation and leakage. Int. J. Pharm. 1997, 146, 193–203. [Google Scholar] [CrossRef]
- Diebold, Y.; Jarrín, M.; Sáez, V.; Carvalho, E.L.; Orea, M.; Calonge, M.; Seijo, B.; Alonso, M.J. Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP). Biomaterials 2007, 28, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Hasanovic, A.; Hollick, C.; Fischinger, K.; Valenta, C. Improvement in physicochemical parameters of DPPC liposomes and increase in skin permeation of aciclovir and minoxidil by the addition of cationic polymers. Eur. J. Pharm. Biopharm. 2010, 75, 148–153. [Google Scholar] [CrossRef]
- Hurler, J.; Sørensen, K.K.; Fallarero, A.; Vuorela, P.; Škalko-Basnet, N. Liposomes-in-hydrogel delivery system with mupirocin: In vitro antibiofilm studies and in vivo evaluation in mice burn model. Biomed. Res. Int. 2013, 2013, 498485. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Rehan, M.; El-Sawy, S.M.; El-bisi, M.K.; Ahmed-Farid, O.A.; Abdel-Mohdy, F.A. Lidocaine/β-cyclodextrin inclusion complex as drug delivery system. Eur. Polym. J. 2018, 108, 304–310. [Google Scholar] [CrossRef]
- Khelghati, N.; Rasmi, Y.; Farahmandan, N.; Sadeghpour, A.; Mir, S.M.; Karimian, A.; Yousefi, B. Hyperbranched polyglycerol β-cyclodextrin as magnetic platform for optimization of doxorubicin cytotoxic effects on Saos-2 bone cancerous cell line. J. Drug Deliv. Sci. Technol. 2020, 57, 101741. [Google Scholar] [CrossRef]
- Namgung, R.; Lee, Y.M.; Kim, J.; Jang, Y.; Lee, B.-H.; Kim, I.-S.; Sokkar, P.; Rhee, Y.M.; Hoffman, A.S.; Kim, W.J. Poly-cyclodextrin and poly-paclitaxel nano-assembly for anticancer therapy. Nat. Commun. 2014, 5, 3702. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liu, X.; Huang, M.; Jiang, J.; Liao, Y.-P.; Liu, Q.; Chang, C.H.; Liao, H.; Lu, J.; Wang, X. Development of self-assembled multi-arm polyrotaxanes nanocarriers for systemic plasmid delivery in vivo. Biomaterials 2019, 192, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.-K.; Costa, A.; De Rossi, C.; de Souza Carvalho-Wodarz, C.; Loretz, B.; Lehr, C.-M. Polysaccharide Submicrocarrier for Improved Pulmonary Delivery of Poorly Soluble Anti-infective Ciprofloxacin: Preparation, Characterization, and Influence of Size on Cellular Uptake. Mol. Pharm. 2018, 15, 1081–1096. [Google Scholar] [CrossRef]
- Haley, R.M.; Zuckerman, S.T.; Gormley, C.A.; Korley, J.N.; von Recum, H.A. Local delivery polymer provides sustained antifungal activity of amphotericin B with reduced cytotoxicity. Exp. Biol. Med. 2019, 244, 526–533. [Google Scholar] [CrossRef] [PubMed]



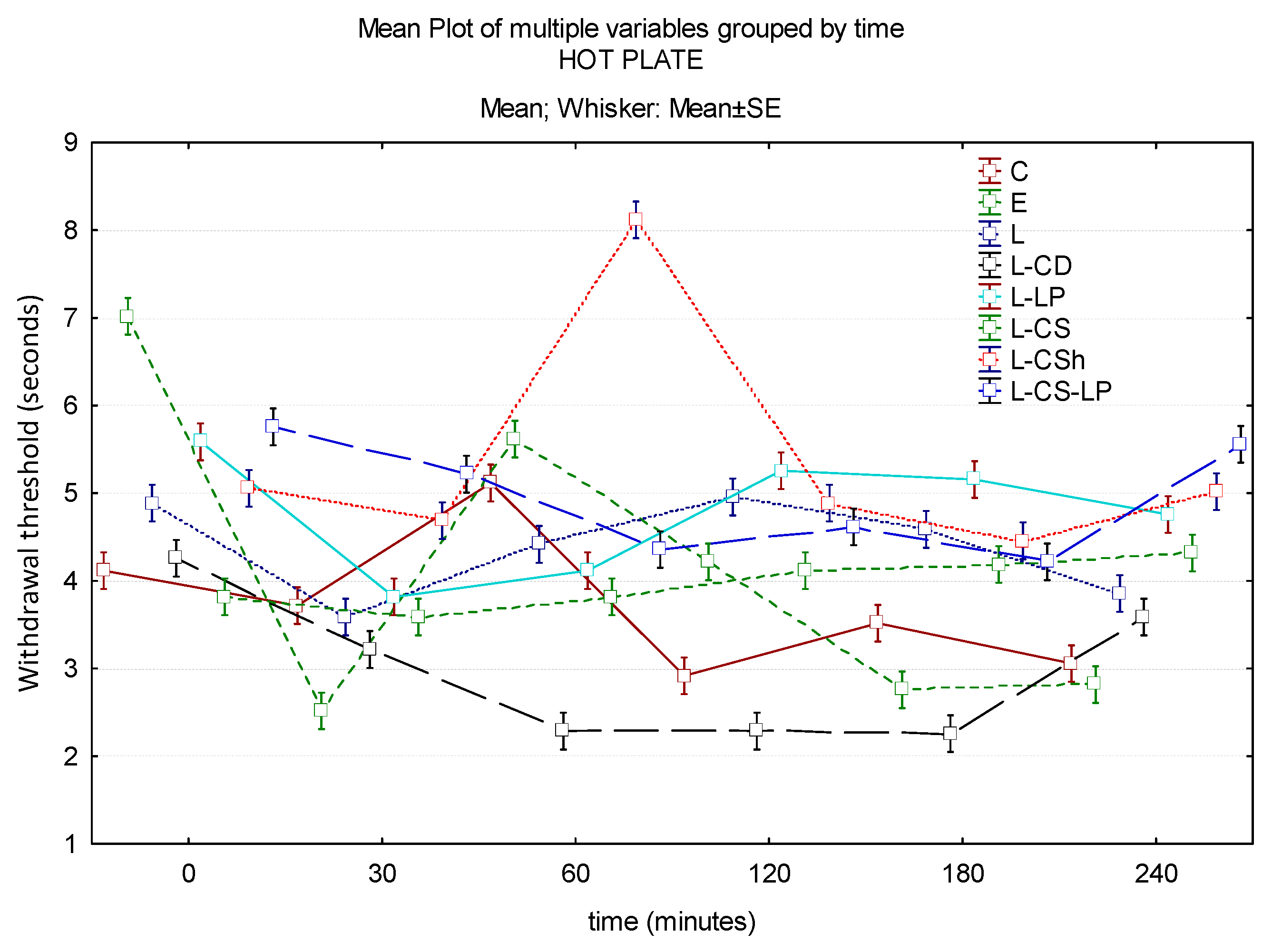

| Control | EMLA | Lidocaine 2% | Lidocaine–Cyclodextrin 2.5% | Lidocaine–Liposomes 1.7% | Lidocaine–Chitosan 4% | Lidocaine–Chitosan Hydrochloride | Lidocaine–Chitosan Liposomes | |
|---|---|---|---|---|---|---|---|---|
| Hot Plate | ||||||||
| Baseline | 4.13 s | 7.03 s | 4.9 s | 4.27 s | 5.6 s | 3.83 s | 5.07 s | 5.77 s |
| 30′ | 3.73 s | 2.53 s | 3.6 s | 3.23 s | 3.83 s | 3.6 s | 4.7 s | 5.23 s |
| 60′ | 5.13 s | 5.63 s | 4.43 s | 2.3 s | 4.13 s | 3.83 s | 8.13 s | 4.37 s |
| 120′ | 2.93 s | 4.23 s | 4.97 s | 2.3 s | 5.27 s | 4.13 s | 4.9 s | 4.63 s |
| 180′ | 3.53 s | 2.77 s | 4.6 s | 2.27 s | 5.17 s | 4.2 s | 4.47 s | 4.23 s |
| 240′ | 3.07 s | 2.83 s | 3.87 s | 3.6 s | 4.77 s | 4.33 s | 5.03 s | 5.57 s |
| Cold Plate | ||||||||
| Baseline | 7.67 s | 3.33 s | 15.33 s | 12.03 s | 10 s | 12.67 s | 8 s | 14 s |
| 30′ | 8.33 s | 4.67 s | 9 s | 13 s | 5.33 s | 13.33 s | 7.33 s | 8.67 s |
| 60′ | 6.33 s | 2.33 s | 2.33 s | 7 s | 3.33 s | 3.33 s | 7.33 s | 6 s |
| 120′ | 5.67 s | 3 s | 1 s | 7 s | 2.33 s | 1 s | 6.67 s | 2.33 s |
| 180′ | 5 s | 2.67 s | 1 s | 6 s | 0.67 s | 3 s | 4.33 s | 2.33 s |
| 240′ | 1.67 s | 2 s | 0.33 s | 4 s | 0.33 s | 2.33 s | 1.33 s | 1.67 s |
| Algesimeter | ||||||||
| Baseline | 10.67 s | 16.17 s | 6.5 s | 13.1 s | 7.83 s | 13.83 s | 11.17 s | 6 s |
| 30′ | 12.67 s | 17 s | 4.33 s | 8.67 s | 8.33 s | 14.5 s | 8.17 s | 5.5 s |
| 60′ | 14.83 s | 18 s | 6.17 s | 6.13 s | 8.67 s | 17 s | 13.83 s | 4.6 s |
| 120′ | 3.67 s | 16.5 s | 4.33 s | 12.5 s | 6.83 s | 10.17 s | 15.83 s | 8.5 s |
| 180′ | 8.4 s | 9.5 s | 5.17 s | 11.57 s | 11 s | 7 s | 11.83 s | 5.67 s |
| 240′ | 9.03 s | 14.83 s | 6.33 s | 10.67 s | 8.83 s | 5.33 s | 15 s | 7.17 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leon, M.M.; Maștaleru, A.; Oancea, A.; Alexa-Stratulat, T.; Peptu, C.A.; Tamba, B.-I.; Harabagiu, V.; Grosu, C.; Alexa, A.I.; Cojocaru, E. Lidocaine–Liposomes—A Promising Frontier for Transdermal Pain Management. J. Clin. Med. 2024, 13, 271. https://doi.org/10.3390/jcm13010271
Leon MM, Maștaleru A, Oancea A, Alexa-Stratulat T, Peptu CA, Tamba B-I, Harabagiu V, Grosu C, Alexa AI, Cojocaru E. Lidocaine–Liposomes—A Promising Frontier for Transdermal Pain Management. Journal of Clinical Medicine. 2024; 13(1):271. https://doi.org/10.3390/jcm13010271
Chicago/Turabian StyleLeon, Maria Magdalena, Alexandra Maștaleru, Andra Oancea, Teodora Alexa-Stratulat, Cătălina Anișoara Peptu, Bogdan-Ionel Tamba, Valeria Harabagiu, Cristina Grosu, Anisia Iuliana Alexa, and Elena Cojocaru. 2024. "Lidocaine–Liposomes—A Promising Frontier for Transdermal Pain Management" Journal of Clinical Medicine 13, no. 1: 271. https://doi.org/10.3390/jcm13010271
APA StyleLeon, M. M., Maștaleru, A., Oancea, A., Alexa-Stratulat, T., Peptu, C. A., Tamba, B.-I., Harabagiu, V., Grosu, C., Alexa, A. I., & Cojocaru, E. (2024). Lidocaine–Liposomes—A Promising Frontier for Transdermal Pain Management. Journal of Clinical Medicine, 13(1), 271. https://doi.org/10.3390/jcm13010271






