Imaging of Multiple Myeloma: Present and Future
Abstract
1. Introduction
2. Imaging Techniques and Algorithm
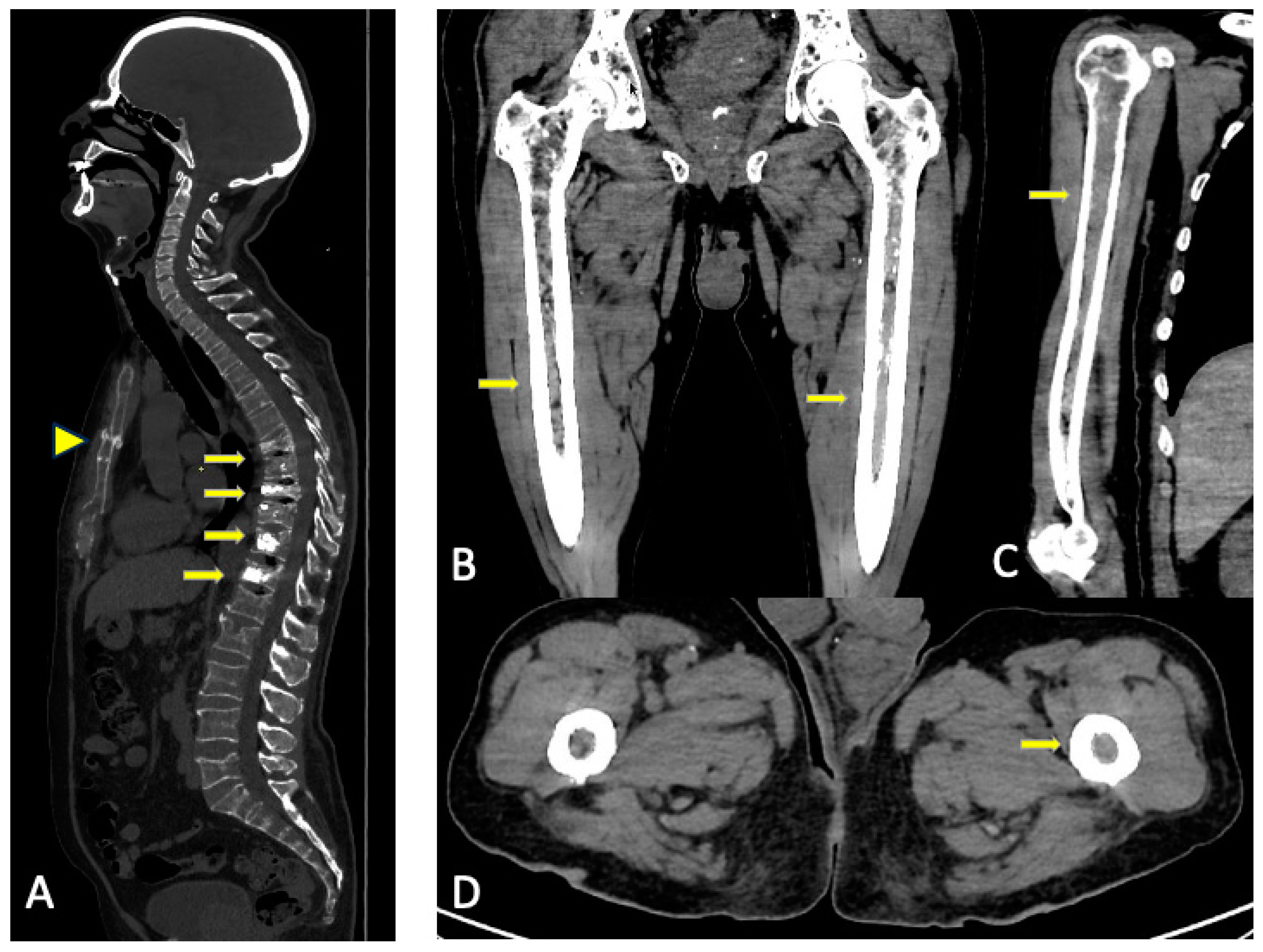
3. WBLDCT
3.1. CT Acquisition Protocol
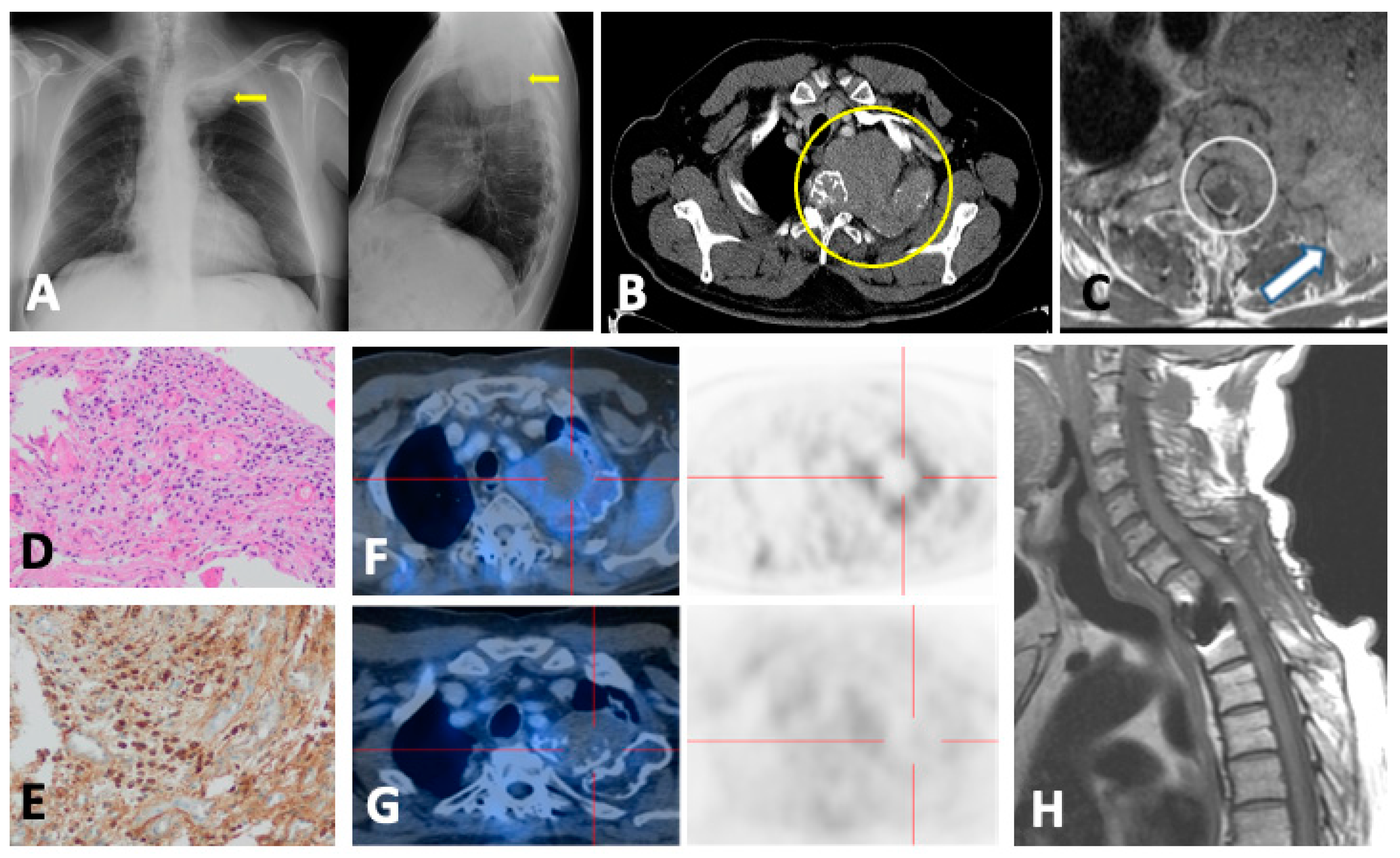
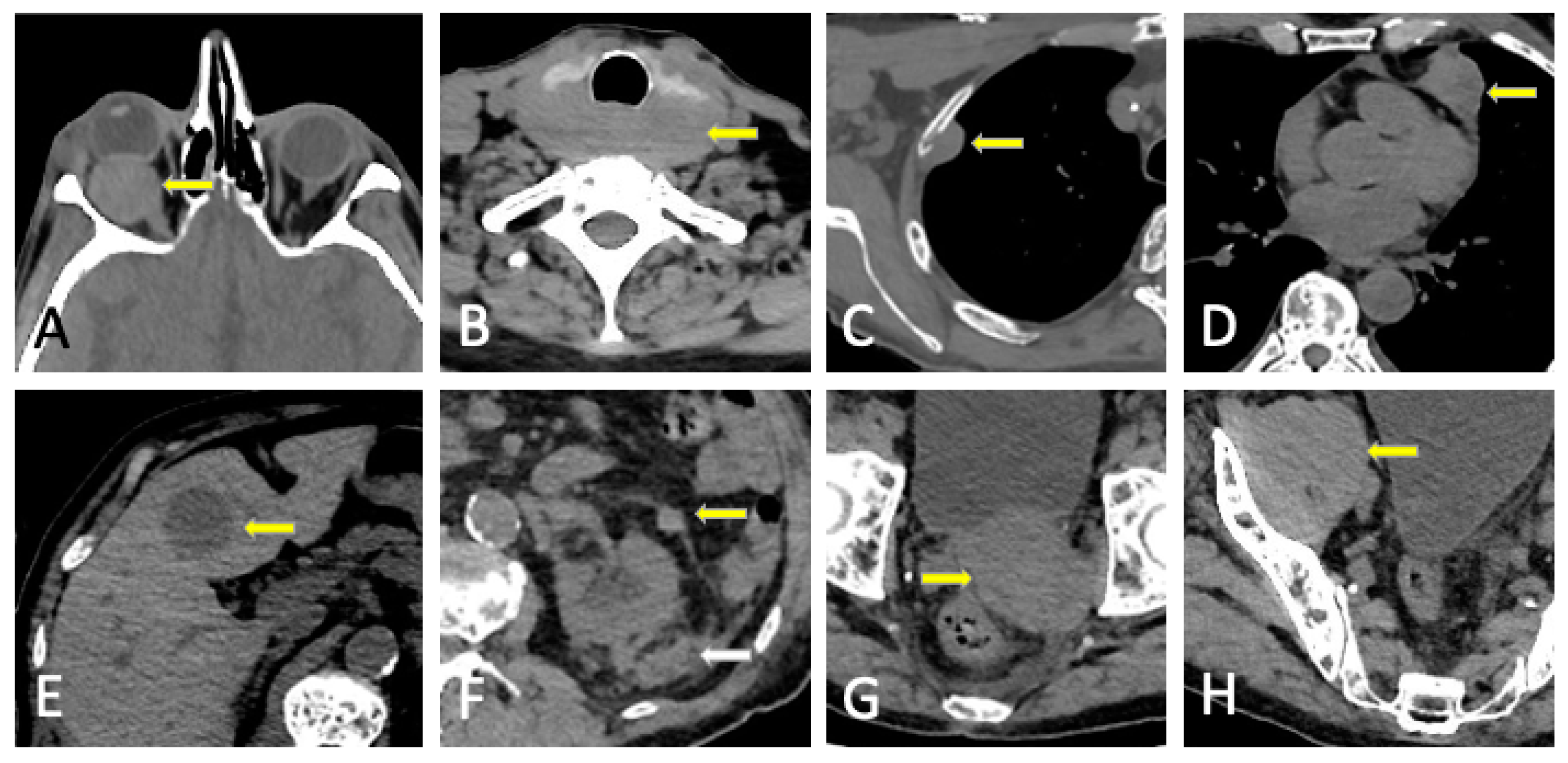
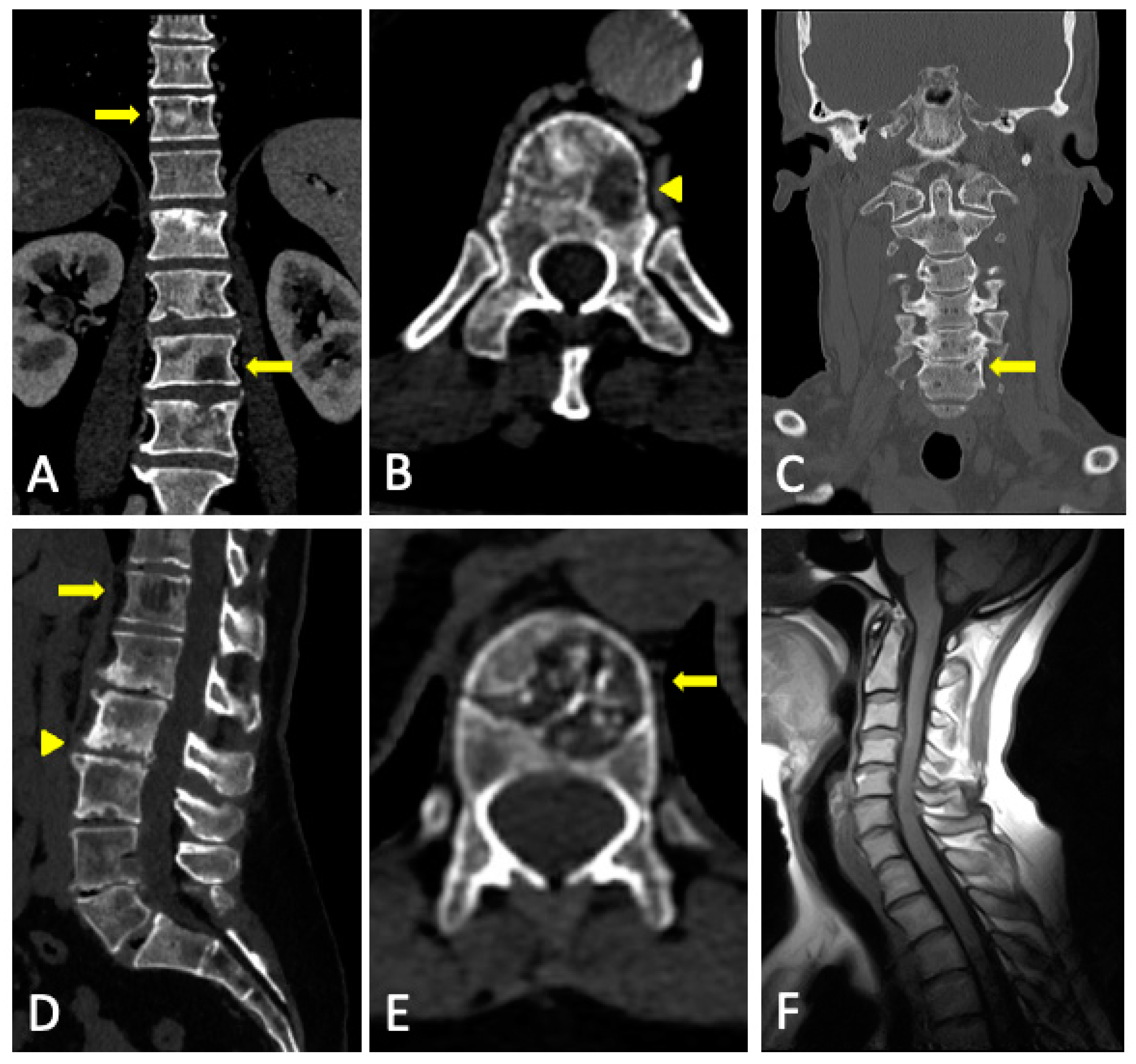
3.2. Imaging Findings
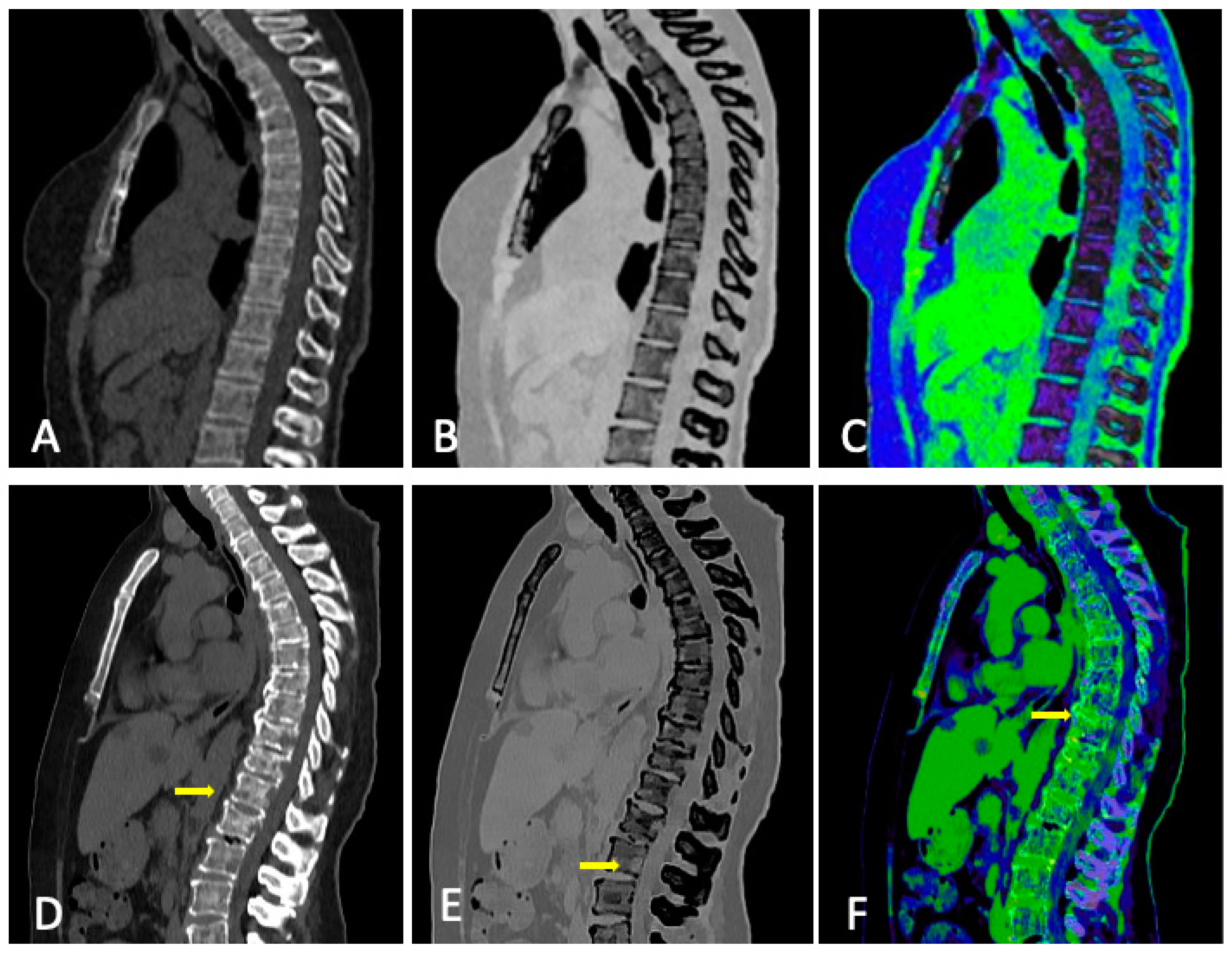
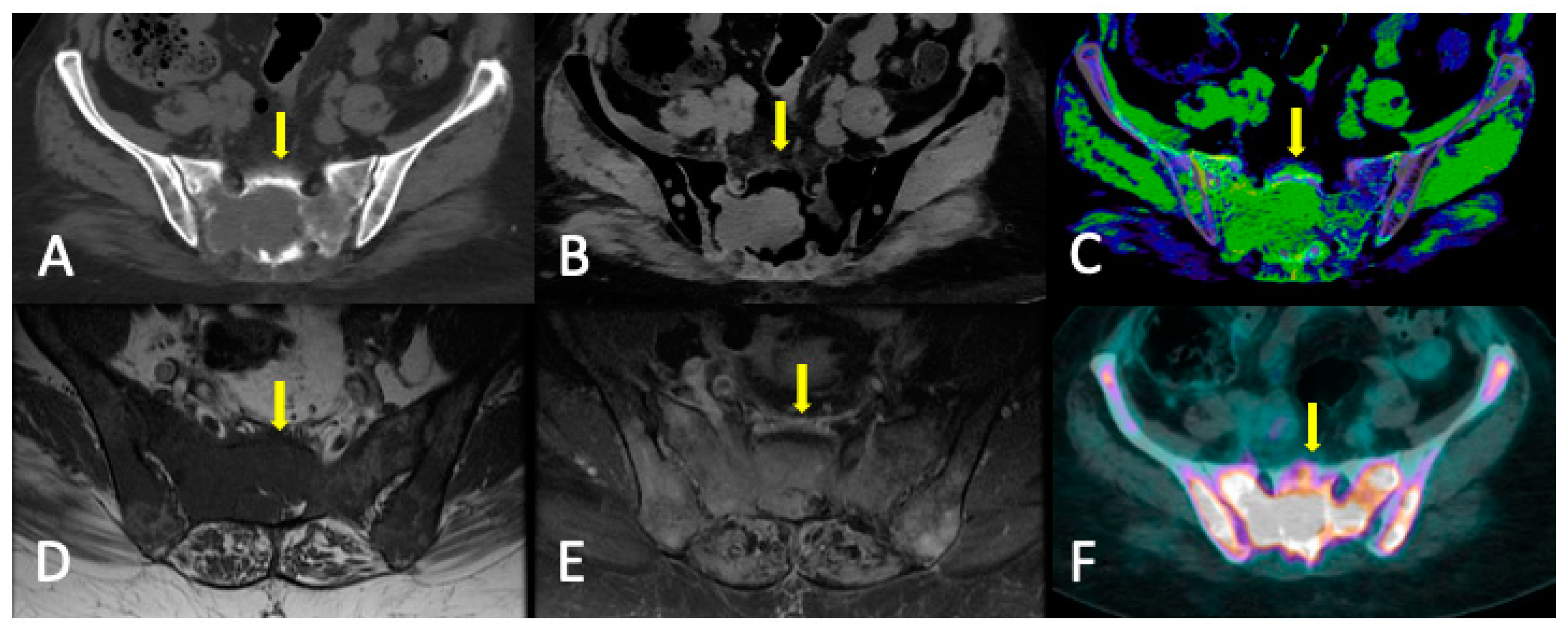
3.3. Role of DECT
3.4. DECT vs. Conventional CT
3.5. Limitations
3.6. Summary
4. WBMRI
4.1. MRI Acquisition Protocol
4.2. Disease Patterns
- Apparently normal bone marrow.
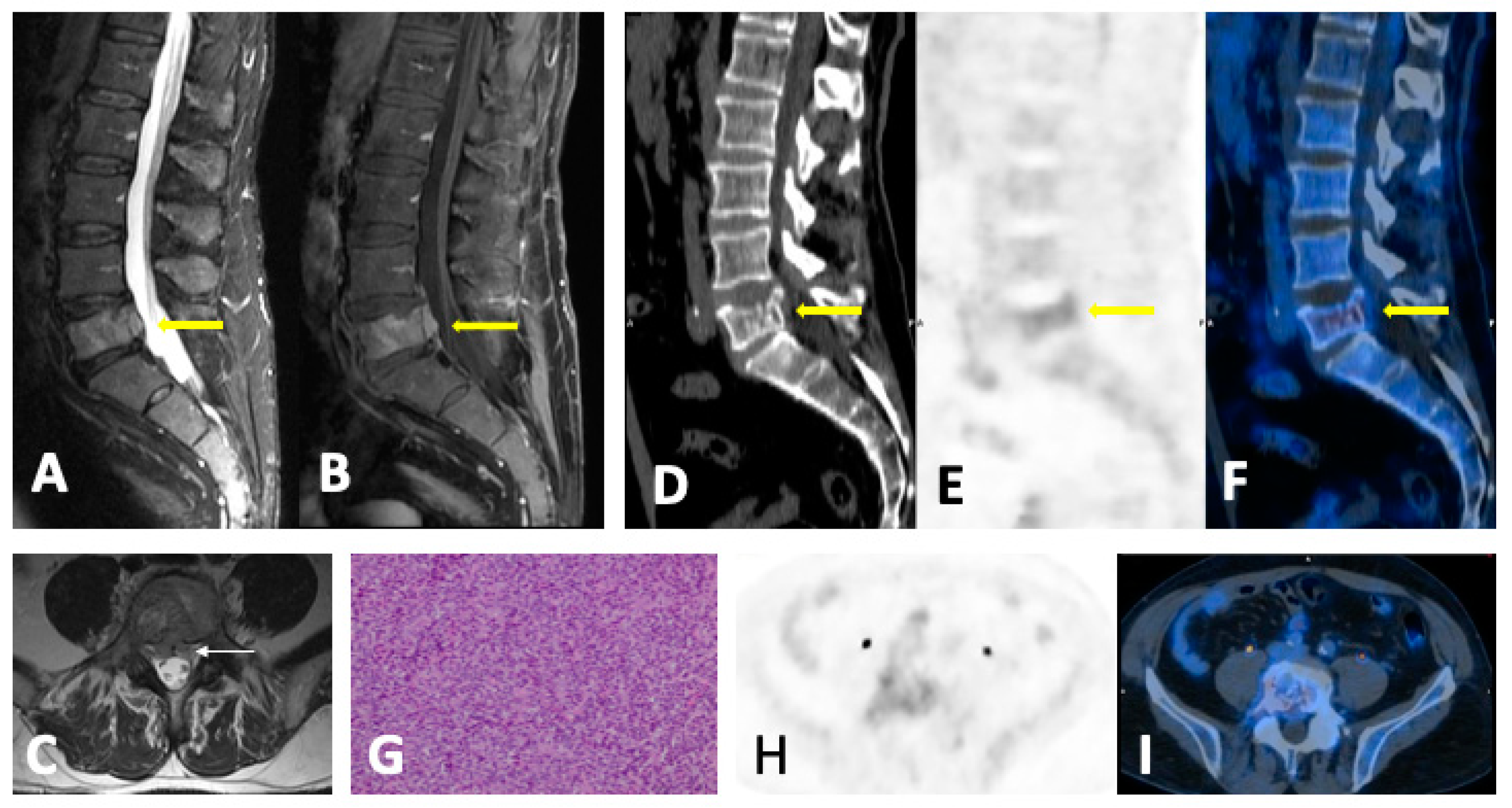
- Focal pattern: Focal myeloma infiltration was defined by circumscribed areas of high SI on STIR and T2WI. These corresponded to areas of low SI or, in a few cases, isointense signal upon an unenhanced T1WI [31]. The definition of focal lesion has evolved lately through the use of sequences such as DWI and Dixon. Therefore, focal lesions are defined as lesions greater than 5 mm hyperintense to background muscle at a b-value of 900 s/mm2, using ADC maps and confirming these findings with the corresponding Dixon sequences [32].
- Micronodular pattern: The micronodular or variegated or “salt and pepper” pattern presents a widespread heterogeneity with tiny nodular areas of altered diffusion signal (<5 mm) and T1WI hypointensities with preserved normal marrow between them [26].
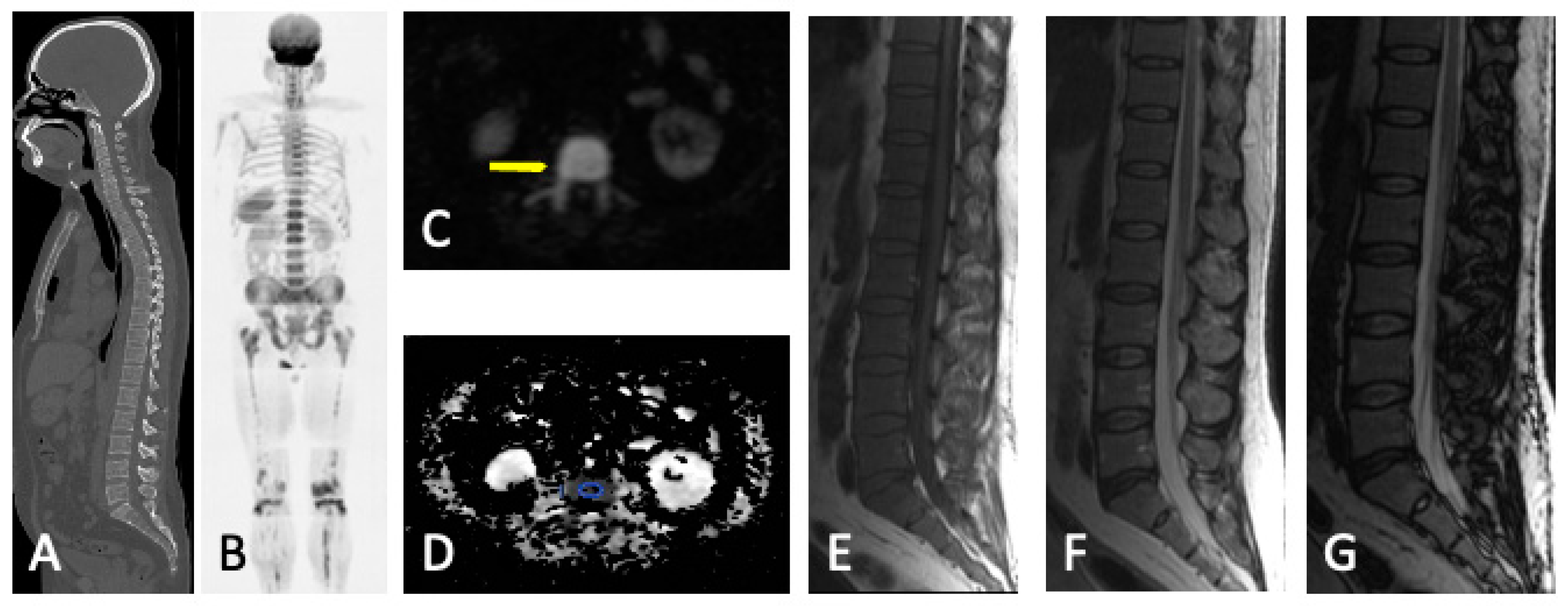
- Diffuse pattern: Diffuse disease can be suspected from a diffuse decreased signal on T1WI (either iso- or hypointense to intervertebral discs and muscle) and a diffuse increased signal throughout the marrow on T2FSWI, STIR, or high b-value DWI. Marrow ADC values above 600–700 μm2/s in a nontreated and newly diagnosed patient with MM could be used to increase confidence for the diagnosis of diffuse marrow involvement [33] (Figure 7). Due to potential false-positive findings, diffuse disease in imaging must be supported by bone marrow trephine biopsy [26].
- Mixed pattern: This pattern combines diffuse and focal patterns.
4.3. Follow-Up Imaging Features
4.4. Summary
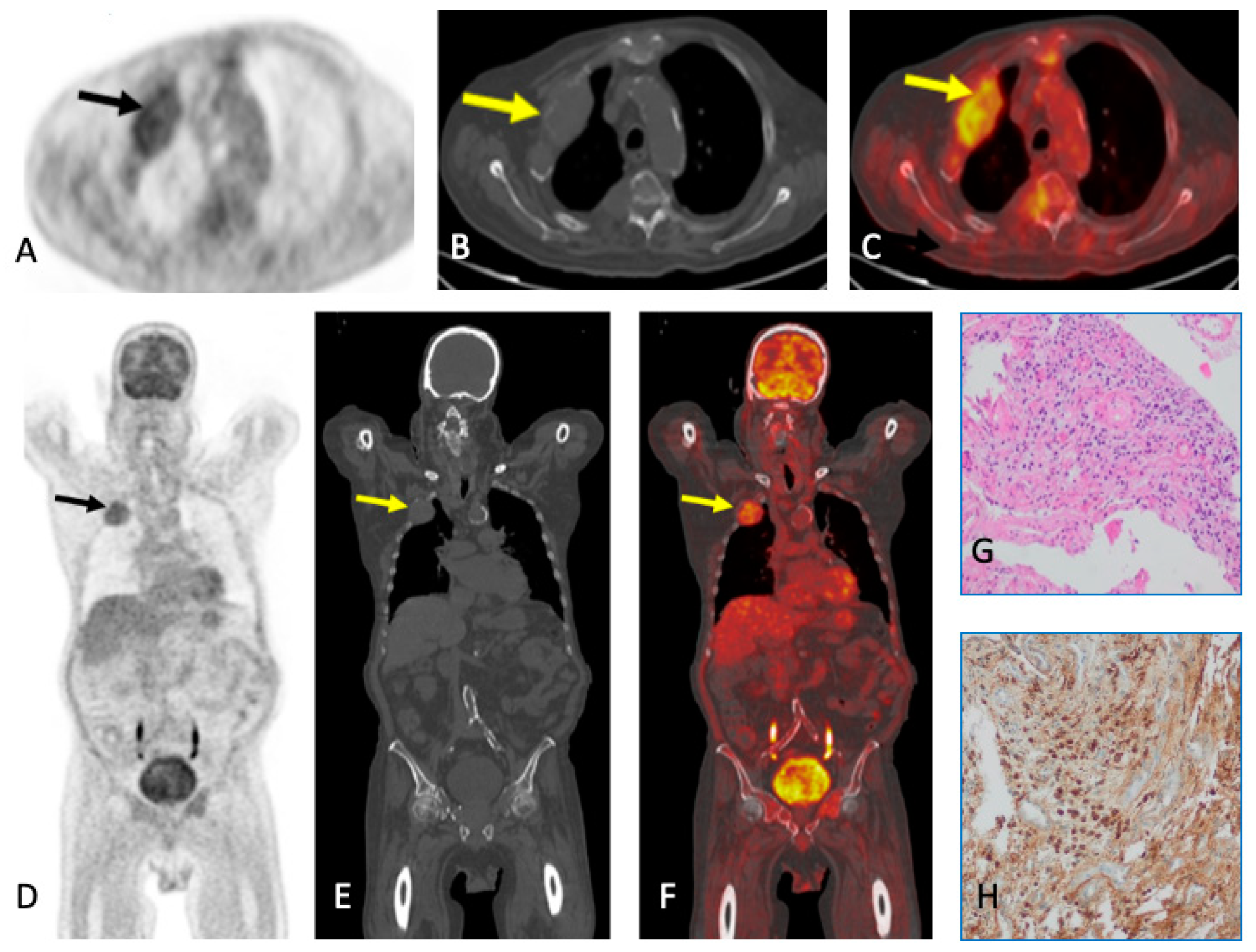
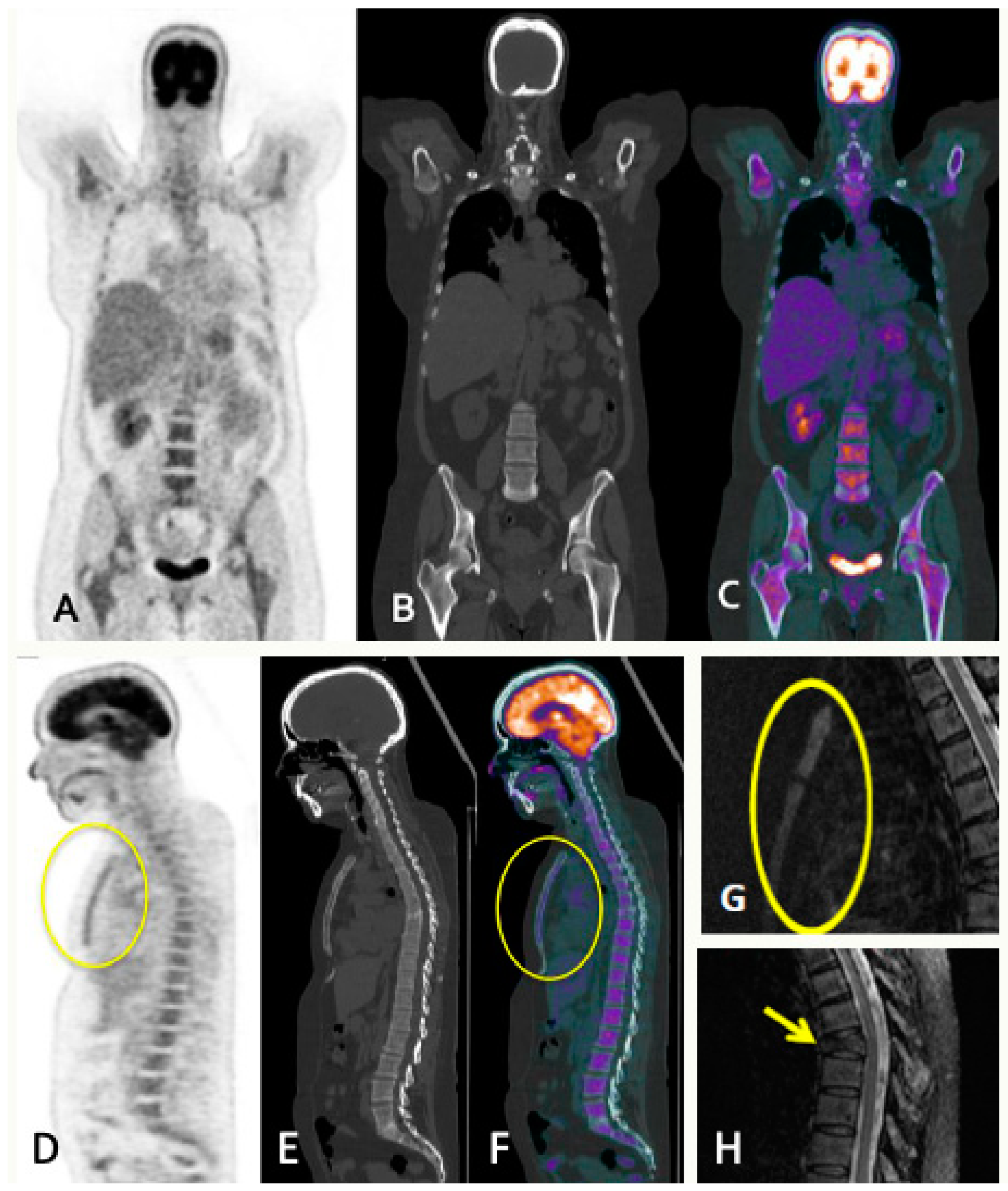
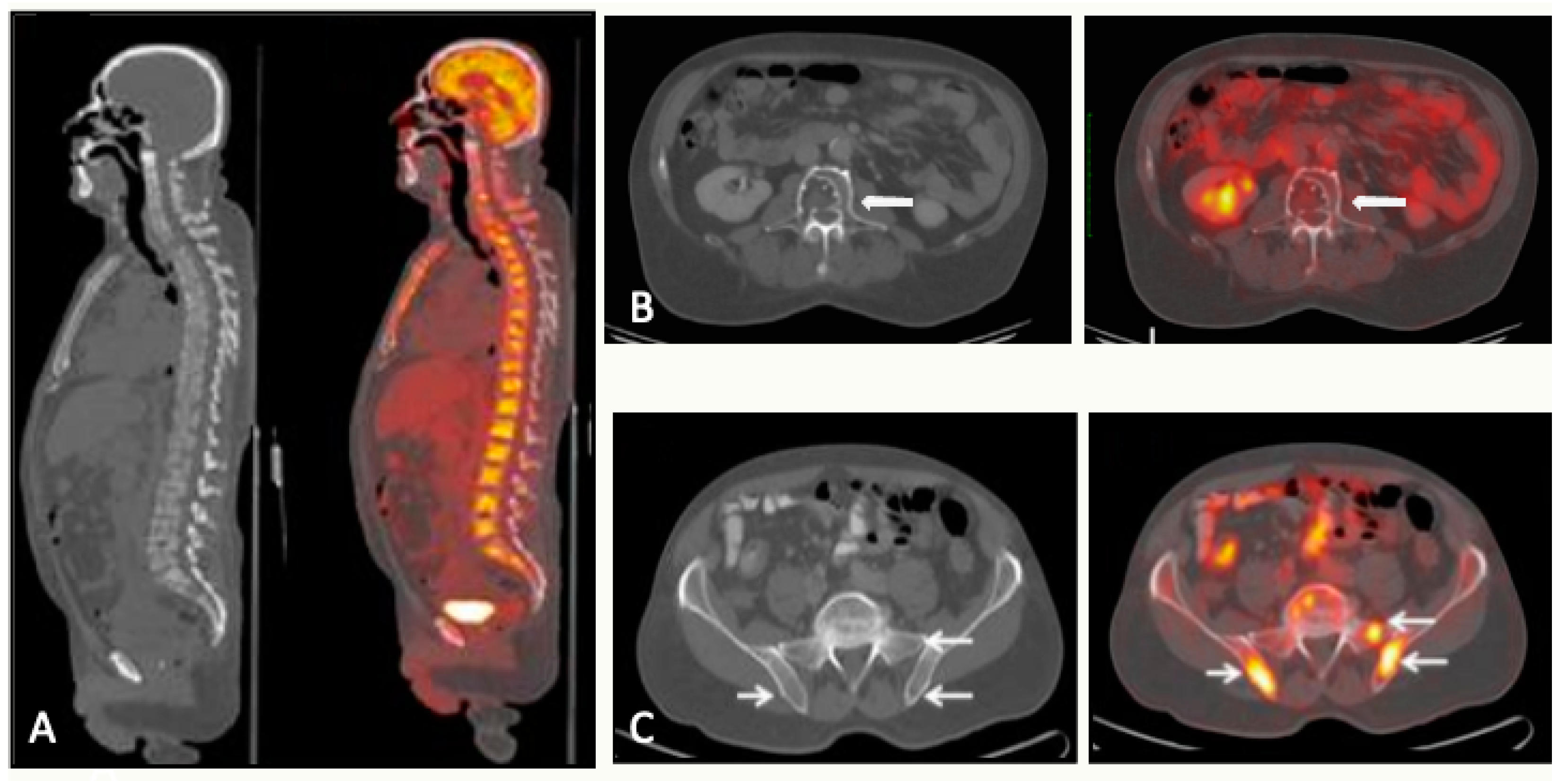
5. PET
5.1. PET/CT
5.1.1. Technique and Image Analysis
5.1.2. Response Measurement
5.1.3. Minimal Residual Disease
5.1.4. Alternative PET Tracers
5.1.5. FDG-PET/CT vs. WBMRI
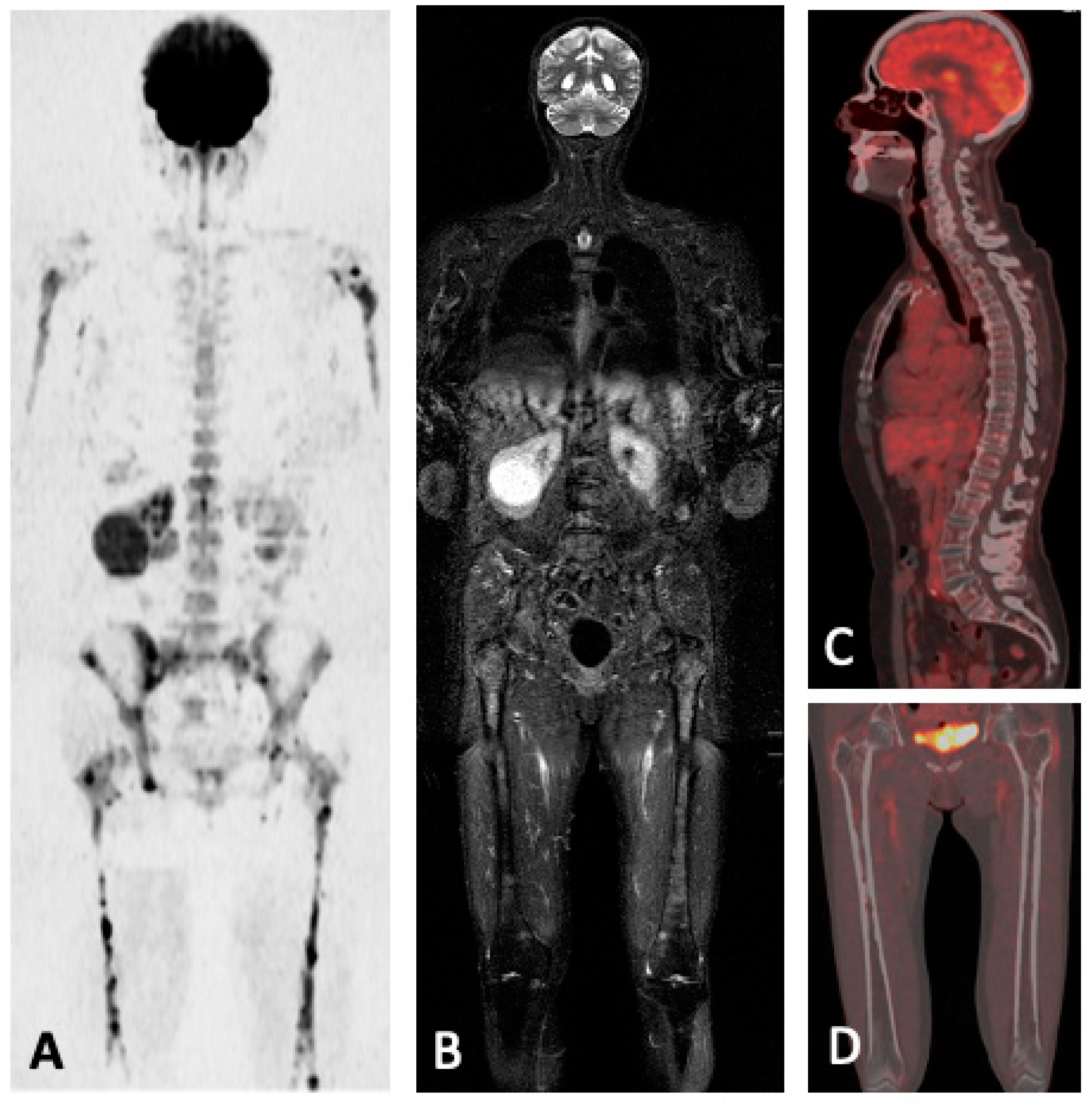
5.2. FDG-PET/MRI
5.3. Summary
6. Plasmacytoma and Extramedullary MM
7. Structured Reporting
| LDWBCT | WBMRI | FDG-PET/CT | |
|---|---|---|---|
| First diagnosis | Lytic lesions: Size measurements of main lesions; if less than 10, specify number and location, if more, describe the most relevant (craniocaudal order; alternatively, largest lesion first); associated soft tissue masses Paramedullary/extraosseous disease (location, extension, and complications) Intramedullary deposits in long bones (extension and endosteal scalloping). Risk of fracture in weight-bearing bones Vertebral fractures (possible etiology, instability, and risk of cord/root compression) Incidental relevant findings (including previous musculoskeletal surgery) | Disease involvement: measurement of 5 lesions and/or associated pattern of marrow infiltration (normal, focal, focal on diffuse, diffuse, micronodular) Extraosseous disease: size of paramedullary or extramedullary involvement Infiltration of long bones Vertebral fractures: Characterize as benign vs. malignant, instability (SINS) [84], cord (ESCC) [85]/nerve root compression Posterior iliac crest disease Incidental findings (i.e., avascular necrosis) | Localization (bone, paramedullary, and extramedullary) extent and intensity of pathological radiotracer accumulations (SUVmax) in relation to (suspected) MM Relevant findings in CT and correlation with metabolic activity Report accumulation as mild, moderate, or intense and compared to the background uptake (liver parenchyma) IMPeTUs (summarized) [80]: -Diffuse bone marrow uptake (Deauville 5 grades). A if limbs and ribs involvement -Focal bone lesions by number group -Skull, spine, and extraspinal -Target (hottest) focal bone lesion (Deauville 5 grades) -Lytic lesions at associated CT by number group -Fractures -Paramedullary disease -Extramedullary disease (specify site) Target (hottest) extramedullary lesion (Deauville 5 grades) |
| Follow-up | New lytic lesions/soft tissue masses Change in features of known lytic lesions Change in the intramedullary attenuation of long bones Risk of fracture in weight-bearing bones Evolution of paramedullary/extraosseous disease New fractures and possible etiology Evolution of known fractures (stability, healing, and osteosynthesis complications) Incidental relevant findings (including previous musculoskeletal surgery) | 5-point scale MY-RADS response assessment categories [26] 1: Highly likely to be responding -Unequivocal ↓ in number—size FL/soft tissue (RECIST: PR, CR) -≥40% ↑ in ADC and ↓ in high b-value SI -ADC: from ≤1400 to >1400 -Intra- or peritumoral fat of previous FL/DI 2: Likely to be responding -Slight ↓ in number—size FL ->25% and <40% ↑ in ADC and ↓ in high b-value SI -ADC: from ≤1000 to <1400 3: Stable 4: Likely to be progressing -Equivocal new FL -↑ in high b-value SI and ADC < 1400 -Relapsed disease -Spinal canal stenosis without symptoms 5: Highly likely to be progressing -Unequivocal new or ↑ in number—size FL/DI/soft tissue (RECIST: PD) -New FL with ADC: 600–1000 -New fracture/cord compression requiring intervention | Evolution of number or extent and intensity of known pathological radiotracer accumulations Evolution of previous relevant findings in CT New accumulations in relation to the suspected evolution/relapse of MM New relevant findings in CT and correlation with metabolic activity IMPeTUs (same as first diagnosis) |
| Conclusion | Clear and concise impression Recommendations for follow-up or need for additional test | ||
8. Pitfalls and Differential Diagnosis
8.1. Diffuse Imaging Findings
8.2. Malignant Focal Lesions
8.3. Benign Entities
9. Complications
10. Future Directions
10.1. Photon-Counting CT
10.2. Artificial Intelligence
11. Conclusions
Supplementary Materials
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 Patients with Newly Diagnosed Multiple Myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Terpos, E.; Comenzo, R.L.; Tosi, P.; Beksac, M.; Sezer, O.; Siegel, D.; Lokhorst, H.; Kumar, S.; Rajkumar, S.V.; et al. International Myeloma Working Group Consensus Statement and Guidelines Regarding the Current Role of Imaging Techniques in the Diagnosis and Monitoring of Multiple Myeloma. Leukemia 2009, 23, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Dimopoulos, M.A.; Meuleman, N.; Belch, A.; Mohty, M.; Chen, W.-M.; Kim, K.; Zamagni, E.; Rodriguez-Otero, P.; Renwick, W.; et al. A Simplified Frailty Scale Predicts Outcomes in Transplant-Ineligible Patients with Newly Diagnosed Multiple Myeloma Treated in the FIRST (MM-020) Trial. Leukemia 2020, 34, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Mateos, M.-V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric Assessment Predicts Survival and Toxicities in Elderly Myeloma Patients: An International Myeloma Working Group Report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.M.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Bernard, S.; Fayad, L.M.; Ilaslan, H.; Messiou, C.; Moulopoulos, L.A.; Mulligan, M.E. Updates and Ongoing Challenges in Imaging of Multiple Myeloma: AJR Expert Panel Narrative Review. Am. J. Roentgenol. 2021, 217, 775–785. [Google Scholar] [CrossRef]
- Hillengass, J.; Usmani, S.; Rajkumar, S.V.; Durie, B.G.M.; Mateos, M.-V.; Lonial, S.; Joao, C.; Anderson, K.C.; García-Sanz, R.; Riva, E.; et al. International Myeloma Working Group Consensus Recommendations on Imaging in Monoclonal Plasma Cell Disorders. Lancet Oncol. 2019, 20, e302–e312. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Hillengass, J.; Usmani, S.; Zamagni, E.; Lentzsch, S.; Davies, F.E.; Raje, N.; Sezer, O.; Zweegman, S.; Shah, J.; et al. Role of Magnetic Resonance Imaging in the Management of Patients with Multiple Myeloma: A Consensus Statement. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 657–664. [Google Scholar] [CrossRef]
- Zamagni, E.; Tacchetti, P.; Cavo, M. Imaging in Multiple Myeloma: How? When? Blood 2019, 133, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Mosebach, J.; Thierjung, H.; Schlemmer, H.-P.; Delorme, S. Multiple Myeloma Guidelines and Their Recent Updates: Implications for Imaging. RöFo-Fortschritte Auf Dem Geb. Röntgenstrahlen Bildgeb. Verfahr. 2019, 191, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, A.; Bley, T.; Petritsch, B. Imaging of Multiple Myeloma. RöFo-Fortschritte Auf Dem Geb. Röntgenstrahlen Bildgeb. Verfahr. 2019, 191, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Moulopoulos, L.A.; Koutoulidis, V.; Hillengass, J.; Zamagni, E.; Aquerreta, J.D.; Roche, C.L.; Lentzsch, S.; Moreau, P.; Cavo, M.; Miguel, J.S.; et al. Recommendations for Acquisition, Interpretation and Reporting of Whole Body Low Dose CT in Patients with Multiple Myeloma and Other Plasma Cell Disorders: A Report of the IMWG Bone Working Group. Blood Cancer J. 2018, 8, 95. [Google Scholar] [CrossRef]
- Ormond Filho, A.G.; Carneiro, B.C.; Pastore, D.; Silva, I.P.; Yamashita, S.R.; Consolo, F.D.; Hungria, V.T.M.; Sandes, A.F.; Rizzatti, E.G.; Nico, M.A.C. Whole-Body Imaging of Multiple Myeloma: Diagnostic Criteria. RadioGraphics 2019, 39, 1077–1097. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Thomas, C.; Schabel, C.; Krauss, B.; Weisel, K.; Bongers, M.; Claussen, C.D.; Horger, M. Dual-Energy CT: Virtual Calcium Subtraction for Assessment of Bone Marrow Involvement of the Spine in Multiple Myeloma. Am. J. Roentgenol. 2015, 204, W324–W331. [Google Scholar] [CrossRef]
- Brandelik, S.C.; Skornitzke, S.; Mokry, T.; Sauer, S.; Stiller, W.; Nattenmüller, J.; Kauczor, H.U.; Weber, T.F.; Do, T.D. Quantitative and Qualitative Assessment of Plasma Cell Dyscrasias in Dual-Layer Spectral CT. Eur. Radiol. 2021, 31, 7664–7673. [Google Scholar] [CrossRef]
- Fervers, P.; Celik, E.; Bratke, G.; Maintz, D.; Baues, C.; Ruffing, S.; Pollman-Schweckhorst, P.; Kottlors, J.; Lennartz, S.; Große Hokamp, N. Radiotherapy Response Assessment of Multiple Myeloma: A Dual-Energy CT Approach with Virtual Non-Calcium Images. Front. Oncol. 2021, 11, 734819. [Google Scholar] [CrossRef]
- Kosmala, A.; Weng, A.M.; Heidemeier, A.; Krauss, B.; Knop, S.; Bley, T.A.; Petritsch, B. Multiple Myeloma and Dual-Energy CT: Diagnostic Accuracy of Virtual Noncalcium Technique for Detection of Bone Marrow Infiltration of the Spine and Pelvis. Radiology 2018, 286, 205–213. [Google Scholar] [CrossRef]
- Gu, R.; Amlani, A.; Haberland, U.; Hodson, D.; Streetly, M.; Antonelli, M.; Dregely, I.; Goh, V. Correlation between Whole Skeleton Dual Energy CT Calcium-Subtracted Attenuation and Bone Marrow Infiltration in Multiple Myeloma. Eur. J. Radiol. 2022, 149, 110223. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krauss, B.; Horger, M. Dual-Energy CT-Based Bone Marrow Imaging in Multiple Myeloma: Assessment of Focal Lesions in Relation to Disease Status and MRI Findings. Acad. Radiol. 2022, 29, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Fervers, P.; Glauner, A.; Gertz, R.; Täger, P.; Kottlors, J.; Maintz, D.; Borggrefe, J. Virtual Calcium-Suppression in Dual Energy Computed Tomography Predicts Metabolic Activity of Focal MM Lesions as Determined by Fluorodeoxyglucose Positron-Emission-Tomography. Eur. J. Radiol. 2021, 135, 109502. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, A.; Weng, A.M.; Krauss, B.; Knop, S.; Bley, T.A.; Petritsch, B. Dual-Energy CT of the Bone Marrow in Multiple Myeloma: Diagnostic Accuracy for Quantitative Differentiation of Infiltration Patterns. Eur. Radiol. 2018, 28, 5083–5090. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.T.; Lloyd, T.B. Utility of Dual Energy Computed Tomography in the Evaluation of Infiltrative Skeletal Lesions and Metastasis: A Literature Review. Skeletal Radiol. 2022, 51, 1731–1741. [Google Scholar] [CrossRef]
- Messiou, C.; Hillengass, J.; Delorme, S.; Lecouvet, F.E.; Moulopoulos, L.A.; Collins, D.J.; Blackledge, M.D.; Abildgaard, N.; Østergaard, B.; Schlemmer, H.-P.; et al. Guidelines for Acquisition, Interpretation, and Reporting of Whole-Body MRI in Myeloma: Myeloma Response Assessment and Diagnosis System (MY-RADS). Radiology 2019, 291, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Hillengass, J.; Landgren, O. Challenges and Opportunities of Novel Imaging Techniques in Monoclonal Plasma Cell Disorders: Imaging “Early Myeloma”. Leuk. Lymphoma 2013, 54, 1355–1363. [Google Scholar] [CrossRef]
- Omoumi, P. The Dixon Method in Musculoskeletal MRI: From Fat-Sensitive to Fat-Specific Imaging. Skeletal Radiol. 2022, 51, 1365–1369. [Google Scholar] [CrossRef]
- Hagmann, P.; Jonasson, L.; Maeder, P.; Thiran, J.-P.; Wedeen, V.J.; Meuli, R. Understanding Diffusion MR Imaging Techniques: From Scalar Diffusion-Weighted Imaging to Diffusion Tensor Imaging and Beyond. RadioGraphics 2006, 26, S205–S223. [Google Scholar] [CrossRef]
- Giles, S.L.; Messiou, C.; Collins, D.J.; Morgan, V.A.; Simpkin, C.J.; West, S.; Davies, F.E.; Morgan, G.J.; deSouza, N.M. Whole-Body Diffusion-Weighted MR Imaging for Assessment of Treatment Response in Myeloma. Radiology 2014, 271, 785–794. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Mosebach, J.; Freitag, M.T.; Wilhelm, T.; Mai, E.K.; Goldschmidt, H.; Haberkorn, U.; Schlemmer, H.-P.; Delorme, S.; Dimitrakopoulou-Strauss, A. Application of 18F-FDG PET and Diffusion Weighted Imaging (DWI) in Multiple Myeloma: Comparison of Functional Imaging Modalities. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 479–492. [Google Scholar] [PubMed]
- Messiou, C.; Porta, N.; Sharma, B.; Levine, D.; Koh, D.-M.; Boyd, K.; Pawlyn, C.; Riddell, A.; Downey, K.; Croft, J.; et al. Prospective Evaluation of Whole-Body MRI versus FDG PET/CT for Lesion Detection in Participants with Myeloma. Radiol. Imaging Cancer 2021, 3, e210048. [Google Scholar] [CrossRef] [PubMed]
- Mai, E.K.; Hielscher, T.; Kloth, J.K.; Merz, M.; Shah, S.; Raab, M.S.; Hillengass, M.; Wagner, B.; Jauch, A.; Hose, D.; et al. A Magnetic Resonance Imaging-Based Prognostic Scoring System to Predict Outcome in Transplant-Eligible Patients with Multiple Myeloma. Haematologica 2015, 100, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Koutoulidis, V.; Fontara, S.; Terpos, E.; Zagouri, F.; Matsaridis, D.; Christoulas, D.; Panourgias, E.; Kastritis, E.; Dimopoulos, M.A.; Moulopoulos, L.A. Quantitative Diffusion-Weighted Imaging of the Bone Marrow: An Adjunct Tool for the Diagnosis of a Diffuse MR Imaging Pattern in Patients with Multiple Myeloma. Radiology 2017, 282, 484–493. [Google Scholar] [CrossRef]
- Dutoit, J.C.; Verstraete, K.L. MRI in Multiple Myeloma: A Pictorial Review of Diagnostic and Post-Treatment Findings. Insights Imaging 2016, 7, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Messiou, C.; Kaiser, M. Whole Body Diffusion Weighted MRI—A New View of Myeloma. Br. J. Haematol. 2015, 171, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, J.; Xu, W.-B.; Guan, Y.-J.; Ling, H.-W.; Mi, J.-Q.; Yan, H. Discriminating Depth of Response to Therapy in Multiple Myeloma Using Whole-Body Diffusion-Weighted MRI with Apparent Diffusion Coefficient. Acad. Radiol. 2018, 25, 904–914. [Google Scholar] [CrossRef]
- Belotti, A.; Ribolla, R.; Cancelli, V.; Villanacci, A.; Angelini, V.; Chiarini, M.; Giustini, V.; Facchetti, G.V.; Roccaro, A.M.; Ferrari, S.; et al. Predictive Role of Diffusion-weighted Whole-body MRI (DW-MRI) Imaging Response According to MY-RADS Criteria after Autologous Stem Cell Transplantation in Patients with Multiple Myeloma and Combined Evaluation with MRD Assessment by Flow Cytometry. Cancer Med. 2021, 10, 5859–5865. [Google Scholar] [CrossRef]
- Wang, K.; Lee, E.; Kenis, S.; Hallam, S.; Haroon, A.; Wan, S.; Rabin, N.; Rojas-Garcia, A.; Padhani, A.; Adeleke, S. Application of Diffusion-Weighted Whole-Body MRI for Response Monitoring in Multiple Myeloma after Chemotherapy: A Systematic Review and Meta-Analysis. Eur. Radiol. 2022, 32, 2135–2148. [Google Scholar] [CrossRef]
- Torkian, P.; Mansoori, B.; Hillengass, J.; Azadbakht, J.; Rashedi, S.; Lee, S.S.; Amini, B.; Bonaffini, P.A.; Chalian, M. Diffusion-Weighted Imaging (DWI) in Diagnosis, Staging, and Treatment Response Assessment of Multiple Myeloma: A Systematic Review and Meta-Analysis. Skeletal Radiol. 2023, 52, 565–583. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wu, X.; Zhao, A.; Feng, J.; Zhang, H.; Cao, X.; Li, S.; Cai, H.; Sun, Z.; et al. Baseline Bone Marrow ADC Value of Diffusion-Weighted MRI: A Potential Independent Predictor for Progression and Death in Patients with Newly Diagnosed Multiple Myeloma. Eur. Radiol. 2021, 31, 1843–1852. [Google Scholar] [CrossRef]
- Van Den Berghe, T.; Verstraete, K.L.; Lecouvet, F.E.; Lejoly, M.; Dutoit, J. Review of Diffusion-Weighted Imaging and Dynamic Contrast–Enhanced MRI for Multiple Myeloma and Its Precursors (Monoclonal Gammopathy of Undetermined Significance and Smouldering Myeloma). Skeletal Radiol. 2022, 51, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Koutoulidis, V.; Terpos, E.; Papanikolaou, N.; Fontara, S.; Seimenis, I.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Bourgioti, C.; Santinha, J.; Moreira, J.M.; et al. Comparison of MRI Features of Fat Fraction and ADC for Early Treatment Response Assessment in Participants with Multiple Myeloma. Radiology 2022, 304, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Matsaridis, D.; Koutoulidis, V.; Zagouri, F.; Christoulas, D.; Fontara, S.; Panourgias, E.; Gavriatopoulou, M.; Kastritis, E.; Dimopoulos, M.A.; et al. Dynamic Contrast-Enhanced Magnetic Resonance Imaging Parameters Correlate with Advanced Revised-ISS and Angiopoietin-1/Angiopoietin-2 Ratio in Patients with Multiple Myeloma. Ann. Hematol. 2017, 96, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- NICE Guideline on Myeloma: Diagnosis and Management. UK National Institute for Health and Care Excellence. Available online: https://www.nice.org.uk/guidance/ng35 (accessed on 30 August 2023).
- Lecouvet, F.E.; Vekemans, M.-C.; Van Den Berghe, T.; Verstraete, K.; Kirchgesner, T.; Acid, S.; Malghem, J.; Wuts, J.; Hillengass, J.; Vandecaveye, V.; et al. Imaging of Treatment Response and Minimal Residual Disease in Multiple Myeloma: State of the Art WB-MRI and PET/CT. Skeletal Radiol. 2022, 51, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Plathow, C.; Weber, W.A. Tumor Cell Metabolism Imaging. J. Nucl. Med. 2008, 49, 43S–63S. [Google Scholar] [CrossRef] [PubMed]
- García Garzón, J.R.; Rodríguez, A.; Cabrera, A. Tomografía por emisión de positrones de cuerpo completo (PET/TAC) con 18F-fluorodesoxiglucosa. Rev. Esp. Med. Nucl. 2009, 28, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Itoh, M.; Ozaki, K.; Ono, S.; Tashiro, M.; Yamaguchi, K.; Akaizawa, T.; Yamada, K.; Fukuda, H. Advantage of Delayed Whole-Body FDG-PET Imaging for Tumour Detection. Eur. J. Nucl. Med. 2001, 28, 696–703. [Google Scholar] [CrossRef]
- Davies, F.E.; Rosenthal, A.; Rasche, L.; Petty, N.M.; McDonald, J.E.; Ntambi, J.A.; Steward, D.M.; Panozzo, S.B.; Van Rhee, F.; Zangari, M.; et al. Treatment to Suppression of Focal Lesions on Positron Emission Tomography-Computed Tomography Is a Therapeutic Goal in Newly Diagnosed Multiple Myeloma. Haematologica 2018, 103, 1047–1053. [Google Scholar] [CrossRef]
- Zamagni, E.; Patriarca, F.; Nanni, C.; Zannetti, B.; Englaro, E.; Pezzi, A.; Tacchetti, P.; Buttignol, S.; Perrone, G.; Brioli, A.; et al. Prognostic Relevance of 18-F FDG PET/CT in Newly Diagnosed Multiple Myeloma Patients Treated with up-Front Autologous Transplantation. Blood 2011, 118, 5989–5995. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Caillot, D.; Macro, M.; Karlin, L.; Garderet, L.; Facon, T.; Benboubker, L.; Escoffre-Barbe, M.; Stoppa, A.-M.; et al. Prospective Evaluation of Magnetic Resonance Imaging and [18F]Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography at Diagnosis and Before Maintenance Therapy in Symptomatic Patients With Multiple Myeloma Included in the IFM/DFCI 2009 Trial: Results of the IMAJEM Study. J. Clin. Oncol. 2017, 35, 2911–2918. [Google Scholar] [PubMed]
- Zamagni, E.; Nanni, C.; Gay, F.; Dozza, L.; Rota Scalabrini, D.; Omedé, P.; Ribolla, R.; Galli, M.; Racca, M.; Zambello, R.; et al. MRD Evaluation By PET/CT According to Deauville Criteria Combined with Multiparameter Flow Cytometry in Newly Diagnosed Transplant Eligible Multiple Myeloma (MM) Patients Enrolled in the Phase II Randomized Forte Trial. Blood 2019, 134, 4321. [Google Scholar] [CrossRef]
- Moreau, P.; Zweegman, S.; Perrot, A.; Hulin, C.; Caillot, D.; Facon, T.; Leleu, X.; Belhadj, K.; Karlin, L.; Benboubker, L.; et al. Evaluation of the Prognostic Value of Positron Emission Tomography-Computed Tomography (PET-CT) at Diagnosis and Follow-up in Transplant-Eligible Newly Diagnosed Multiple Myeloma (TE NDMM) Patients Treated in the Phase 3 Cassiopeia Study: Results of the Cassiopet Companion Study. Blood 2019, 134, 692. [Google Scholar]
- Rasche, L.; Alapat, D.; Kumar, M.; Gershner, G.; McDonald, J.; Wardell, C.P.; Samant, R.; Van Hemert, R.; Epstein, J.; Williams, A.F.; et al. Combination of Flow Cytometry and Functional Imaging for Monitoring of Residual Disease in Myeloma. Leukemia 2019, 33, 1713–1722. [Google Scholar] [CrossRef]
- Nanni, C.; Zamagni, E.; Cavo, M.; Rubello, D.; Tacchetti, P.; Pettinato, C.; Farsad, M.; Castellucci, P.; Ambrosini, V.; Montini, G.C.; et al. 11C-Choline vs. 18F-FDG PET/CT in Assessing Bone Involvement in Patients with Multiple Myeloma. World J. Surg. Oncol. 2007, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Knop, S.; Schreder, M.; Rudelius, M.; Knott, M.; Jörg, G.; Samnick, S.; Herrmann, K.; Buck, A.K.; Einsele, H.; et al. 11C-Methionine-PET in Multiple Myeloma: Correlation with Clinical Parameters and Bone Marrow Involvement. Theranostics 2016, 6, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Schreder, M.; Schirbel, A.; Samnick, S.; Kortüm, K.M.; Herrmann, K.; Kropf, S.; Einsele, H.; Buck, A.K.; Wester, H.-J.; et al. [68Ga]Pentixafor-PET/CT for Imaging of Chemokine Receptor CXCR4 Expression in Multiple Myeloma—Comparison to [18F]FDG and Laboratory Values. Theranostics 2017, 7, 205–212. [Google Scholar] [CrossRef]
- Pawlyn, C.; Fowkes, L.; Otero, S.; Jones, J.R.; Boyd, K.D.; Davies, F.E.; Morgan, G.J.; Collins, D.J.; Sharma, B.; Riddell, A.; et al. Whole-Body Diffusion-Weighted MRI: A New Gold Standard for Assessing Disease Burden in Patients with Multiple Myeloma? Leukemia 2016, 30, 1446–1448. [Google Scholar] [CrossRef]
- Rama, S.; Suh, C.H.; Kim, K.W.; Durieux, J.C.; Ramaiya, N.H.; Tirumani, S.H. Comparative Performance of Whole-Body MRI and FDG PET/CT in Evaluation of Multiple Myeloma Treatment Response: Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2022, 218, 602–613. [Google Scholar] [CrossRef]
- Yokoyama, K.; Tsuchiya, J.; Tateishi, U. Comparison of [18F]FDG PET/CT and MRI for Treatment Response Assessment in Multiple Myeloma: A Meta-Analysis. Diagnostics 2021, 11, 706. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, K.W.; Yoon, M.A.; Lee, M.H.; Chae, E.J.; Lee, J.H.; Chung, H.W.; Yoon, D.H. Role of Whole-Body MRI for Treatment Response Assessment in Multiple Myeloma: Comparison between Clinical Response and Imaging Response. Cancer Imaging 2020, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Paternain, A.; García-Velloso, M.J.; Rosales, J.J.; Ezponda, A.; Soriano, I.; Elorz, M.; Rodríguez-Otero, P.; Aquerreta, J.D. The Utility of ADC Value in Diffusion-Weighted Whole-Body MRI in the Follow-up of Patients with Multiple Myeloma. Correlation Study with 18F-FDG PET-CT. Eur. J. Radiol. 2020, 133, 109403. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Terpos, E.; Nanni, C.; Moreau, P.; Lentzsch, S.; Zweegman, S.; Hillengass, J.; Engelhardt, M.; Usmani, S.Z.; Vesole, D.H.; et al. Role of 18F-FDG PET/CT in the Diagnosis and Management of Multiple Myeloma and Other Plasma Cell Disorders: A Consensus Statement by the International Myeloma Working Group. Lancet Oncol. 2017, 18, e206–e217. [Google Scholar] [CrossRef] [PubMed]
- Mulé, S.; Reizine, E.; Blanc-Durand, P.; Baranes, L.; Zerbib, P.; Burns, R.; Nouri, R.; Itti, E.; Luciani, A. Whole-Body Functional MRI and PET/MRI in Multiple Myeloma. Cancers 2020, 12, 3155. [Google Scholar] [CrossRef]
- Shah, S.N.; Oldan, J.D. PET/MR Imaging of Multiple Myeloma. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Hillengass, J.; Goldschmidt, H.; Mosebach, J.; Pan, L.; Schlemmer, H.-P.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Comparison of 18F-FDG PET/CT and PET/MRI in Patients with Multiple Myeloma. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 469–478. [Google Scholar] [PubMed]
- Ooi, G.C.; Chim, J.C.-S.; Au, W.-Y.; Khong, P.-L. Radiologic Manifestations of Primary Solitary Extramedullary and Multiple Solitary Plasmacytomas. Am. J. Roentgenol. 2006, 186, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, S.; Wu, C.; Wang, J.; Li, J.; Chen, L. Survival Trends and Prognostic Factors in Patients with Solitary Plasmacytoma of Bone: A Population-based Study. Cancer Med. 2021, 10, 462–470. [Google Scholar] [CrossRef]
- Caers, J.; Paiva, B.; Zamagni, E.; Leleu, X.; Bladé, J.; Kristinsson, S.Y.; Touzeau, C.; Abildgaard, N.; Terpos, E.; Heusschen, R.; et al. Diagnosis, Treatment, and Response Assessment in Solitary Plasmacytoma: Updated Recommendations from a European Expert Panel. J. Hematol. Oncol. 2018, 11, 10. [Google Scholar] [CrossRef]
- Zuo, Z.; Tang, Y.; Bi, C.-F.; Zhang, W.-Y.; Zhao, S.; Wang, X.-Q.; Yang, Q.-P.; Zou, L.-Q.; Liu, W.-P. Extraosseous (Extramedullary) Plasmacytomas: A Clinicopathologic and Immunophenotypic Study of 32 Chinese Cases. Diagn. Pathol. 2011, 6, 123. [Google Scholar] [CrossRef]
- Salaun, P.-Y.; Gastinne, T.; Frampas, E.; Bodet-Milin, C.; Moreau, P.; Bodere-Kraeber, F. FDG-Positron-Emission Tomography for Staging and Therapeutic Assessment in Patients with Plasmacytoma. Haematologica 2008, 93, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, G.; Guidez, S.; Herbaux, C.; Van De Wyngaert, Z.; Bonnet, S.; Beauvais, D.; Demarquette, H.; Adib, S.; Hivert, B.; Wemeau, M.; et al. Impact of Initial FDG-PET/CT and Serum-Free Light Chain on Transformation of Conventionally Defined Solitary Plasmacytoma to Multiple Myeloma. Clin. Cancer Res. 2014, 20, 3254–3260. [Google Scholar] [CrossRef] [PubMed]
- Galán González, I.; Santos Salas, X.; Campos Rivas, R.; Idoate Ortueta, C.; Muñoz Olmedo, J.M.; Gómez León, N.N. Comparación Entre La RM y La 18FDG PET/TC En El Diagnóstico de Los Plasmocitomas Con Correlación Anatomo Patológica. Seram 2018. Available online: https://www.piper.espacio-seram.com/index.php/seram/article/view/2921 (accessed on 30 August 2023).
- Chantry, A.; Kazmi, M.; Barrington, S.; Goh, V.; Mulholland, N.; Streetly, M.; Lai, M.; Pratt, G.; the British Society for Haematology Guidelines. Guidelines for the Use of Imaging in the Management of Patients with Myeloma. Br. J. Haematol. 2017, 178, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.C.; Brown, T.L.; Jones-Jackson, L.B.; De Blanche, L.; Bartel, T. Imaging of Multiple Myeloma and Related Plasma Cell Dyscrasias. J. Nucl. Med. 2012, 53, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Abdel Razek, A.A.K.; Castillo, M. Imaging Appearance of Primary Bony Tumors and Pseudo-Tumors of the Spine. J. Neuroradiol. 2010, 37, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Rodallec, M.H.; Feydy, A.; Larousserie, F.; Anract, P.; Campagna, R.; Babinet, A.; Zins, M.; Drapé, J.-L. Diagnostic Imaging of Solitary Tumors of the Spine: What to Do and Say. RadioGraphics 2008, 28, 1019–1041. [Google Scholar] [CrossRef]
- Hall, M.N.; Jagannathan, J.P.; Ramaiya, N.H.; Shinagare, A.B.; Van Den Abbeele, A.D. Imaging of Extraosseous Myeloma: CT, PET/CT, and MRI Features. Am. J. Roentgenol. 2010, 195, 1057–1065. [Google Scholar] [CrossRef]
- Varettoni, M.; Corso, A.; Pica, G.; Mangiacavalli, S.; Pascutto, C.; Lazzarino, M. Incidence, Presenting Features and Outcome of Extramedullary Disease in Multiple Myeloma: A Longitudinal Study on 1003 Consecutive Patients. Ann. Oncol. 2010, 21, 325–330. [Google Scholar] [CrossRef]
- Cho, R.; Myers, D.T.; Onwubiko, I.N.; Williams, T.R. Extraosseous Multiple Myeloma: Imaging Spectrum in the Abdomen and Pelvis. Abdom. Radiol. 2021, 46, 1194–1209. [Google Scholar] [CrossRef]
- Nanni, C. PET-FDG: Impetus. Cancers 2020, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, E.; Nanni, C.; Dozza, L.; Carlier, T.; Bailly, C.; Tacchetti, P.; Versari, A.; Chauvie, S.; Gallamini, A.; Gamberi, B.; et al. Standardization of 18F-FDG–PET/CT According to Deauville Criteria for Metabolic Complete Response Definition in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2021, 39, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A Novel Classification System for Spinal Instability in Neoplastic Disease: An Evidence-Based Approach and Expert Consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [PubMed]
- Bilsky, M.H.; Laufer, I.; Fourney, D.R.; Groff, M.; Schmidt, M.H.; Varga, P.P.; Vrionis, F.D.; Yamada, Y.; Gerszten, P.C.; Kuklo, T.R. Reliability Analysis of the Epidural Spinal Cord Compression Scale. J. Neurosurg. Spine 2010, 13, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Huang, W.; Ji, X.; Huang, L.; Zou, D.; Hao, M.; Deng, S.; Shen, Z.; Lu, X.; Wang, J.; et al. Prediction of Early Treatment Response in Multiple Myeloma Using MY-RADS Total Burden Score, ADC, and Fat Fraction from Whole-Body MRI: Impact of Anemia on Predictive Performance. AJR Am. J. Roentgenol. 2022, 218, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.K.; Saifuddin, A.; Price, G.J. Magnetic Resonance Imaging of Spinal Plasmacytoma. Clin. Radiol. 2000, 55, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Rasche, L.; Angtuaco, E.; McDonald, J.E.; Buros, A.; Stein, C.; Pawlyn, C.; Thanendrarajan, S.; Schinke, C.; Samant, R.; Yaccoby, S.; et al. Low Expression of Hexokinase-2 Is Associated with False-Negative FDG-Positron Emission Tomography in Multiple Myeloma. Blood 2017, 130, 30–34. [Google Scholar] [CrossRef]
- Gaudino, S.; Martucci, M.; Colantonio, R.; Lozupone, E.; Visconti, E.; Leone, A.; Colosimo, C. A Systematic Approach to Vertebral Hemangioma. Skeletal Radiol. 2015, 44, 25–36. [Google Scholar] [CrossRef]
- Bredella, M.A.; Vande Berg, B.C. Metabolic-Endocrine. In Musculoskeletal Diseases 2021–2024 Diagnostic Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; p. 175. ISBN 978-3-030-71281-5. [Google Scholar]
- Toci, G.R.; Bressner, J.A.; Morris, C.D.; Fayad, L.; Levin, A.S. Can a Novel Scoring System Improve on the Mirels Score in Predicting the Fracture Risk in Patients with Multiple Myeloma? Clin. Orthop. 2021, 479, 521–530. [Google Scholar] [CrossRef]
- Mauch, J.T.; Carr, C.M.; Cloft, H.; Diehn, F.E. Review of the Imaging Features of Benign Osteoporotic and Malignant Vertebral Compression Fractures. Am. J. Neuroradiol. 2018, 39, 1584–1592. [Google Scholar] [CrossRef]
- Fisher, C.G.; Schouten, R.; Versteeg, A.L.; Boriani, S.; Varga, P.P.; Rhines, L.D.; Kawahara, N.; Fourney, D.; Weir, L.; Reynolds, J.J.; et al. Reliability of the Spinal Instability Neoplastic Score (SINS) among Radiation Oncologists: An Assessment of Instability Secondary to Spinal Metastases. Radiat. Oncol. 2014, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Zadnik, P.L.; Goodwin, C.R.; Karami, K.J.; Mehta, A.I.; Amin, A.G.; Groves, M.L.; Wolinsky, J.-P.; Witham, T.F.; Bydon, A.; Gokaslan, Z.L.; et al. Outcomes Following Surgical Intervention for Impending and Gross Instability Caused by Multiple Myeloma in the Spinal Column. J. Neurosurg. Spine 2015, 22, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Balagamwala, E.H.; Chao, S.T.; Emch, T.; Suh, J.H.; Djemil, T.; Angelov, L. Spine Stereotactic Radiosurgery for the Treatment of Multiple Myeloma. J. Neurosurg. Spine 2017, 26, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Serratrice, N.; Faddoul, J.; Tarabay, B.; Attieh, C.; Chalah, M.A.; Ayache, S.S.; Abi Lahoud, G.N. Ten Years After SINS: Role of Surgery and Radiotherapy in the Management of Patients with Vertebral Metastases. Front. Oncol. 2022, 12, 802595. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Rajendran, K.; Ferrero, A.; Dhillon, P.; Kumar, S.; Baffour, F. Photon Counting Detector Computed Tomography: A New Frontier of Myeloma Bone Disease Evaluation. Acta Haematol. 2023, 146, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, M.T.; Hagen, F.; Le-Yannou, L.; Weiss, J.; Riffel, P.; Gutjahr, R.; Faby, S.; Nikolaou, K.; Horger, M. Myeloma Bone Disease Imaging on a 1st-Generation Clinical Photon-Counting Detector CT vs. 2nd-Generation Dual-Source Dual-Energy CT. Eur. Radiol. 2022, 33, 2415–2425. [Google Scholar] [CrossRef]
- Baffour, F.I.; Huber, N.R.; Ferrero, A.; Rajendran, K.; Glazebrook, K.N.; Larson, N.B.; Kumar, S.; Cook, J.M.; Leng, S.; Shanblatt, E.R.; et al. Photon-Counting Detector CT with Deep Learning Noise Reduction to Detect Multiple Myeloma. Radiology 2023, 306, 229–236. [Google Scholar] [CrossRef]
- Sieren, M.M.; Brenne, F.; Hering, A.; Kienapfel, H.; Gebauer, N.; Oechtering, T.H.; Fürschke, A.; Wegner, F.; Stahlberg, E.; Heldmann, S.; et al. Rapid Study Assessment in Follow-up Whole-Body Computed Tomography in Patients with Multiple Myeloma Using a Dedicated Bone Subtraction Software. Eur. Radiol. 2020, 30, 3198–3209. [Google Scholar] [CrossRef]
- Horger, M.; Thaiss, W.M.; Ditt, H.; Weisel, K.; Fritz, J.; Nikolaou, K.; Liao, S.; Kloth, C. Improved MDCT Monitoring of Pelvic Myeloma Bone Disease through the Use of a Novel Longitudinal Bone Subtraction Post-Processing Algorithm. Eur. Radiol. 2017, 27, 2969–2977. [Google Scholar] [CrossRef]
- Horger, M.; Ditt, H.; Liao, S.; Weisel, K.; Fritz, J.; Thaiss, W.M.; Kaufmann, S.; Nikolaou, K.; Kloth, C. Automated “Bone Subtraction” Image Analysis Software Package for Improved and Faster CT Monitoring of Longitudinal Spine Involvement in Patients with Multiple Myeloma. Acad. Radiol. 2017, 24, 623–632. [Google Scholar] [CrossRef]
- Gong, H.; Tao, S.; Rajendran, K.; Zhou, W.; McCollough, C.H.; Leng, S. Deep-learning-based Direct Inversion for Material Decomposition. Med. Phys. 2020, 47, 6294–6309. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Baffour, F.I.; Glazebrook, K.N.; Rhodes, N.G.; Tiegs-Heiden, C.A.; Thorne, J.E.; Cook, J.M.; Kumar, S.; Fletcher, J.G.; McCollough, C.H.; et al. Deep Learning-based Virtual Noncalcium Imaging in Multiple Myeloma Using Dual-energy CT. Med. Phys. 2022, 49, 6346–6358. [Google Scholar] [CrossRef] [PubMed]
- Reinert, C.P.; Krieg, E.; Esser, M.; Nikolaou, K.; Bösmüller, H.; Horger, M. Role of Computed Tomography Texture Analysis Using Dual-Energy-Based Bone Marrow Imaging for Multiple Myeloma Characterization: Comparison with Histology and Established Serologic Parameters. Eur. Radiol. 2021, 31, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Reinert, C.P.; Krieg, E.-M.; Bösmüller, H.; Horger, M. Mid-Term Response Assessment in Multiple Myeloma Using a Texture Analysis Approach on Dual Energy-CT-Derived Bone Marrow Images—A Proof of Principle Study. Eur. J. Radiol. 2020, 131, 109214. [Google Scholar] [CrossRef] [PubMed]
- Özgül, H.A.; Akin, I.B.; Mutlu, U.; Balci, A. Diagnostic Value of Machine Learning-Based Computed Tomography Texture Analysis for Differentiating Multiple Myeloma from Osteolytic Metastatic Bone Lesions in the Peripheral Skeleton. Skeletal Radiol. 2023, 52, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Huang, D.; Wu, J.; Chen, X.; Chen, Y.; Huang, C. 18F-FDG PET/CT Based Radiomics Features Improve Prediction of Prognosis: Multiple Machine Learning Algorithms and Multimodality Applications for Multiple Myeloma. BMC Med. Imaging 2023, 23, 87. [Google Scholar] [CrossRef]
- Almeida, S.D.; Santinha, J.; Oliveira, F.P.M.; Ip, J.; Lisitskaya, M.; Lourenço, J.; Uysal, A.; Matos, C.; João, C.; Papanikolaou, N. Quantification of Tumor Burden in Multiple Myeloma by Atlas-Based Semi-Automatic Segmentation of WB-DWI. Cancer Imaging 2020, 20, 6. [Google Scholar] [CrossRef]
- Ekert, K.; Hinterleitner, C.; Baumgartner, K.; Fritz, J.; Horger, M. Extended Texture Analysis of Non-Enhanced Whole-Body MRI Image Data for Response Assessment in Multiple Myeloma Patients Undergoing Systemic Therapy. Cancers 2020, 12, 761. [Google Scholar] [CrossRef]


| LDWBCT | DECT | WBMRI | FDG-PET/CT | PET/MRI | |
|---|---|---|---|---|---|
| Advantages |
|
|
|
|
|
| Disadvantages |
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Laval, V.; Lumbreras-Fernández, B.; Aguado-Bueno, B.; Gómez-León, N. Imaging of Multiple Myeloma: Present and Future. J. Clin. Med. 2024, 13, 264. https://doi.org/10.3390/jcm13010264
Rodríguez-Laval V, Lumbreras-Fernández B, Aguado-Bueno B, Gómez-León N. Imaging of Multiple Myeloma: Present and Future. Journal of Clinical Medicine. 2024; 13(1):264. https://doi.org/10.3390/jcm13010264
Chicago/Turabian StyleRodríguez-Laval, Víctor, Blanca Lumbreras-Fernández, Beatriz Aguado-Bueno, and Nieves Gómez-León. 2024. "Imaging of Multiple Myeloma: Present and Future" Journal of Clinical Medicine 13, no. 1: 264. https://doi.org/10.3390/jcm13010264
APA StyleRodríguez-Laval, V., Lumbreras-Fernández, B., Aguado-Bueno, B., & Gómez-León, N. (2024). Imaging of Multiple Myeloma: Present and Future. Journal of Clinical Medicine, 13(1), 264. https://doi.org/10.3390/jcm13010264






