Therapeutic Management of Children with Vesicoureteral Reflux
Abstract
:1. Introduction
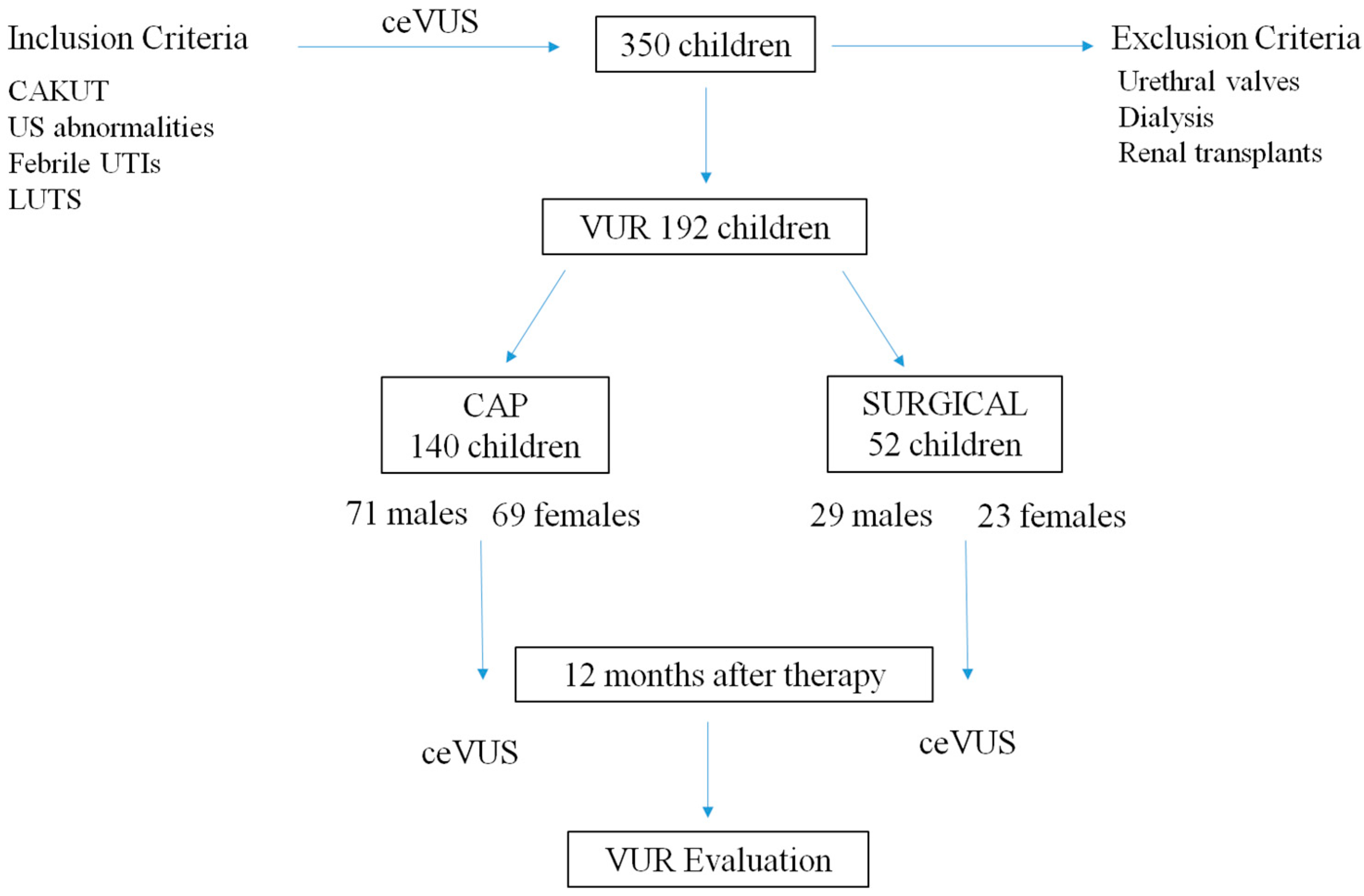
2. Materials and Methods
2.1. Patient and Study Design
2.2. Outcomes and Follow-Up
2.3. Statistical Analyses
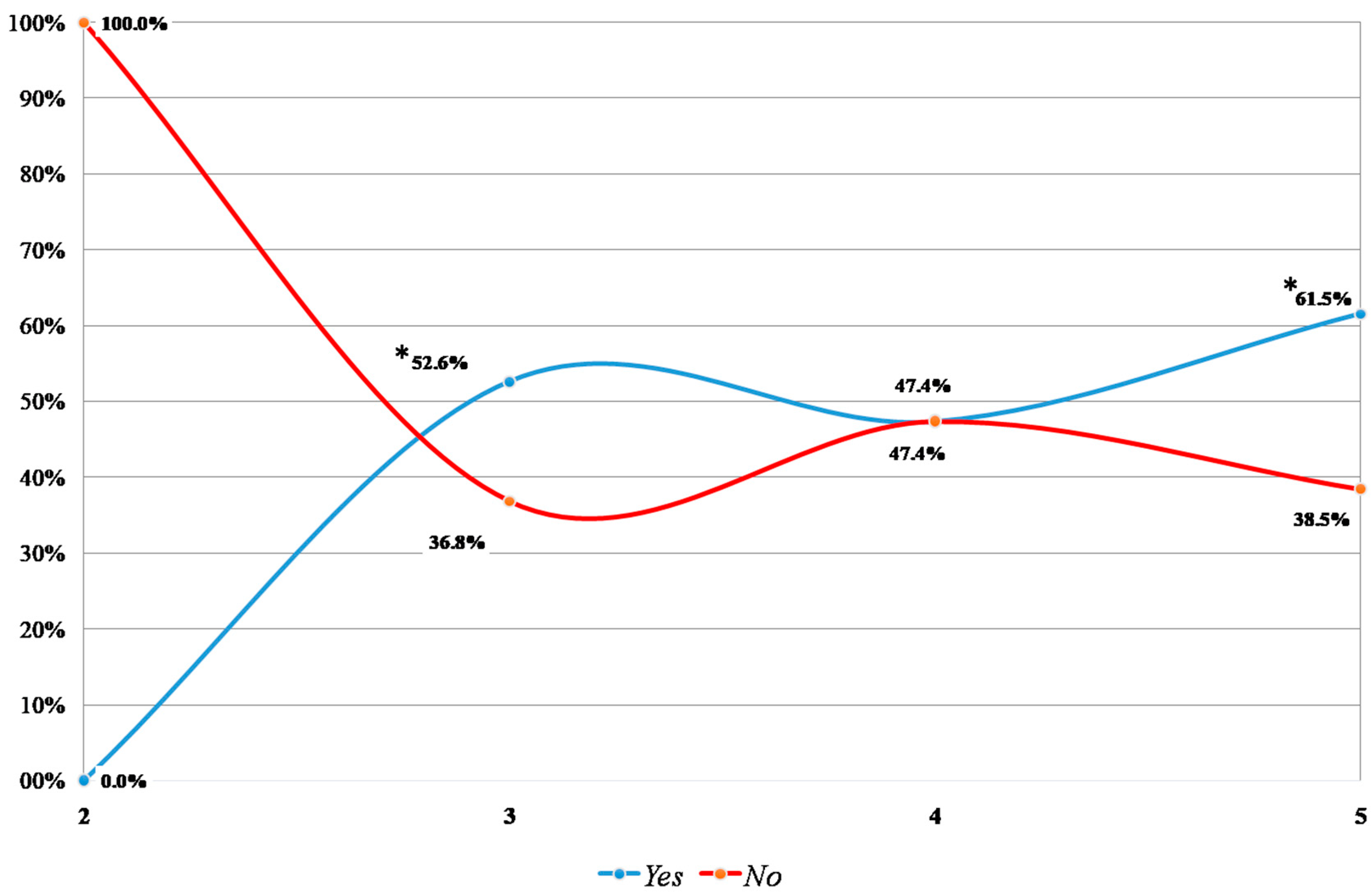
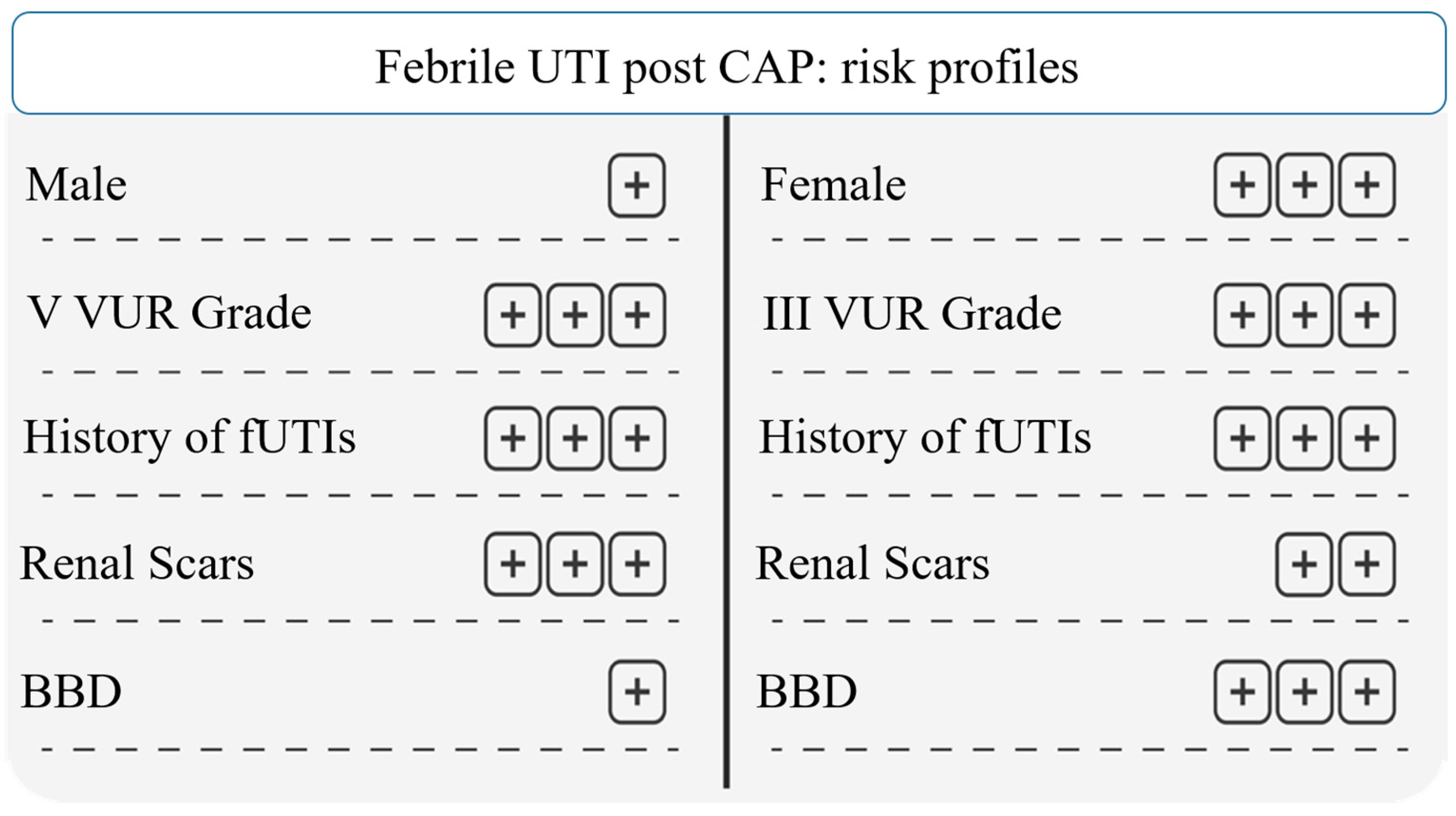
3. Results
3.1. Epidemiologic Data
3.2. Surgical Approach
3.3. Continuous Antibiotic Prophylaxis (CAP)
3.4. VUR and ceVUS after 12 Months
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cendron, M. Reflux nephropathy. J. Pediatr. Urol. 2008, 4, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, T.K.; Mohammad, D. Primary Vesicoureteral Reflux and Renal Scarring. Pediatr. Clin. N. Am. 2022, 69, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Sargent, M.A. What is the normal prevalence of vesicoureteral reflux? Pediatr. Radiol. 2000, 30, 587–593. [Google Scholar] [CrossRef]
- Liang, D.; McHugh, K.M.; Brophy, P.D.; Shaikh, N.; Manak, J.R.; Andrews, P.; Hakker, I.; Wang, Z.; Schwaderer, A.L.; Hains, D.S. DNA copy number variations in children with vesicoureteral reflux and urinary tract infections. PLoS ONE 2019, 14, e0220617. [Google Scholar] [CrossRef] [PubMed]
- Capozza, N.; Gulia, C.; Heidari Bateni, Z.; Zangari, A.; Gigli, S.; Briganti, V.; Tursini, S.; Koh, C.J.; Gaffi, M.; Baldassarra, S.; et al. Vesicoureteral reflux in infants: What do we know about the gender prevalence by age? Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5321–5329. [Google Scholar]
- Tekgül, S.; Riedmiller, H.; Hoebeke, P.; Kočvara, R.; Nijman, R.J.; Radmayr, C.; Stein, R.; Dogan, H.S. European Association of Urology. EAU guidelines on vesicoureteral reflux in children. Eur. Urol. 2012, 62, 534–542. [Google Scholar] [CrossRef]
- Miyakita, H.; Hayashi, Y.; Mitsui, T.; Okawada, M.; Kinoshita, Y.; Kimata, T.; Koikawa, Y.; Sakai, K.; Satoh, H.; Tokunaga, M.; et al. Guidelines for the medical management of pediatric vesicoureteral reflux. Int. J. Urol. 2020, 27, 480–490. [Google Scholar] [CrossRef]
- Kurt-Sukur, E.D.; Özçakar, Z.B.; Haznedar-Karakaya, P.; Yılmaz, S.; Elhan, A.H.; Çakar, N.; Yalçınkaya, F. Clinical characteristics and outcome of childhood vesicoureteral reflux. Arch. Argent. Pediatr. 2020, 118, e16–e21. [Google Scholar]
- Silva, J.M.; Diniz, J.S.; Silva, A.C.; Azevedo, M.V.; Pimenta, M.R.; Oliveira, E.A. Predictive factors of chronic kidney disease in severe vesicoureteral reflux. Pediatr. Nephrol. 2006, 21, 1285–1292. [Google Scholar] [CrossRef]
- Alberici, I.; La Manna, A.; Pennesi, M.; Starc, M.; Scozzola, F.; Nicolini, G.; Toffolo, A.; Marra, G.; Chimenz, R.; Sica, F.; et al. First urinary tract infections in children: The role of the risk factors proposed by the Italian recommendations. Acta Paediatr. 2019, 108, 544–550. [Google Scholar] [CrossRef]
- Hewitt, I.; Montini, G. Vesicoureteral reflux is it important to find? Pediatr. Nephrol. 2021, 36, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Chimenz, R.; Chirico, V.; Cuppari, C.; Sallemi, A.; Cardile, D.; Baldari, S.; Ascenti, G.; Monardo, P.; Lacquaniti, A. Febrile Urinary Tract Infections in Children: The Role of High Mobility Group Box-1. Children 2022, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- La Scola, C.; De Mutiis, C.; Hewitt, I.K.; Puccio, G.; Toffolo, A.; Zucchetta, P.; Mencarelli, F.; Marsciani, M.; Dall’amico, R.; Montini, G. Different guidelines for imaging after first UTI in febrile infants: Yield, cost, and radiation. Pediatrics 2013, 131, e665–e671. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Saha, S.; Maji, B.; Tse, Y. Antibiotics for performing voiding cystourethrogram: A randomised control trial. Arch. Dis. Child. 2018, 103, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Tzanis, E.; Raissaki, M.; Konstantinos, A.; Damilakis, J.; Perisinakis, K. Radiation exposure to infants undergoing voiding cystourethrography: The importance of the digital imaging technology. Phys. Med. 2021, 85, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Kis, E.; Nyitrai, A.; Varkonyi, I.; Mattyus, I.; Cseprekal, O.; Reusz, G.; Szabó, A. Voiding urosonography with second-generation contrast agent versus voiding cystourethrography. Pediatr. Nephrol. 2010, 25, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ma, T.; Huang, S.; Chen, G.; Dietrich, C.F.; Peng, Y.; Cui, X. A narrative review on the applications of intracavitary contrast-enhanced ultrasonography in pediatric lower genitourinary anomalies. Front. Pediatr. 2023, 11, 984643. [Google Scholar] [CrossRef]
- Colceriu, M.-C.; Aldea, P.L.; Boț, A.-L.; Bulată, B.; Delean, D.; Grama, A.; Mititelu, A.; Decea, R.M.; Sevastre-Berghian, A.; Clichici, S.; et al. The Utility of Noninvasive Urinary Biomarkers for the Evaluation of Vesicoureteral Reflux in Children. Int. J. Mol. Sci. 2023, 24, 17579. [Google Scholar] [CrossRef]
- Hewitt, I.K.; Roebuck, D.J.; Montini, G. Conflicting views of physicians and surgeons concerning pediatric urinary tract infection: A comparative review. Pediatr. Radiol. 2023, 53, 2651–2661. [Google Scholar] [CrossRef]
- Toffolo, A.; Ammenti, A.; Montini, G. Long-term clinical consequences of urinary tract infections during childhood: A review. Acta Paediatr. 2012, 101, 1018–1031. [Google Scholar] [CrossRef]
- Pepper, R.J.; Trompeter, R.S. The causes and consequences of paediatric kidney disease on adult nephrology care. Pediatr. Nephrol. 2022, 37, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, T.K. Medical management of vesicoureteral reflux--quiz within the article. Don’t overlook placebos. Pediatr. Nephrol. 2007, 22, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Läckgren, G.; Cooper, C.S.; Neveus, T.; Kirsch, A.J. Management of Vesicoureteral Reflux: What Have We Learned Over the Last 20 Years? Front. Pediatr. 2021, 9, 650326. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.W., Jr.; Wu, H.Y.; Selman, H.; Smith, G.H.; Snyder, H.M.; Canning, D.A. Spontaneous resolution of vesicoureteral reflux: A 15-year perspective. J. Urol. 2002, 168, 2594–2599. [Google Scholar] [CrossRef] [PubMed]
- Meena, J.; Mathew, G.; Hari, P.; Sinha, A.; Bagga, A. Prevalence of Bladder and Bowel Dysfunction in Toilet-Trained Children With Urinary Tract Infection and/or Primary Vesicoureteral Reflux: A Systematic Review and Meta-Analysis. Front. Pediatr. 2020, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.G.; de Winter, J.P.; Milani, G.P. Vesicoureteral reflux: We have yet to complete our learning. Eur. J. Pediatr. 2021, 180, 1381–1382. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.C.; Simpson, J.M.; Williams, G.J.; Lowe, A.; Reynolds, G.J.; McTaggart, S.J.; Hodson, E.M.; Carapetis, J.R.; Cranswick, N.E.; Smith, G.; et al. Prevention of Recurrent Urinary Tract Infection in Children with Vesicoureteric Reflux and Normal Renal Tracts (PRIVENT) Investigators. Antibiotic prophylaxis and recurrent urinary tract infection in children. N. Engl. J. Med. 2009, 361, 1748–1759. [Google Scholar] [CrossRef]
- Holmdahl, G.; Brandström, P.; Läckgren, G.; Sillén, U.; Stokland, E.; Jodal, U.; Hansson, S. The Swedish reflux trial in children: II. Vesicoureteral reflux outcome. J. Urol. 2010, 184, 280–285. [Google Scholar] [CrossRef]
- Keren, R.; Carpenter, M.A.; Hoberman, A.; Shaikh, N.; Matoo, T.K.; Chesney, R.W.; Matthews, R.; Gerson, A.C.; Greenfield, S.P.; Fivush, B.; et al. Rationale and design issues of the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) study. Pediatrics 2008, 122, S240–S250. [Google Scholar] [CrossRef]
- Alberici, I.; Bayazit, A.K.; Drozdz, D.; Emre, S.; Fischbach, M.; Harambat, J.; Jankauskiene, A.; Litwin, M.; Mir, S.; Morello, W.; et al. ESCAPE Study Group; PREDICT Trial. Pathogens causing urinary tract infections in infants: A European overview by the ESCAPE study group. Eur. J. Pediatr. 2015, 174, 783–790. [Google Scholar] [CrossRef]
- Morello, W.; D’Amico, F.; Serafinelli, J.; Turroni, S.; Abati, I.; Fiori, J.; Baskin, E.; Yalcinkaya, F.; Jankauskiene, A.; Pennesi, M.; et al. Low-Dose Antibiotic Prophylaxis Induces Rapid Modifications of the Gut Microbiota in Infants With Vesicoureteral Reflux. Front. Pediatr. 2021, 9, 674716. [Google Scholar] [CrossRef] [PubMed]
- Pensabene, M.; Cimador, M.; Spataro, B.; Serra, G.; Baldanza, F.; Grasso, F.; Corsello, G.; Salerno, S.; Di Pace, M.R.; Sergio, M. Intraoperative ultrasound-assisted endoscopic treatment of primary intermediate and high-grade vesicoureteral reflux in children in a long-term follow-up. J. Pediatr. Urol. 2023; In Press. [Google Scholar] [CrossRef]
- Austin, J.C.; Cooper, C.S. Vesicoureteral reflux: Surgical approaches. Urol. Clin. N. Am. 2004, 31, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Liu, B.; Luo, L.; Duan, X.; Cai, C.; Zhao, Z.; Zhu, W.; Wu, W.; Zeng, G. Robot-assisted laparoscopic versus open ureteral reimplantation for pediatric vesicoureteral reflux: A systematic review and meta-analysis. World J. Urol. 2018, 36, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, Y.S.; Han, S.W. Endoscopic injection therapy. Investig. Clin. Urol. 2017, 58, S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Escolino, M.; Kalfa, N.; Castagnetti, M.; Caione, P.; Esposito, G.; Florio, L.; Esposito, C. Endoscopic injection of bulking agents in pediatric vesicoureteral reflux: A narrative review of the literature. Pediatr. Surg. Int. 2023, 39, 133. [Google Scholar] [CrossRef]
- Hari, P.; Meena, J.; Kumar, M.; Sinha, A.; Iyengar, A.; Khandelwal, P.; Ekambaram, S.; Pais, P.; Sharma, J.; Kanitkar, M.; et al. Evidence-based clinical practice guideline for management of urinary tract infection and primary vesicoureteric reflux. Pediatr. Nephrol. 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mina-Riascos, S.H.; Fernández, N.; García-Perdomo, H.A. Effectiveness and risks of endoscopic management compared to vesicoureteral reimplantation in patients with high-grade vesicoureteral reflux: Systematic review and network meta-analysis. Eur. J. Pediatr. 2021, 180, 1383–1391. [Google Scholar] [CrossRef]
- Williams, G.; Hodson, E.M.; Craig, J.C. Interventions for primary vesicoureteric reflux. Cochrane Database Syst. Rev. 2019, 2, CD001532. [Google Scholar] [CrossRef]
- Ammenti, A.; Alberici, I.; Brugnara, M.; Chimenz, R.; Guarino, S.; La Manna, A.; La Scola, C.; Maringhini, S.; Marra, G.; Materassi, M.; et al. Updated Italian recommendations for the diagnosis, treatment and follow-up of the first febrile urinary tract infection in young children. Acta Paediatr. 2020, 109, 236–247. [Google Scholar] [CrossRef]
- Austin, P.F.; Bauer, S.B.; Bower, W.; Chase, J.; Franco, I.; Hoebeke, P.; Rittig, S.; Walle, J.V.; von Gontard, A.; Wright, A.; et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the standardization committee of the International Children’s Continence Society. Neurourol. Urodyn. 2016, 35, 471–481. [Google Scholar] [CrossRef]
- Drudi, F.M.; Angelini, F.; Bertolotto, M.; Granata, A.; Pierro, G.B.D.; Lai, Q.; D’Ermo, G.; Pretagostini, R.; Cantisani, V. Role of contrast-enhanced voiding urosonography in the evaluation of renal transplant reflux-comparison with voiding cystourethrography and a new classification. Ultraschall Med. 2022, 43, e73–e80. [Google Scholar] [CrossRef] [PubMed]
- Zimbaro, G.; Ascenti, G.; Visalli, C.; Bottari, A.; Zimbaro, F.; Martino, N.; Mazziotti, S. Contrast-enhanced ultrasonography (voiding urosonography) of vesicoureteral reflux: State of the art. Radiol. Med. 2007, 112, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.C.; Lee, K.H.; Park, J.S. Primary Vesico-Ureteral Reflux: Comparison of Factors between Infants and Children. Korean J. Urol. 2011, 52, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Brakeman, P. Vesicoureteral reflux, reflux nephropathy, and end-stage renal disease. Adv. Urol. 2008, 2008, 508949. [Google Scholar] [CrossRef] [PubMed]
- Chimenz, R.; Chirico, V.; Basile, P.; Carcione, A.; Conti, G.; Monardo, P.; Lacquaniti, A. HMGB-1 and TGFβ-1 highlight immuno-inflammatory and fibrotic processes before proteinuria onset in pediatric patients with Alport syndrome. J. Nephrol. 2021, 34, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Ardissino, G.; Dacco, V.; Testa, S.; Bonaudo, R.; Claris-Appiani, A.; Taioli, E.; Marra, G.; Edefonti, A.; Sereni, F. Epidemiology of Chronic Renal Failure in Children: Data From the ItalKid Project. Pediatrics 2003, 111, e382–e387. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lai, J.N.; Haw, Y.; Chiu, L.T.; Huang, S.M.; Cheng, K.L.; Chew, F.Y. Vesicoureteral reflux is associated with increased risk of chronic kidney disease: A nationwide cohort study. Medicine 2023, 102, e34867. [Google Scholar] [CrossRef]
- Routh, J.C. Endoscopic Therapy for High Grade Vesicoureteral Reflux-First Line Therapy or Too Good to be True? J. Urol. 2018, 200, 510–511. [Google Scholar] [CrossRef]
- Nordenström, J.; Holmdahl, G.; Brandström, P.; Sixt, R.; Stokland, E.; Sillén, U.; Sjöström, S. The Swedish infant high-grade reflux trial: Study presentation and vesicoureteral reflux outcome. J. Pediatr. Urol. 2017, 13, 130–138. [Google Scholar] [CrossRef]
- Roupakias, S.; Sinopidis, X.; Spyridakis, I.; Tsikopoulos, G.; Karatza, A.; Varvarigou, A. Endoscopic Injection Treatment of Vesicoureteral Reflux in Children: Meeting with the Factors Involved in the Success Rate. Acta Medica 2021, 64, 193–199. [Google Scholar] [CrossRef]
- Craig, J.C.; Irwig, L.M.; Knight, J.F.; Roy, L.P. Does treatment of vesicoureteric reflux in childhood prevent end-stage renal disease attributable to reflux nephropathy? Pediatrics 2000, 105, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.J.; Wei, L.; Lee, A.; Craig, J.C. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst. Rev. 2006, 4, CD001534. [Google Scholar]
- Pennesi, M.; Travan, L.; Peratoner, L.; Bordugo, A.; Cattaneo, A.; Ronfani, L.; Minisini, S.; Ventura, A.; North East Italy Prophylaxis in VUR Study Group. Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics 2008, 121, e1489–e1494. [Google Scholar] [CrossRef] [PubMed]
- Morello, W.; Baskin, E.; Jankauskiene, A.; Yalcinkaya, F.; Zurowska, A.; Puccio, G.; Serafinelli, J.; La Manna, A.; Krzemień, G.; Pennesi, M.; et al. Antibiotic Prophylaxis in Infants with Grade III, IV, or V Vesicoureteral Reflux. N. Engl. J. Med. 2023, 389, 987–997. [Google Scholar] [CrossRef]
- Loukogeorgakis, S.P.; Burnand, K.; MacDonald, A.; Wessely, K.; De Caluwe, D.; Rahman, N.; Farrugia, M.-K. Renal scarring is the most significant predictor of breakthrough febrile urinary tract infection in patients with simplex and duplex primary vesico-ureteral reflux. J. Pediatr. Urol. 2020, 16, e1–e189. [Google Scholar] [CrossRef]
- Palmer, L.S.; Seideman, C.A.; Lotan, Y. Cost-effectiveness of antimicrobial prophylaxis for children in the RIVUR trial. World J. Urol. 2018, 36, 1441–1447. [Google Scholar] [CrossRef]
- Shaikh, N.; Rajakumar, V.; Peterson, C.G.; Gorski, J.; Ivanova, A.; Gravens Muller, L.; Miyashita, Y.; Smith, K.J.; Mattoo, T.; Pohl, H.G.; et al. Cost-Utility of Antimicrobial Prophylaxis for Treatment of Children With Vesicoureteral Reflux. Front. Pediatr. 2020, 7, 530. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirico, V.; Tripodi, F.; Lacquaniti, A.; Monardo, P.; Conti, G.; Ascenti, G.; Chimenz, R. Therapeutic Management of Children with Vesicoureteral Reflux. J. Clin. Med. 2024, 13, 244. https://doi.org/10.3390/jcm13010244
Chirico V, Tripodi F, Lacquaniti A, Monardo P, Conti G, Ascenti G, Chimenz R. Therapeutic Management of Children with Vesicoureteral Reflux. Journal of Clinical Medicine. 2024; 13(1):244. https://doi.org/10.3390/jcm13010244
Chicago/Turabian StyleChirico, Valeria, Filippo Tripodi, Antonio Lacquaniti, Paolo Monardo, Giovanni Conti, Giorgio Ascenti, and Roberto Chimenz. 2024. "Therapeutic Management of Children with Vesicoureteral Reflux" Journal of Clinical Medicine 13, no. 1: 244. https://doi.org/10.3390/jcm13010244
APA StyleChirico, V., Tripodi, F., Lacquaniti, A., Monardo, P., Conti, G., Ascenti, G., & Chimenz, R. (2024). Therapeutic Management of Children with Vesicoureteral Reflux. Journal of Clinical Medicine, 13(1), 244. https://doi.org/10.3390/jcm13010244






