Pediatric Psoriasis with or without Arthritis: Does It Make a Difference?
Abstract
:1. Introduction
2. Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
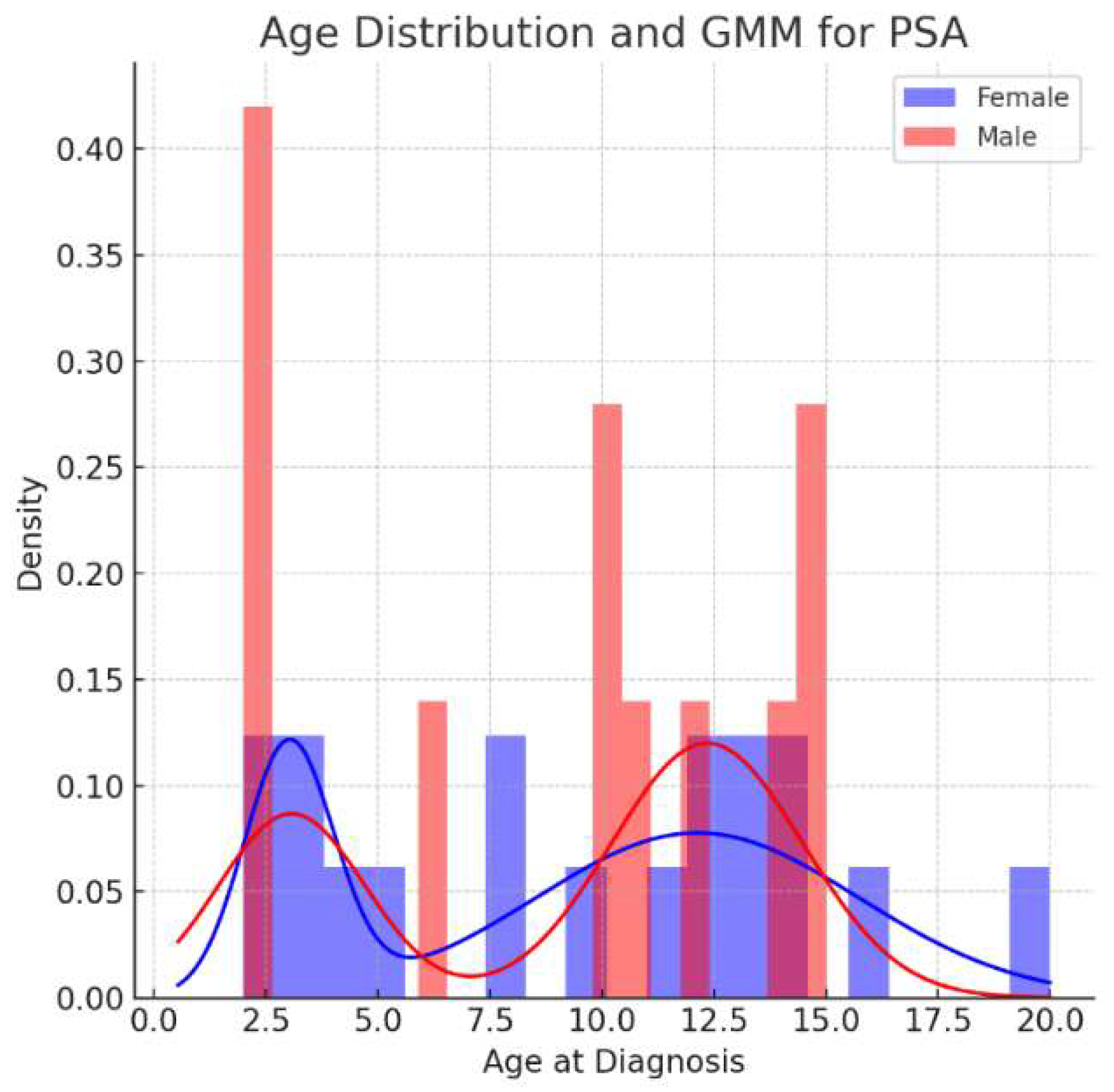
3.1.1. Psoriatic Arthritis Group (PSA)
GMM, Gaussian Mixture Model, PSA—Psoriatic Arthritis
3.1.2. Psoriasis-Only Group (PSO)
3.2. Comparison of PSA and PSO Groups
3.2.1. Body Surface Area Affected by Psoriasis
3.2.2. Anatomical Distribution of Psoriasis
3.2.3. Severity and Response to Treatment
3.2.4. Adverse Events
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menter, A.; Cordoro, K.M.; Davis, D.M.R.; Kroshinsky, D.; Paller, A.S.; Armstrong, A.W.; Connor, C.; Elewski, B.E.; Gelfand, J.M.; Gordon, K.B.; et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J. Am. Acad. Dermatol. 2020, 82, 161–201. [Google Scholar] [CrossRef]
- Imhof, R.L.; Eton, D.T.; Tollefson, M.M. The impact of childhood psoriasis on the quality of life of parents and caregivers. Pediatr. Dermatol. 2023, 40, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Mahe, E.; Maccari, F.; Beauchet, A.; Lahfa, M.; Barthelemy, H.; Reguiai, Z.; Beneton, N.; Estève, E.; Chaby, G.; Ruer-Mulard, M.; et al. Childhood-onset psoriasis: Association with future cardiovascular and metabolic comorbidities. Br. J. Dermatol. 2013, 169, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Rustad, A.M.; Nolan, B.E.; Ollech, A.; Boctor, M.J.; Paller, A.S. Incorporating joint pain screening into the pediatric dermatologic examination. Pediatr. Dermatol. 2021, 38, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Mercy, K.; Kwasny, M.; Cordoro, K.M.; Menter, A.; Tom, W.L.; Korman, N.; Belazarian, L.; Armstrong, A.W.; Levy, M.L.; Paller, A.S. Clinical manifestations of pediatric psoriasis: Results of a multicenter study in the United States. Pediatr. Dermatol. 2013, 30, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Tollefson, M.M.; Crowson, C.S.; McEvoy, M.T.; Maradit Kremers, H. Incidence of psoriasis in children: A population-based study. J. Am. Acad. Dermatol. 2010, 62, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Brunello, F.; Tirelli, F.; Pegoraro, L.; Dell’Apa, F.; Alfisi, A.; Calzamatta, G.; Folisi, C.; Zulian, F. New Insights on Juvenile Psoriatic Arthritis. Front. Pediatr. 2022, 10, 884727. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.-M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar] [PubMed]
- Shore, A.; Ansell, B.M. Juvenile psoriatic arthritis--an analysis of 60 cases. J. Pediatr. 1982, 100, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Stoll, M.L.; Zurakowski, D.; Nigrovic, L.E.; Nichols, D.P.; Sundel, R.P.; Nigrovic, P.A. Patients with juvenile psoriatic arthritis comprise two distinct populations. Arthritis Rheum. 2006, 54, 3564–3572. [Google Scholar] [CrossRef] [PubMed]
- Zisman, D.; Gladman, D.D.; Stoll, M.L.; Strand, V.; Lavi, I.; Hsu, J.J.; Mellins, E.D.; CARRA Legacy Registry Investigators. The Juvenile Psoriatic Arthritis Cohort in the CARRA Registry: Clinical Characteristics, Classification, and Outcomes. J. Rheumatol. 2017, 44, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Brandon, T.G.; Manos, C.K.; Xiao, R.; Ogdie, A.; Weiss, P.F. Pediatric psoriatic arthritis: A population-based cohort study of risk factors for onset and subsequent risk of inflammatory comorbidities. J. Psoriasis Psoriatic Arthritis 2018, 3, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Etzel, C.J.; Huster, W.J.; Muram, T.M.; Armstrong, A.W.; Lisse, J.R.; Rebello, S.; Dodge, R.; Murage, M.J.; Greenberg, J.D.; et al. Understanding the association between skin involvement and joint activity in patients with psoriatic arthritis: Experience from the Corrona Registry. RMD Open 2019, 5, e000867. [Google Scholar] [CrossRef] [PubMed]
- Pouw, J.N.; Jacobs, M.E.; Balak, D.M.W.; van Laar, J.M.; Welsing, P.M.J.; Leijten, E.F.A. Do Patients with Psoriatic Arthritis Have More Severe Skin Disease than Patients with Psoriasis Only? A Systematic Review and Meta-Analysis. Dermatology 2022, 238, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Southwood, T.R.; Petty, R.E.; Malleson, P.N.; Delgado, E.A.; Hunt, D.W.; Wood, B.; Schroeder, M.-L. Psoriatic arthritis in children. Arthritis Rheum. 1989, 32, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Zabotti, A.; De Marco, G.; Gossec, L.; Baraliakos, X.; Aletaha, D.; Iagnocco, A.; Gisondi, P.; Balint, P.V.; Bertheussen, H.; Boehncke, W.-H.; et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann. Rheum. Dis. 2023, 82, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Butbul Aviel, Y.; Tyrrell, P.; Schneider, R.; Dhillon, S.; Feldman, B.M.; Laxer, R.; Saurenmann, R.K.; Spiegel, L.; Cameron, B.; Tse, S.M.L.; et al. Juvenile Psoriatic Arthritis (JPsA): Juvenile arthritis with psoriasis? Pediatr. Rheumatol. Online J. 2013, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Bronckers, I.; Paller, A.S.; West, D.P.; Lara-Corrales, I.; Tollefson, M.M.; Tom, W.L.; Hogeling, M.; Belazarian, L.; Zachariae, C.; Mahé, E.; et al. A Comparison of Psoriasis Severity in Pediatric Patients Treated With Methotrexate vs. Biologic Agents. JAMA Dermatol. 2020, 156, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Camela, E.; Battista, T.; Genco, L.; Martora, F.; Noto, M.; Picone, P.; Ruggiero, A.; Monfrecola, G.; Fabbrocini, G.; et al. Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part I: Focus on pediatric patients. Expert Opin. Drug Saf. 2023, 22, 25–41. [Google Scholar] [CrossRef] [PubMed]


| Psoriatic Arthritis (n, %) | Psoriasis Only (n, %) | |
|---|---|---|
| Total | 29 | 64 |
| Past medical history | Depression 2 (6.8) Hypermobility 2 (6.8) Other 1 each (3.4) (ADHD, HS, celiac, CRPS, precocious puberty) Uveitis 4 (13.7) | Atopy (asthma, AD) 4 (6.3) ADHD 3 (4.7) Down syndrome (4.7) Eating disorder 2 (3.1) Short stature 2 (3.1) Other 1 each (1.5) (migraine, celiac, Crohn’s, hypothyroidism, metabolic syndrome, seizures) |
| Labs | Positive ANA 7 (24.1) | Positive strep A test 5 (7.8) |
| Family history psoriasis | 20 (68) 1st 10 (34.4) 2nd 6 (20.6) 1st + 2nd 4 (13.7) | 48 (75) 1st 37 (57.8) 2nd 11 (17.2) |
| Family history arthritis | 6 (20%) RA 3 (10%) | NA |
| Psoriasis subtype | Plaque 23 (79) No skin lesions 6 (20.6) | Plaque 51 (79.7) Guttate 5 (7.8) Inversa 3 (4.7) Nail 2 (3.1) Palmoplantar 2 (3.1) |
| Psoriasis involvement | Scalp 13 (44.8) Face, ears 4 (13.8) Trunk 5 (17.2) Extremities 12 (41.3) Inversa 6 (20.7) Nails 6 (20.7) Dactylitis 10 (34.4) | Scalp 39 (60.9) Face, ears 21 (32.8) Trunk 37 (57.8) Extremities 28 (75) Inversa 24 (37.5) Nails 8 (12.5) |
| Skin involvement severity | Severe 6 (30%) Mild–moderate 14 (70%) | Severe 14 (22%) Mild–moderate 46 (76%) |
| Psoriatic arthritis subtype | Oligoarthritic 19 (65.5) Polyarthritis 8 (27.5) Sacroiliitis 2 (0.07) | |
| Joint involvement | Elbow-4 (6.5) Hands-7 (11.4) Wrists-8 (13.2) Feet-5 (8.1) Ankle 13 (21.3) | |
| Joint disease severity | Mild–moderate 7 (24%) Severe 22 (76%) |
| Treatment | Disease | No Response (n, %) | Complete Response (n, %) | Partial Response (n, %) | Missing Data (n, % of Total) | Total Patients |
|---|---|---|---|---|---|---|
| MTX | PSO | 1 (25%) | 1 (25%) | 2 (50%) | 3 (43%) | 7 (11%) |
| PSA | 1 (20%) | 1 (20%) | 3 (60%) | - | 5 (17% | |
| Biological | PSO | - | 3 (60%) | 2 (40%) | - | 5 (8%) |
| PSA | 1 (25%) | - | 3 (75%) | - | 4 (13.8%) | |
| Phototherapy | PSO | 1 (3%) | 12 (35%) | 21 (62%) | 6 (15%) | 40 (61.5%) |
| PSA | - | 2 (40%) | 3 (60%) | - | 5 (17%) | |
| Acitretin | PSO | 1 (25%) | 2 (50%) | 1 (25%) | 4 (50%) | 8 (12.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ollech, A.; Rotenberg, M.; Tirosh, I.; Bar-Ilan, E.; Solomon, M.; Greenberger, S.; Pavlotsky, F. Pediatric Psoriasis with or without Arthritis: Does It Make a Difference? J. Clin. Med. 2024, 13, 242. https://doi.org/10.3390/jcm13010242
Ollech A, Rotenberg M, Tirosh I, Bar-Ilan E, Solomon M, Greenberger S, Pavlotsky F. Pediatric Psoriasis with or without Arthritis: Does It Make a Difference? Journal of Clinical Medicine. 2024; 13(1):242. https://doi.org/10.3390/jcm13010242
Chicago/Turabian StyleOllech, Ayelet, Mor Rotenberg, Irit Tirosh, Efrat Bar-Ilan, Michal Solomon, Shoshana Greenberger, and Felix Pavlotsky. 2024. "Pediatric Psoriasis with or without Arthritis: Does It Make a Difference?" Journal of Clinical Medicine 13, no. 1: 242. https://doi.org/10.3390/jcm13010242
APA StyleOllech, A., Rotenberg, M., Tirosh, I., Bar-Ilan, E., Solomon, M., Greenberger, S., & Pavlotsky, F. (2024). Pediatric Psoriasis with or without Arthritis: Does It Make a Difference? Journal of Clinical Medicine, 13(1), 242. https://doi.org/10.3390/jcm13010242







