The Role of High-Flow Nasal Cannula Oxygen Therapy in Exercise Testing and Pulmonary Rehabilitation: A Review of the Current Literature
Abstract
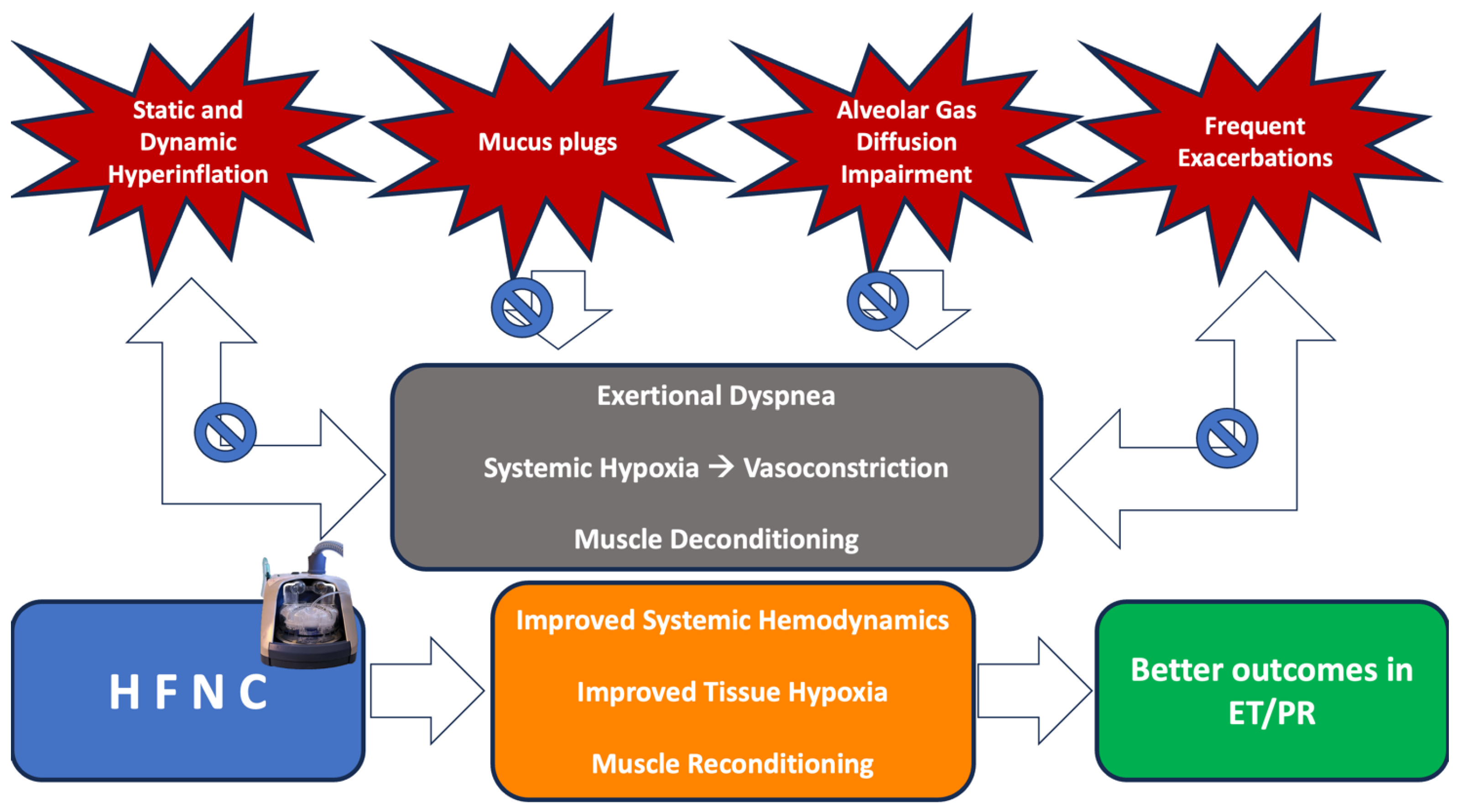
:1. Introduction
- humidify the epithelium of the airways, thus improving ciliary motion and mucus clearance [4];
- wash out the dead space, removing excess carbon dioxide (CO2), thus improving hypercapnia [5];
- reduce the work of breathing [6];
- generate a small end-expiratory positive pressure (PEEP), which helps wash out CO2 and prevents the airways from collapsing during the expiratory phase [7].
2. Search Strategy
3. Rationale for the Application of HFNC during ET or PR
4. Effects of HFNC on ET and PR in COPD Patients
5. HFNC and PR in Other Respiratory Diseases
5.1. HFNC during ET and PR in ILDs
5.2. HFNC and ET in Patients with Primary or Secondary Lung Cancer
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boccatonda, A.; Groff, P. High-flow nasal cannula oxygenation utilization in respiratory failure. Eur. J. Intern. Med. 2019, 64, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.G. High-Flow Nasal Cannula Oxygen in Adults: An Evidence-based Assessment. Ann. Am. Thorac. Soc. 2018, 15, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Spoletini, G.; Alotaibi, M.; Blasi, F.; Hill, N.S. Heated Humidified High-Flow Nasal Oxygen in Adults: Mechanisms of Action and Clinical Implications. Chest 2015, 148, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Chikata, Y.; Izawa, M.; Okuda, N.; Itagaki, T.; Nakataki, E.; Onodera, M.; Imanaka, H.; Nishimura, M. Humidification performance of two high-flow nasal cannula devices: A bench study. Respir. Care 2014, 59, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Moller, W.; Feng, S.; Domanski, U.; Franke, K.J.; Celik, G.; Bartenstein, P.; Becker, S.; Meyer, G.; Schmid, O.; Eickelberg, O.; et al. Nasal high flow reduces dead space. J. Appl. Physiol. 2017, 122, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Turrini, C.; Eronia, N.; Grasselli, G.; Volta, C.A.; Bellani, G.; Pesenti, A. Physiologic Effects of High-Flow Nasal Cannula in Acute Hypoxemic Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Vega, M.L. Use of Nasal High Flow in Stable COPD: Rationale and Physiology. COPD 2017, 14, 346–350. [Google Scholar] [CrossRef]
- Moreel, L.; Proesmans, M. High flow nasal cannula as respiratory support in treating infant bronchiolitis: A systematic review. Eur. J. Pediatr. 2020, 179, 711–718. [Google Scholar] [CrossRef]
- Nishimura, M. High-flow nasal cannula oxygen therapy in adults. J. Intensive Care 2015, 3, 15. [Google Scholar] [CrossRef]
- Lee, J.H.; Rehder, K.J.; Williford, L.; Cheifetz, I.M.; Turner, D.A. Use of high flow nasal cannula in critically ill infants, children, and adults: A critical review of the literature. Intensive Care Med. 2013, 39, 247–257. [Google Scholar] [CrossRef]
- Crimi, C.; Pierucci, P.; Renda, T.; Pisani, L.; Carlucci, A. High-Flow Nasal Cannula and COVID-19: A Clinical Review. Respir. Care 2022, 67, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Tascón, G.A.; Martínez, D.; Gempeler, A. High-Flow Oxygen vs Conventional Oxygen and Invasive Mechanical Ventilation and Clinical Recovery in Patients with Severe COVID-19-Reply. JAMA 2022, 327, 1092–1093. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Ji, C.; Connolly, B.A.; Couper, K.; Lall, R.; Baillie, J.K.; Bradley, J.M.; Dark, P.; Dave, C.; De Soyza, A.; et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients with Acute Hypoxemic Respiratory Failure and COVID-19, The RECOVERY-RS Randomized Clinical Trial. JAMA 2022, 327, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Crimi, C.; Noto, A.; Madotto, F.; Ippolito, M.; Nolasco, S.; Campisi, R.; De Vuono, S.; Fiorentino, G.; Pantazopoulos, I.; Chalkias, A.; et al. High-flow nasal oxygen versus conventional oxygen therapy in patients with COVID-19 pneumonia and mild hypoxaemia: A randomised controlled trial. Thorax 2023, 78, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Simioli, F.; Fiorentino, G.; Cauteruccio, R.; Coppola, A.; Imitazione, P.; Marotta, A.; Di Spirito, V.; Annunziata, A. Long-Term High Flow Nasal Cannula Therapy in Primary and Secondary Bronchiectasis. Healthcare 2023, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Good, W.R.; Garrett, J.; Hockey, H.U.P.; Jayaram, L.; Wong, C.; Rea, H. The role of high-flow nasal therapy in bronchiectasis: A post hoc analysis. ERJ Open Res. 2021, 7, 00711-2020. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Kikuchi, T.; Horie, T.; Shiraki, A.; Kitajima, T.; Kadowaki, T.; Tokioka, F.; Chohnabayashi, N.; Watanabe, A.; Sato, S.; et al. Domiciliary High-Flow Nasal Cannula Oxygen Therapy for Patients with Stable Hypercapnic Chronic Obstructive Pulmonary Disease. A Multicenter Randomized Crossover Trial. Ann. Am. Thorac. Soc. 2018, 15, 432–439. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Ye, Y.; Gao, J.; Zhu, F.; Min, L. Comparison of High-Flow Nasal Cannula with Conventional Oxygen Therapy in Patients with Hypercapnic Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Int. J. Chron. Obstruct Pulmon Dis. 2023, 18, 895–906. [Google Scholar] [CrossRef]
- Oczkowski, S.; Ergan, B.; Bos, L.; Chatwin, M.; Ferrer, M.; Gregoretti, C.; Heunks, L.; Frat, J.P.; Longhini, F.; Nava, S.; et al. ERS clinical practice guidelines: High-flow nasal cannula in acute respiratory failure. Eur. Respir. J. 2022, 59, 2101574. [Google Scholar] [CrossRef]
- Elshof, J.; Duiverman, M.L. Clinical Evidence of Nasal High-Flow Therapy in Chronic Obstructive Pulmonary Disease Patients. Respiration 2020, 99, 140–153. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, R.; ZuWallack, R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N. Engl. J. Med. 2009, 360, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Clofent, D.; Alvarez, A.; Traversi, L.; Culebras, M.; Loor, K.; Polverino, E. Comorbidities and mortality risk factors for patients with bronchiectasis. Expert. Rev. Respir. Med. 2021, 15, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Crimi, C.; Ferri, S.; Campisi, R.; Crimi, N. The Link between Asthma and Bronchiectasis: State of the Art. Respiration 2020, 99, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Bocchino, M.; Alicante, P.; Capitelli, L.; Stanziola, A.A.; Gallotti, L.; Di Gregorio, A.; Rea, G.; Sanduzzi Zamparelli, A.; Scalfi, L. Dynapenia is highly prevalent in older patients with advanced idiopathic pulmonary fibrosis. Sci. Rep. 2021, 11, 17884. [Google Scholar] [CrossRef] [PubMed]
- Braunlich, J.; Beyer, D.; Mai, D.; Hammerschmidt, S.; Seyfarth, H.J.; Wirtz, H. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration 2013, 85, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, U.M.; Burchardt, C.; Huremovic, J. The effect of domiciliary high flow nasal cannula treatment on dyspnea and walking distance in patients with interstitial lung disease — A pilot study. Chron. Respir. Dis. 2022, 19, 14799731221137085. [Google Scholar] [CrossRef]
- Maggiore, S.M.; Idone, F.A.; Vaschetto, R.; Festa, R.; Cataldo, A.; Antonicelli, F.; Montini, L.; De Gaetano, A.; Navalesi, P.; Antonelli, M. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am. J. Respir. Crit. Care Med. 2014, 190, 282–288. [Google Scholar] [CrossRef]
- Holland, A.E. Exercise limitation in interstitial lung disease - mechanisms, significance and therapeutic options. Chron. Respir. Dis. 2010, 7, 101–111. [Google Scholar] [CrossRef]
- Kent, B.D.; Mitchell, P.D.; McNicholas, W.T. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chron. Obstruct Pulmon Dis. 2011, 6, 199–208. [Google Scholar]
- Neunhauserer, D.; Steidle-Kloc, E.; Weiss, G.; Kaiser, B.; Niederseer, D.; Hartl, S.; Tschentscher, M.; Egger, A.; Schonfelder, M.; Lamprecht, B.; et al. Supplemental Oxygen During High-Intensity Exercise Training in Nonhypoxemic Chronic Obstructive Pulmonary Disease. Am. J. Med. 2016, 129, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.Y.; Liu, W.L.; Nassef, Y.; Tseng, C.W.; Wang, J.S. Effects of high-flow nasal cannula with oxygen on self-paced exercise performance in COPD: A randomized cross-over trial. Medicine 2021, 100, e28032. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Chihara, Y.; Tsuboi, T.; Sumi, K.; Sato, A. Effectiveness of high-flow nasal cannula on pulmonary rehabilitation in subjects with chronic respiratory failure. Respir. Investig. 2022, 60, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Cirio, S.; Piran, M.; Vitacca, M.; Piaggi, G.; Ceriana, P.; Prazzoli, M.; Paneroni, M.; Carlucci, A. Effects of heated and humidified high flow gases during high-intensity constant-load exercise on severe COPD patients with ventilatory limitation. Respir. Med. 2016, 118, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Liu, X.; Zhu, Q.; Wu, X.; Hao, S.; Xie, L.; Li, S. Efficiency of High-Flow Nasal Cannula on Pulmonary Rehabilitation in COPD Patients: A Meta-Analysis. Biomed. Res. Int. 2020, 2020, 7097243. [Google Scholar] [CrossRef] [PubMed]
- Vitacca, M.; Paneroni, M.; Zampogna, E.; Visca, D.; Carlucci, A.; Cirio, S.; Banfi, P.; Pappacoda, G.; Trianni, L.; Brogneri, A.; et al. High-Flow Oxygen Therapy During Exercise Training in Patients with Chronic Obstructive Pulmonary Disease and Chronic Hypoxemia: A Multicenter Randomized Controlled Trial. Phys. Ther. 2020, 100, 1249–1259. [Google Scholar] [CrossRef]
- Volpi, V.; Volpato, E.; Compalati, E.; Lebret, M.; Russo, G.; Sciurello, S.; Pappacoda, G.; Nicolini, A.; Banfi, P. Efficacy of Nasal High-Flow Oxygen Therapy in Chronic Obstructive Pulmonary Disease Patients in Long-Term Oxygen and Nocturnal Non-Invasive Ventilation during Exercise Training. Healthcare 2022, 10, 2001. [Google Scholar] [CrossRef]
- Harada, J.; Nagata, K.; Morimoto, T.; Iwata, K.; Matsunashi, A.; Sato, Y.; Tachikawa, R.; Ishikawa, A.; Tomii, K. Effect of high-flow nasal cannula oxygen therapy on exercise tolerance in patients with idiopathic pulmonary fibrosis: A randomized crossover trial. Respirology 2022, 27, 144–151. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Suzuki, A.; Ando, M.; Kimura, T.; Kataoka, K.; Yokoyama, T.; Shiroshita, E.; Kondoh, Y. The impact of high-flow nasal cannula oxygen therapy on exercise capacity in fibrotic interstitial lung disease: A proof-of-concept randomized controlled crossover trial. BMC Pulm. Med. 2020, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Mahler, D.A.; Larsson, L.; Wu, J.; Thomas, S.; Harrison, C.A.; Hess, K.; Lopez-Mattei, J.; Thompson, K.; Gomez, D.; et al. High-Flow Nasal Cannula Therapy for Exertional Dyspnea in Patients with Cancer: A Pilot Randomized Clinical Trial. Oncologist 2021, 26, e1470–e1479. [Google Scholar] [CrossRef] [PubMed]
| Study | Subjects | Design | Intervention | Performance Tests | Main Findings |
|---|---|---|---|---|---|
| Chao et al. (2021) [32] | 30 stable COPD patients at discharge from PR | Randomized crossover trial | HFNC vs. COT | 6MWT | ↑ 6MWD *, ↔ Dyspnea, ↔ HR, ↔ BP, ↔ RR |
| Chihara et al. (2022) [34] | 13 COPD patients with CRF | Single-center RCT/crossover trial | HFNC at 50 L/min and FiO2 100% vs. Oxygen at 6 L/min through nasal cannula during PR | 6MWT, Constant-load exercise testing at 80% maximal capacity | ↑ 6MWD, ↔ Endurance time, ↔ Dyspnea, ↔ HR, ↔ BP, ↔ RR |
| Cirio et al. (2016) [35] | 12 severe, stable, ventilatory-limited COPD patients | Randomized crossover trial | HFNC vs. VM at the same FiO2 | Constant-load exercise testing at 75% maximal capacity | ↑ Endurance Time, ↑ SpO2, ↓ Dyspnea |
| Fu et al. (2020) [36] | 600 Stable COPD patients from eight studies | Metanalysis | HFNC vs. COT (six studies) HFNC vs. NIV (two studies) | 6MWT, Constant-load exercise testing | ↓ RR ^, ↔ PaO2, ↑ SGRQ, ↑ 6MWD, ↔ Endurance Time |
| Vitacca et al. (2020) [37] | 171 COPD patients undergoing PR | Multicenter RCT | HFNC vs. VM during PR | 6MWT, Constant-load exercise testing | ↑ 6MWD, ↔ Endurance Time |
| Volpi et al. (2022) [38] | 31 COPD patients with nocturnal NIV undergoing PR | Single-center RCT | HFNC vs. COT during PR | 6MWT, Constant-load exercise testing | ↔ 6MWD, ↓ Dyspnea, ↓ Fatigue |
| Study | Subjects | Design | Intervention | Performance Tests | Main Findings |
|---|---|---|---|---|---|
| Chihara et al. (2022) [34] | 15 IPF patients with CRF | Single-center RCT/crossover trial | HFNC at 50 L/min and FiO2 100% vs. Oxygen at 6 L/min through nasal cannula during PR | 6MWT, Constant-load exercise testing at 80% maximal capacity | ↑ 6MWD, ↔ Endurance time, ↔ Dyspnea, ↔ HR, ↔ BP, ↔ RR |
| Harada et al. (2022) [39] | 24 IPF patients with exertional dyspnea and desaturation | Randomized crossover trial | HFNC vs. VM at the same FiO2 | Constant-load exercise testing at 80% maximal capacity | ↑ Endurance Time, ↑ SpO2, ↓ Leg Fatigue, ↔ Dyspnea, ↔ HR, ↔ Comfort |
| Suzuki et al. (2020) [41] | 20 fibrotic ILDs patients with mild exertional desaturation | Randomized crossover trial | HFNC vs. VM at the same FiO2 | Constant-load exercise testing | ↔ Endurance Time, ↔ SpO2, ↔ Dyspnea, ↔ HR, ↔ Comfort |
| Hui et al. (2021) [42] | 45 non-hypoxemic patients with primary or secondary Lung Cancer | Single-center RCT | HFNC with FiO2 100% vs. HFNC without O2 vs. COT vs. Low-Flow Air at 2 L/min | Constant-load exercise testing | ↑ Endurance Time +, ↓ Dyspnea + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candia, C.; Lombardi, C.; Merola, C.; Ambrosino, P.; D’Anna, S.E.; Vicario, A.; De Marco, S.; Molino, A.; Maniscalco, M. The Role of High-Flow Nasal Cannula Oxygen Therapy in Exercise Testing and Pulmonary Rehabilitation: A Review of the Current Literature. J. Clin. Med. 2024, 13, 232. https://doi.org/10.3390/jcm13010232
Candia C, Lombardi C, Merola C, Ambrosino P, D’Anna SE, Vicario A, De Marco S, Molino A, Maniscalco M. The Role of High-Flow Nasal Cannula Oxygen Therapy in Exercise Testing and Pulmonary Rehabilitation: A Review of the Current Literature. Journal of Clinical Medicine. 2024; 13(1):232. https://doi.org/10.3390/jcm13010232
Chicago/Turabian StyleCandia, Claudio, Carmen Lombardi, Claudia Merola, Pasquale Ambrosino, Silvestro Ennio D’Anna, Aldo Vicario, Stefania De Marco, Antonio Molino, and Mauro Maniscalco. 2024. "The Role of High-Flow Nasal Cannula Oxygen Therapy in Exercise Testing and Pulmonary Rehabilitation: A Review of the Current Literature" Journal of Clinical Medicine 13, no. 1: 232. https://doi.org/10.3390/jcm13010232
APA StyleCandia, C., Lombardi, C., Merola, C., Ambrosino, P., D’Anna, S. E., Vicario, A., De Marco, S., Molino, A., & Maniscalco, M. (2024). The Role of High-Flow Nasal Cannula Oxygen Therapy in Exercise Testing and Pulmonary Rehabilitation: A Review of the Current Literature. Journal of Clinical Medicine, 13(1), 232. https://doi.org/10.3390/jcm13010232










