Factors Contributing to the Development of Choroidal Microvasculature Dropout in Glaucoma Suspects and Patients with Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Definition of Central VF Progression
2.3. HRV (Heart Rate Variability) Assessment
2.4. OCT Examination
2.5. Analysis of OCT Images for Determining LC Parameters
2.6. OCT-A Examination
2.7. Confocal Scanning Laser Ophthalmoscopy to Measure ONH Surface Depression
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Faiq, M.A.; Sofi, R.A. A Glimpse into the mysteries of glaucoma: From theories to clinics. Oman J. Ophthalmol. 2019, 12, 1–3. [Google Scholar] [CrossRef]
- Ahmad, S.S. Controversies in the vascular theory of glaucomatous optic nerve degeneration. Taiwan J. Ophthalmol. 2016, 6, 182–186. [Google Scholar] [CrossRef]
- O’Brart, D.P.; de Souza Lima, M.; Bartsch, D.U.; Freeman, W.; Weinreb, R.N. Indocyanine green angiography of the peripapillary region in glaucomatous eyes by confocal scanning laser ophthalmoscopy. Am. J. Ophthalmol. 1997, 123, 657–666. [Google Scholar] [CrossRef]
- Flammer, J. The vascular concept of glaucoma. Surv. Ophthalmol. 1994, 38, S3–S6. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, S.; Hwang, S.; Han, J.C.; Kee, C. Microvascular Compromise Develops Following Nerve Fiber Layer Damage in Normal-Tension Glaucoma Without Choroidal Vasculature Involvement. J. Glaucoma 2017, 26, 216–222. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, S.H.; Kim, J.A.; Kim, T.W. Parapapillary Deep-Layer Microvasculature Dropout in Glaucoma: Topographic Association With Glaucomatous Damage. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3004–3010. [Google Scholar] [CrossRef]
- Kim, G.N.; Lee, E.J.; Kim, T.W. Parapapillary choroidal microvasculature dropout in nonglaucomatous healthy eyes. Acta Ophthalmol. 2020, 98, e754–e760. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, T.W.; Kim, J.A. Central Visual Field Damage and Parapapillary Choroidal Microvasculature Dropout in Primary Open-Angle Glaucoma. Ophthalmology 2018, 125, 588–596. [Google Scholar] [CrossRef]
- Park, H.L.; Kim, J.W.; Park, C.K. Choroidal Microvasculature Dropout Is Associated with Progressive Retinal Nerve Fiber Layer Thinning in Glaucoma with Disc Hemorrhage. Ophthalmology 2018, 125, 1003–1013. [Google Scholar] [CrossRef]
- Shin, J.W.; Jo, Y.H.; Song, M.K.; Won, H.J.; Kook, M.S. Nocturnal blood pressure dip and parapapillary choroidal microvasculature dropout in normal-tension glaucoma. Sci. Rep. 2021, 11, 206. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.A.; Kim, T.W.; Kim, H.; Yang, H.K.; Hwang, J.M. Glaucoma-like Parapapillary Choroidal Microvasculature Dropout in Patients with Compressive Optic Neuropathy. Ophthalmology 2020, 127, 1652–1662. [Google Scholar] [CrossRef]
- Jung, Y.; Park, H.L.; Shin, H.; Oh, S.E.; Kim, S.A.; Lee, J.Y.; Shin, D.Y.; Jeon, S.J.; Kim, Y.C.; Shin, H.Y.; et al. Microvasculature Dropout and Development of Normal Tension Glaucoma in Glaucoma Suspects: The Normal Tension Glaucoma Suspect Cohort Study. Am. J. Ophthalmol. 2022, 243, 135–148. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Durbin, M.K.; Budenz, D.L.; Sayyad, F.E.; Chang, R.T.; Neelakantan, A.; Godfrey, D.G.; Carter, R.; Crandall, A.S. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: Comparison with nerve fiber layer and optic nerve head. Ophthalmology 2012, 119, 1151–1158. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Oakley, J.D.; Budenz, D.L.; Anderson, D.R. Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology 2011, 118, 241–248.e241. [Google Scholar] [CrossRef]
- Park, H.Y.; Shin, H.Y.; Jung, K.I.; Park, C.K. Changes in the lamina and prelamina after intraocular pressure reduction in patients with primary open-angle glaucoma and acute primary angle-closure. Investig. Ophthalmol. Vis. Sci. 2014, 55, 233–239. [Google Scholar] [CrossRef]
- Chauhan, B.C.; Hutchison, D.M.; Artes, P.H.; Caprioli, J.; Jonas, J.B.; LeBlanc, R.P.; Nicolela, M.T. Optic disc progression in glaucoma: Comparison of confocal scanning laser tomography to optic disc photographs in a prospective study. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1682–1691. [Google Scholar] [CrossRef]
- Chauhan, B.C.; Nicolela, M.T.; Artes, P.H. Incidence and rates of visual field progression after longitudinally measured optic disc change in glaucoma. Ophthalmology 2009, 116, 2110–2118. [Google Scholar] [CrossRef]
- Fleiss, J.L. The Design and Analysis of Clinical Experiments; Wiley Series in Probability and Mathematical Statistics Applied Probability and Statistics; Wiley: New York, NY, USA, 1986; p. xiv. 432p. [Google Scholar]
- Hood, D.C.; Raza, A.S.; de Moraes, C.G.; Odel, J.G.; Greenstein, V.C.; Liebmann, J.M.; Ritch, R. Initial arcuate defects within the central 10 degrees in glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 940–946. [Google Scholar] [CrossRef]
- Ichiyama, Y.; Minamikawa, T.; Niwa, Y.; Ohji, M. Capillary Dropout at the Retinal Nerve Fiber Layer Defect in Glaucoma: An Optical Coherence Tomography Angiography Study. J. Glaucoma 2017, 26, e142–e145. [Google Scholar] [CrossRef]
- Suh, M.H.; Zangwill, L.M.; Manalastas, P.I.; Belghith, A.; Yarmohammadi, A.; Medeiros, F.A.; Diniz-Filho, A.; Saunders, L.J.; Weinreb, R.N. Deep Retinal Layer Microvasculature Dropout Detected by the Optical Coherence Tomography Angiography in Glaucoma. Ophthalmology 2016, 123, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.H.; Zangwill, L.M.; Manalastas, P.I.; Belghith, A.; Yarmohammadi, A.; Medeiros, F.A.; Diniz-Filho, A.; Saunders, L.J.; Yousefi, S.; Weinreb, R.N. Optical Coherence Tomography Angiography Vessel Density in Glaucomatous Eyes with Focal Lamina Cribrosa Defects. Ophthalmology 2016, 123, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, C.K.; Park, H.L. Determinants of vessel defects in superficial and deep vascular layers in normal-tension glaucoma using optical coherence tomography angiography. Sci. Rep. 2021, 11, 9941. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.C.; Blanchard, J.W.; Hamilton, D.C.; LeBlanc, R.P. Technique for detecting serial topographic changes in the optic disc and peripapillary retina using scanning laser tomography. Investig. Ophthalmol. Vis. Sci. 2000, 41, 775–782. [Google Scholar]
- Bowd, C.; Balasubramanian, M.; Weinreb, R.N.; Vizzeri, G.; Alencar, L.M.; O’Leary, N.; Sample, P.A.; Zangwill, L.M. Performance of confocal scanning laser tomograph Topographic Change Analysis (TCA) for assessing glaucomatous progression. Investig. Ophthalmol. Vis. Sci. 2009, 50, 691–701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, G.; Chen, Z. Corneal hysteresis as a risk factor for optic nerve head surface depression and retinal nerve fiber layer thinning in glaucoma patients. Sci. Rep. 2021, 11, 11677. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Weinreb, R.N.; Leung, C.K. Optic nerve head deformation in glaucoma: The temporal relationship between optic nerve head surface depression and retinal nerve fiber layer thinning. Ophthalmology 2014, 121, 2362–2370. [Google Scholar] [CrossRef]
- Park, S.C.; De Moraes, C.G.; Teng, C.C.; Tello, C.; Liebmann, J.M.; Ritch, R. Initial parafoveal versus peripheral scotomas in glaucoma: Risk factors and visual field characteristics. Ophthalmology 2011, 118, 1782–1789. [Google Scholar] [CrossRef]
- Kosior-Jarecka, E.; Wróbel-Dudzińska, D.; Łukasik, U.; Żarnowski, T. Ocular and Systemic Risk Factors of Different Morphologies of Scotoma in Patients with Normal-Tension Glaucoma. J. Ophthalmol. 2017, 2017, 1480746. [Google Scholar] [CrossRef]
- Jeon, S.J.; Park, H.L.; Park, C.K. Effect of Macular Vascular Density on Central Visual Function and Macular Structure in Glaucoma Patients. Sci. Rep. 2018, 8, 16009. [Google Scholar] [CrossRef]
- Kurysheva, N.I.; Ryabova, T.Y.; Shlapak, V.N. Heart rate variability: The comparison between high tension and normal tension glaucoma. EPMA J. 2018, 9, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Park, S.H.; Park, C.K. Central visual field progression in normal-tension glaucoma patients with autonomic dysfunction. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
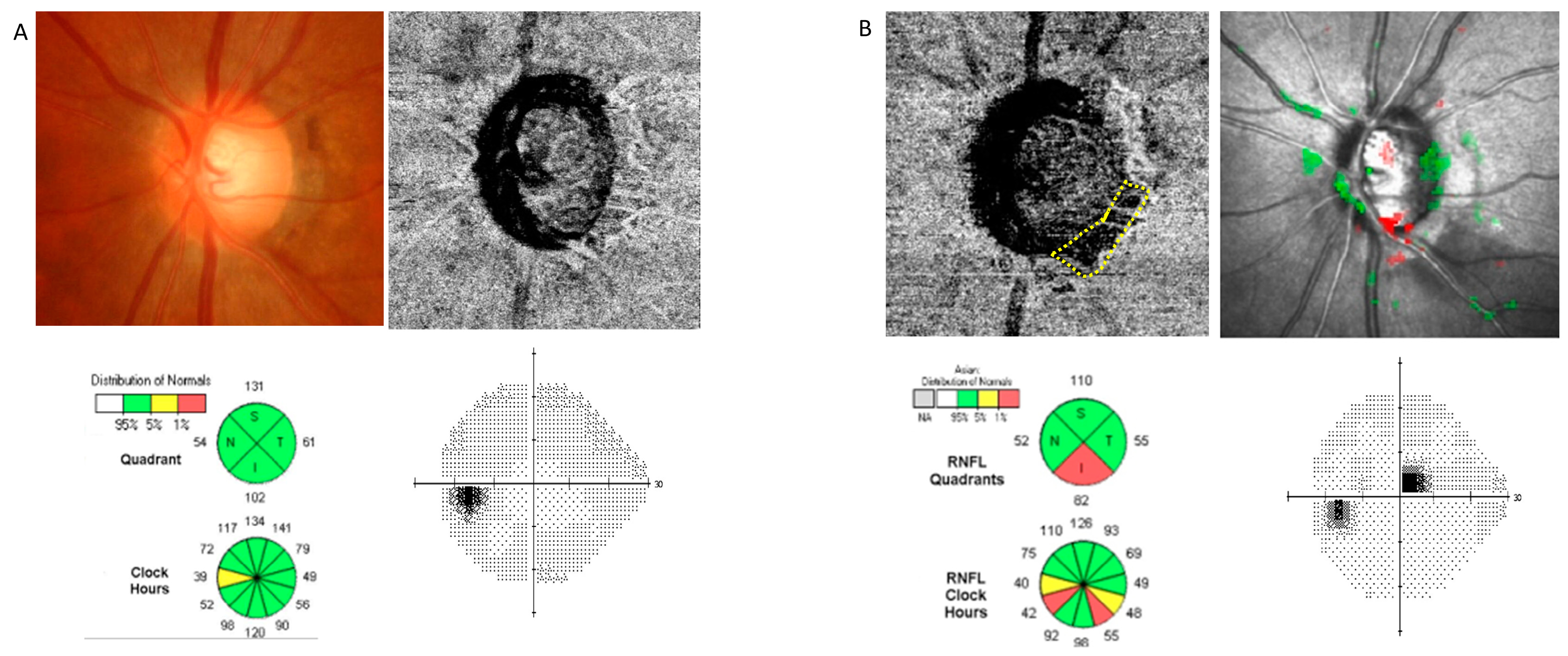
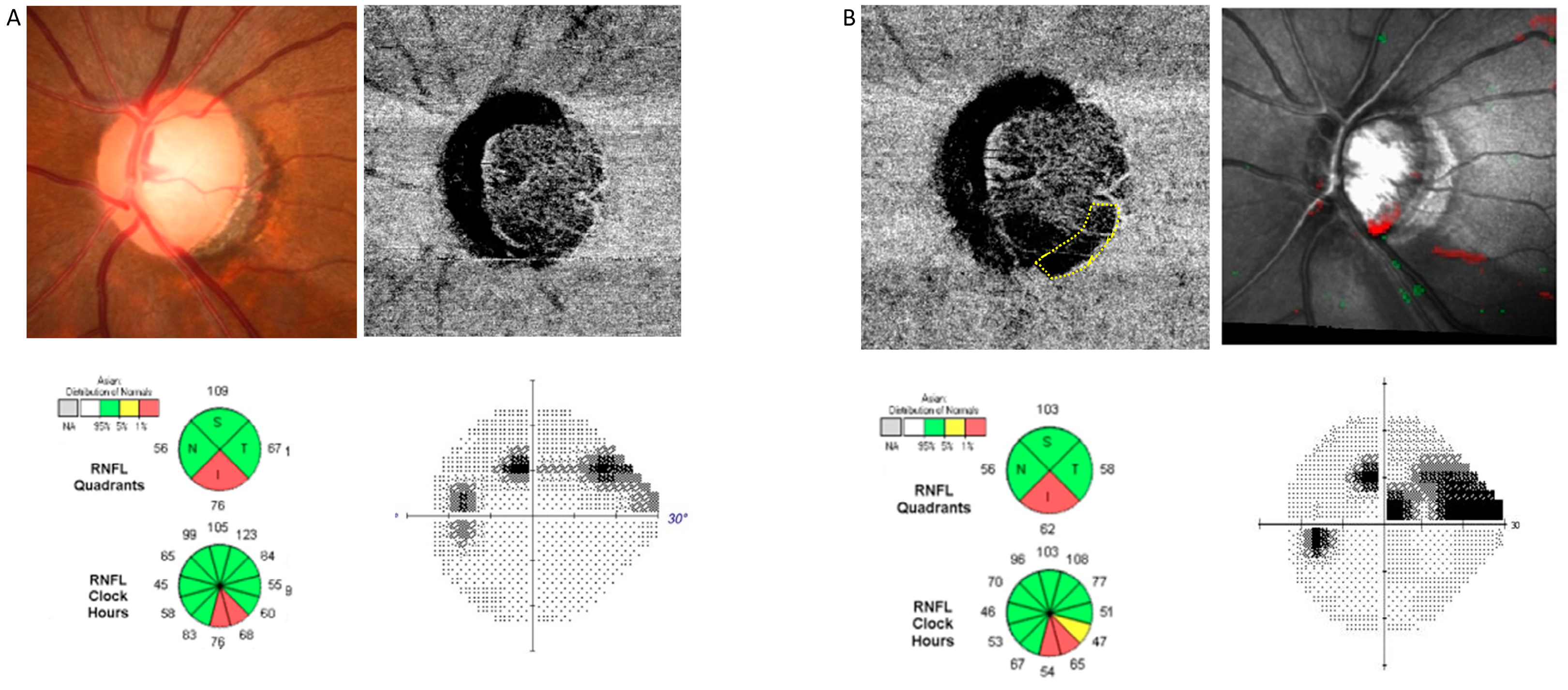
| Demographics | Total (n = 150) | Glaucoma Suspect (n = 68) | Glaucoma (n = 82) |
|---|---|---|---|
| Age at diagnosis, y | 56.32 ± 13.91 | 54.48 ± 12.77 | 57.83 ± 12.58 |
| Female, no. (%) | 62 (41.3%) | 35 (73.5%) | 27 (32.9%) |
| Systemic demographics | 7 (4.7%) | 4 (5.9%) | 3 (3.7%) |
| Medication of DM, no. (%) | 39 (26.0%) | 17 (25.0%) | 22 (26.8%) |
| Medication of HTN, no. (%) | 21 (14.0%) | 2 (2.9%) | 19 (23.2%) |
| Vascular symptoms, no. (%) | 37.36 ± 25.20 | 36.96 ± 25.11 | 37.96 ± 23.17 |
| SDNN of heart rate variability test | |||
| Ocular demographics | 24.26 ± 4.34 | 24.96 ± 4.56 | 24.93 ± 5.11 |
| Axial length, mm | 540.13 ± 39.45 | 552.3 ± 39.52 | 531.26 ± 38.60 |
| Central corneal thickness, μm | 35 (23.3%) | 0 | 35 (42.7%) |
| Presence of DH, no. (%) | |||
| IOP parameters | 14.89 ± 2.87 | 15.08 ± 2.77 | 14.73 ± 2.95 |
| Baseline IOP, mmHg | 14.45 ± 2.79 | 14.49 ± 2.84 | 14.40 ± 3.01 |
| IOP during follow-up, mmHg | 1.91 ± 2.38 | 1.59 ± 2.32 | 2.00 ± 2.37 |
| IOP fluctuation during follow-up, mmHg | |||
| OCT parameters | |||
| Rim area, mm2 | 0.89 ± 0.25 | 0.95 ± 0.25 | 0.88 ± 0.24 |
| Cup volume, mm3 | 0.49 ± 0.26 | 0.49 ± 0.23 | 0.54 ± 0.27 |
| Average pRNFL thickness, μm | 80.37 ± 12.99 | 87.12 ± 12.84 | 74.94 ± 13.01 |
| Average mGC/IPL thickness, μm | 73.07 ± 9.20 | 75.65 ± 9.18 | 70.98 ± 9.62 |
| VF parameters | |||
| MD, dB | −3.36 ± 4.43 | −1.60 ± 2.78 | −4.90 ± 5.01 |
| PSD, dB | 3.82 ± 3.76 | 2.14 ± 3.03 | 5.19 ± 4.40 |
| Disc parameters by HRT | |||
| Disc area, mm2 | 2.23 ± 0.52 | 2.36 ± 0.48 | 2.13 ± 0.47 |
| Cup shape measures | −0.09 ± 0.07 | −0.09 ± 0.07 | −0.10 ± 0.06 |
| Cup depth | 0.64 ± 0.17 | 0.63 ±0.15 | 0.64 ± 0.16 |
| Measured ONH parameters | |||
| LCD, μm | 436.32 ± 118.45 | 427.54 ± 119.32 | 451.27 ± 120.44 |
| LCT, μm | 208.17 ± 47.60 | 208.58 ± 48.16 | 207.47 ± 46.23 |
| MvD on OCT-A, no. (%) | 77 (51.3%) | 32 (47.1%) | 45 (54.9%) |
| Follow-up duration, y | 8.62 ± 2.20 | 8.60 ± 2.19 | 9.09 ± 2.18 |
| Variables | MvD Development (n = 77) | No MvD Development (n = 73) | p Value |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis, y | 54.33 ± 14.52 | 58.41 ± 12.99 | 0.072 * |
| Female, no. (%) | 33 (42.9%) | 29 (39.7%) | 0.135 † |
| Systemic demographics | |||
| Medication of DM, no. (%) | 2 (2.6%) | 5 (3.8%) | 0.200 † |
| Medication of HTN, no. (%) | 22 (28.5%) | 17 (23.3%) | 0.291 † |
| Vascular symptoms, no. (%) | 13 (16.9%) | 8 (11.0%) | 0.209 † |
| SDNN of heart rate variability test | 38.92 ± 27.20 | 35.55 ± 23.08 | 0.516 * |
| Ocular demographics | |||
| Axial length, mm | 24.53 ± 4.29 | 23.97 ± 4.38 | 0.706 * |
| Central corneal thickness, μm | 536.56 ± 37.66 | 543.51 ± 41.02 | 0.756 * |
| Presence of DH, no. (%) | 22 (48.9%) | 13 (35.1%) | 0.152 † |
| IOP parameters | |||
| Baseline IOP, mmHg | 15.02 ± 3.04 | 14.74 ± 2.68 | 0.292 * |
| IOP during follow-up, mmHg | 14.51 ± 2.64 | 14.39 ± 2.96 | 0.292 * |
| IOP fluctuation during follow-up, mmHg | 1.73 ± 2.55 | 2.10 ± 2.18 | 0.160 * |
| OCT parameters | |||
| Baseline rim area, mm2 | 0.85 ± 0.21 | 0.95 ± 0.28 | 0.212 * |
| Slope of rim area, mm2/y | −0.06 ± 0.12 | −0.04 ± 0.13 | 0.768 * |
| Baseline cup volume, mm3 | 0.50 ± 0.27 | 0.48 ± 0.25 | 0.350 * |
| Slope of cup volume, mm3/y | 0.04 ± 0.08 | 0.03 ± 0.07 | 0.010 * |
| Baseline average pRNFL thickness, μm | 78.51 ± 11.95 | 82.33 ± 13.82 | 0.073 * |
| Slope of RNFL thickness, μm/y | −0.54 ± 0.59 | −0.46 ± 0.70 | 0.438 * |
| Baseline average mGC/IPL thickness, μm | 70.96 ± 8.55 | 75.35 ± 9.39 | 0.003 * |
| Slope of mGC/IPL thickness | −0.12 ± 1.36 | −0.25 ± 0.71 | 0.496 * |
| VF parameters | |||
| Baseline MD, dB | −3.63 ± 4.61 | −3.08 ± 4.26 | 0.447 * |
| Slope of MD, dB/y | −0.25 ± 1.10 | −0.04 ± 0.54 | 0.134 * |
| Baseline PSD, dB | 4.36 ± 4.10 | 3.25 ± 3.30 | 0.071 * |
| Slope of PSD, dB/y | 0.61 ± 2.57 | 0.13 ± 0.39 | 0.116 * |
| Central progression, n (%) | 31 (40.3%) | 4 (5.6%) | <0.001 † |
| Disc parameters using HRT | |||
| HRT ONH depression, n (%) | 51 (66.2%) | 30 (41.1%) | 0.002 † |
| Disc area, mm2 | 2.13 ± 0.49 | 2.13 ± 0.45 | 0.410 * |
| Cup shape measures | −0.11 ± 0.07 | −0.10 ± 0.10 | 0.868 * |
| Slope of cup shape measures | 0.01 ± 0.03 | −0.00 ± 0.04 | 0.063 * |
| Cup depth | 0.62 ± 0.11 | 0.64 ± 0.16 | 0.493 * |
| Slope of cup depth | 0.01 ± 0.03 | 0.00 ± 0.03 | 0.880 * |
| Measured ONH parameters | |||
| LCD, μm | 440.80 ± 114.51 | 430.45 ± 124.89 | 0.700 * |
| Slope of LCD, μm/y | 0.69 ± 3.40 | 0.67 ± 3.22 | 0.973 * |
| LCT, μm | 203.19 ± 37.00 | 214.70 ± 58.67 | 0.284 * |
| Slope of LCT, μm/y | −3.08 ± 6.84 | −0.96 ± 8.00 | 0.194 * |
| Follow-up duration, y | 8.57 ± 2.00 | 8.67 ± 2.41 | 0.783 * |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Beta (95% CI) | p Value | Beta (95% CI) | p Value | |
| Age at diagnosis | 0.979 (0.956–1.002) | 0.075 | 0.999 (0.958–1.043) | 0.980 |
| Diagnosis of glaucoma suspect vs. glaucoma | 1.368 (0.718–2.608) | 0.341 | ||
| Female | 1.526 (0.794–2.933) | 0.205 | ||
| Medication of DM | 0.363 (0.068–1.931) | 0.235 | ||
| Medication of HTN | 1.318 (0.632–2.746) | 0.461 | ||
| Vascular symptoms | 1.650 (0.641–4.250) | 0.299 | ||
| SDNN of heart rate variability test | 1.005 (0.989–1.022) | 0.513 | ||
| Axial length | 1.032 (0.954–1.115) | 0.433 | ||
| Central corneal thickness | 1.005 (0.996–1.013) | 0.281 | ||
| Baseline IOP | 1.036 (0.926–1.159) | 0.540 | ||
| IOP during follow-up | 1.014 (0.904–1.138) | 0.810 | ||
| IOP fluctuation during follow-up | 0.935 (0.812–1.077) | 0.351 | ||
| Baseline rim area | 0.181 (0.045–0.733) | 0.017 | 1.242 (0.255–2.233) | 0.242 |
| Slope of rim area | 0.266 (0.019–3.737) | 0.326 | ||
| Baseline cup volume | 1.453 (0.422–5.005) | 0.554 | ||
| Slope of cup volume | 2.340 (0.230–21.729) | 0.183 | ||
| Presence of DH | 1.766 (0.723–4.312) | 0.212 | ||
| Baseline average pRNFL thickness | 0.977 (0.952–1.002) | 0.075 | 0.996 (0.921–1.077) | 0.914 |
| Slope of RNFL thickness | 0.819 (0.496–1.353) | 0.436 | ||
| Baseline average mGC/IPL thickness | 0.946 (0.911–0.983) | 0.005 | 0.895 (0.813–0.987) | 0.026 |
| Slope of mGC/IPL thickness | 0.899 (0.661–1.224) | 0.500 | ||
| Baseline MD of VF | 0.972 (0.903–1.046) | 0.446 | ||
| Slope of MD | 0.733 (0.482–1.116) | 0.148 | ||
| Baseline PSD of VF | 1.086 (0.991–1.190) | 0.076 | 1.222 (0.978–1.528) | 0.078 |
| Slope of PSD | 1.876 (1.013–3.474) | 0.045 | 2.088 (0.838–5.205) | 0.114 |
| Central progression | 1.087 (1.029–2.640) | <0.001 | 1.370 (1.032–1.594) | 0.008 |
| HRT ONH depression | 2.812 (1.448–5.460) | 0.002 | 0.979 (0.956–1.002) | 0.182 |
| HRT depression location | 2.250 (0.891–5.685) | 0.086 | 0.829 (0.223–3.088) | 0.780 |
| Disc area | 1.300 (0.699–2.417) | 0.408 | ||
| Baseline cup shape measures | 1.429 (0.022–92.698) | 0.867 | ||
| Slope of cup shape measures | 4.270 (0.564–13.723) | 0.066 | 2.348 (1.868–4.242) | 0.038 |
| Baseline cup depth | 0.191 (0.026–1.384) | 0.101 | ||
| Slope of cup depth | 0.742 (0.016–3.697) | 0.879 | ||
| Baseline LCD | 1.001 (0.997–1.005) | 0.696 | ||
| Slope of LCD | 1.002 (0.877–1.146) | 0.973 | ||
| Baseline LCT | 0.995 (0.985–1.004) | 0.284 | ||
| Slope of LCT | 0.961 (0.904–1.021) | 0.197 | ||
| Follow-up duration | 0.980 (0.847–1.133) | 0.781 | ||
| Variables | MvD Development (n = 32) | No MvD Development (n = 36) | p Value |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis, y | 53.59 ± 14.89 | 55.31 ± 13.83 | 0.626 * |
| Female, no. (%) | 15 (46.9%) | 20 (55.6%) | 0.902 † |
| Systemic demographics | |||
| Medication of DM, no. (%) | 2 (6.2%) | 2 (5.6%) | 0.647 † |
| Medication of HTN, no. (%) | 9 (28.1%) | 8 (22.2%) | 0.389 † |
| Vascular symptoms, no. (%) | 1 (3.1%) | 1 (2.8%) | 0.723 † |
| SDNN of heart rate variability test | 34.64 ± 23.56 | 37.14 ± 30.67 | 0.811 * |
| Ocular demographics | |||
| Axial length, mm | 25.40 ± 1.77 | 24.51 ± 1.84 | 0.046 * |
| Central corneal thickness, μm | 540.69 ± 43.35 | 562.21 ± 41.20 | 0.040 * |
| Presence of DH, no. (%) | 0 | 0 | |
| IOP parameters | |||
| Baseline IOP, mmHg | 15.87 ± 3.28 | 14.36 ± 2.11 | 0.026 * |
| IOP during follow-up, mmHg | 14.59 ± 2.69 | 14.44 ± 1.79 | 0.787 * |
| IOP fluctuation during follow-up, mmHg | 1.59 ± 1.79 | 1.47 ± 1.55 | 0.766 * |
| OCT parameters | |||
| Baseline rim area, mm2 | 0.86 ± 0.15 | 0.99 ± 0.28 | 0.028 * |
| Slope of rim area, mm2/y | −0.03 ± 0.11 | −0.00 ± 0.14 | 0.420 * |
| Baseline cup volume, mm3 | 0.62 ± 0.27 | 0.48 ± 0.18 | 0.012 * |
| Slope of cup volume, mm3/y | 0.01 ± 0.08 | 0.02 ± 0.08 | 0.505 * |
| Baseline average pRNFL thickness, μm | 84.31 ± 7.45 | 89.25 ± 8.95 | 0.017 * |
| Slope of RNFL thickness, μm/y | −0.49 ± 0.62 | −0.34 ± 0.73 | 0.356 * |
| Baseline average mGC/IPL thickness, μm | 73.46 ± 6.82 | 77.71 ± 8.27 | 0.027 * |
| Slope of mGC/IPL thickness | −0.55 ± 0.82 | −0.10 ± 0.50 | 0.009 * |
| VF parameters | |||
| Baseline MD of SAP, dB | −1.27 ± 1.77 | −1.71 ± 2.05 | 0.455 * |
| Slope of MD, dB/y | −0.14 ± 0.55 | −0.04 ± 0.55 | 0.438 * |
| Baseline PSD of SAP, dB | 2.09 ± 1.22 | 2.24 ± 1.23 | 0.630 * |
| Slope of PSD, dB/y | 0.09 ± 0.43 | 0.01 ± 0.23 | 0.316 * |
| Presence of central scotoma, n (%) | 11 (34.4%) | 1 (2.8%) | 0.001 † |
| Disc parameters using HRT | |||
| HRT ONH depression, n (%) | 16 (50.0%) | 17 (47.2%) | 0.506 † |
| Disc area, mm2 | 2.45 ± 0.52 | 2.26 ± 0.57 | 0.172 * |
| Cup shape measures | −0.08 ± 0.05 | −0.09 ± 0.07 | 0.398 * |
| Slope of cup shape measures | 0.01 ± 0.04 | −0.00 ± 0.04 | 0.345 * |
| Cup depth | 0.61 ± 0.18 | 0.68 ± 0.21 | 0.152 * |
| Slope of cup depth | 0.04 ± 0.10 | 0.05 ± 0.13 | 0.764 * |
| Measured ONH parameters | |||
| LCD, μm | 461.21 ± 154.24 | 442.56 ± 138.47 | 0.730 * |
| Slope of LCD, μm/y | 0.97 ± 3.28 | 0.81 ± 2.13 | 0.875 * |
| LCT, μm | 208.00 ± 41.97 | 207.00 ± 72.72 | 0.964 * |
| Slope of LCT, μm/y | −3.10 ± 7.55 | −2.07 ± 5.20 | 0.665 * |
| Conversion to glaucoma, n (%) | 11 (34.4%) | 4 (11.1%) | 0.021 † |
| Follow-up duration, y | 7.41 ± 1.29 | 7.50 ± 1.21 | 0.758 * |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Beta (95% CI) | p Value | Beta (95% CI) | p Value | |
| Age at diagnosis | 0.991 (0.959–1.026) | 0.619 | ||
| Female | 0.900 (0.771–1.060) | 0.702 | ||
| Medication of DM | 1.133 (0.150–8.548) | 0.903 | ||
| Medication of HTN | 1.370 (0.456–4.117) | 0.575 | ||
| Vascular symptoms | 1.129 (0.068–1.822) | 0.933 | ||
| SDNN of heart rate variability test | 0.966 (0.969–1.025) | 0.802 | ||
| Axial length | 1.322 (1.003–1.752) | 0.050 | 1.520 (1.008–2.291) | 0.046 |
| Central corneal thickness | 0.912 (0.823–0.998) | 0.045 | 1.000 (0.980–1.019) | 0.963 |
| Baseline IOP | 1.237 (1.016–1.505) | 0.034 | 1.232 (0.930–1.631) | 0.145 |
| Baseline rim area | 0.074 (0.006–0.869) | 0.038 | 0.559 (0.031–1.044) | 0.693 |
| Baseline cup volume | 1.669 (1.006–16.674) | 0.017 | 3.993 (1.292–12.345) | 0.035 |
| Baseline average pRNFL thickness | 0.928 (0.871–0.990) | 0.023 | 0.941 (0.863–1.027) | 0.174 |
| Baseline average mGC/IPL thickness, | 0.927 (0.864–0.994) | 0.034 | 0.966 (0.885–1.054) | 0.437 |
| Baseline MD of VF | 1.131 (0.873–1.464) | 0.351 | ||
| Baseline PSD of VF | 0.905 (0.606–1.351) | 0.625 | ||
| Disc area | 1.877 (0.758–4.648) | 0.173 | ||
| Cup shape measures | 2.608 (0.013–6.983) | 0.383 | ||
| Cup depth | 0.158 (0.013–1.994) | 0.154 | ||
| LCD | 1.001 (0.996–1.006) | 0.719 | ||
| LCT | 1.000 (0.988–1.013) | 0.963 | ||
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Beta (95% CI) | p Value | Beta (95% CI) | p Value | |
| IOP during follow-up | 1.030 (0.832–1.276) | 0.783 | ||
| IOP fluctuation during follow-up | 1.046 (0.783–1.396) | 0.762 | ||
| Slope of rim area | 0.190 (0.003–1.055) | 0.418 | ||
| Slope of cup volume | 0.122 (0.000–5.657) | 0.502 | ||
| Slope of RNFL thickness | 0.712 (0.349–1.455) | 0.352 | ||
| Slope of mGC/IPL thickness | 0.286 (0.096–0.855) | 0.025 | 0.027 (0.072–0.851) | 0.027 |
| Slope of MD | 0.701 (0.289–1.703) | 0.433 | ||
| Slope of PSD | 2.143 (0.473–9.700) | 0.322 | ||
| Central progression | 1.333 (2.206–5.234) | 0.007 | 7.040 (1.781–16.306) | 0.014 |
| HRT ONH depression | 1.118 (0.431–2.899) | 0.819 | ||
| Slope of cup shape measures | 2.232 (0.003–5.429) | 0.341 | ||
| Slope of cup depth | 0.520 (0.008–3.445) | 0.760 | ||
| Slope of LCD | 1.023 (0.779–1.343) | 0.869 | ||
| Slope of LCT | 0.974 (0.866–1.094) | 0.653 | ||
| Glaucoma conversion | 4.190 (1.177–14.920) | 0.027 | 1.686 (0.364–7.813) | 0.504 |
| Follow-up duration | 0.940 (0.638–1.394) | 0.754 | ||
| Variables | MvD Development (n = 45) | No MvD Development (n = 37) | p Value |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis, y | 54.86 ± 14.41 | 61.43 ± 11.51 | 0.028 * |
| Female, no. (%) | 18 (40.0%) | 9 (24.3%) | 0.102 † |
| Systemic demographics | |||
| Medication of DM, no. (%) | 0 (0%) | 3 (8.1%) | 0.088 † |
| Medication of HTN, no. (%) | 13 (28.9%) | 9 (24.3%) | 0.417 † |
| Vascular symptoms, no. (%) | 12 (26.7%) | 7 (18.9%) | 0.288 † |
| SDNN of heart rate variability test | 40.50 ± 28.55 | 34.83 ± 19.29 | 0.350 * |
| Ocular demographics | |||
| Axial length, mm | 23.92 ± 5.36 | 23.45 ± 5.88 | 0.706 * |
| Central corneal thickness, μm | 551.61 ± 41.41 | 554.27 ± 38.13 | 0.756 * |
| Presence of DH, no. (%) | 22 (48.9%) | 13 (35.1%) | 0.152 † |
| IOP parameters | |||
| Baseline IOP, mmHg | 14.42 ± 2.73 | 15.11 ± 3.12 | 0.292 * |
| IOP fluctuation during follow-up, mmHg | 1.84 ± 2.99 | 2.72 ± 2.53 | 0.160 * |
| OCT parameters | |||
| Baseline rim area, mm2 | 0.84 ± 0.24 | 0.92 ± 0.27 | 0.212 * |
| Slope of rim area, mm2/y | −0.08 ± 0.13 | −0.07 ± 0.11 | 0.768 * |
| Baseline cup volume, mm3 | 0.42 ± 0.25 | 0.48 ± 0.29 | 0.350 * |
| Slope of cup volume, mm3/y | 0.06 ± 0.06 | 0.03 ± 0.63 | 0.010 * |
| Baseline average pRNFL thickness, μm | 74.40 ± 12.89 | 75.59 ± 14.47 | 0.674 * |
| Slope of RNFL thickness, μm/y | −0.58 ± 0.56 | −0.58 ± 0.66 | 0.987 * |
| Baseline average mGC/IPL thickness, μm | 69.17 ± 9.25 | 73.18 ± 9.94 | 0.063 * |
| Slope of mGC/IPL thickness | −0.61 ± 1.46 | −0.57 ± 0.73 | 0.890 * |
| VF parameters | |||
| Baseline MD, dB | −5.30 ± 5.25 | −4.41 ± 5.34 | 0.446 * |
| Slope of MD, dB/y | −0.33 ± 1.37 | −0.03 ± 0.53 | 0.219 * |
| Baseline PSD, dB | 5.96 ± 4.64 | 4.23 ± 4.27 | 0.085 * |
| Slope of PSD, dB/y | 0.97 ± 3.31 | 0.25 ± 0.48 | 0.187 * |
| Central progression, n (%) | 20 (44.4%) | 3 (8.3%) | <0.001 † |
| Disc parameters using HRT | |||
| HRT ONH depression, n (%) | 35 (77.8%) | 13 (35.1%) | <0.001 † |
| Disc area, mm2 | 2.13 ± 0.49 | 2.13 ± 0.45 | 0.941 * |
| Cup shape measures | −0.11 ± 0.07 | −0.10 ± 0.10 | 0.795 * |
| Slope of cup shape measures | 0.01 ± 0.03 | −0.00 ± 0.04 | 0.077 * |
| Cup depth | 0.62 ± 0.11 | 0.64 ± 0.16 | 0.450 * |
| Slope of cup depth | 0.01 ± 0.03 | 0.00 ± 0.03 | 0.277 * |
| Measured ONH parameters | |||
| LCD, μm | 431.86 ± 93.74 | 420.25 ± 115.08 | 0.696 * |
| Slope of LCD, μm/y | 0.58 ± 3.49 | 0.55 ± 3.97 | 0.983 * |
| LCT, μm | 201.09 ± 35.12 | 221.18 ± 44.68 | 0.081 * |
| Slope of LCT, μm/y | −3.08 ± 6.64 | −0.68 ± 9.73 | 0.183 * |
| Follow-up duration, y | 9.40 ± 2.01 | 9.81 ± 2.73 | 0.437 * |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Beta (95% CI) | p Value | Beta (95% CI) | p Value | |
| Presence of DH | 1.766 (0.723–4.312) | 0.212 | ||
| IOP during follow-up | 1.009 (0.880–1.158) | 0.895 | ||
| IOP fluctuation during follow-up | 0.886 (0.742–1.057) | 0.179 | ||
| Slope of rim area | 0.567 (0.014–3.253) | 0.765 | ||
| Slope of cup volume | 3.380 (0.479–5.280) | 0.015 | 3.460 (0.025–6.480) | 0.230 |
| Slope of RNFL thickness | 1.006 (0.487–2.077) | 0.987 | ||
| Slope of mGC/IPL thickness | 1.027 (0.706–1.494) | 0.888 | ||
| Slope of MD | 0.757 (0.476–1.203) | 0.238 | ||
| Slope of PSD | 1.764 (0.875–3.558) | 0.113 | ||
| Central progression | 8.800 (2.351–32.945) | 0.001 | 5.985 (1.474–24.083) | 0.012 |
| HRT ONH depression | 6.462 (2.439–17.120) | <0.001 | 3.765 (1.301–10.895) | 0.014 |
| Slope of cup shape measures | 6.351 (0.153–12.541) | 0.086 | 2.079 (0.001–5.572) | 0.530 |
| Slope of cup depth | 1.648 (0.003–3.548) | 0.278 | ||
| Slope of LCD | 1.002 (0.856–1.172) | 0.982 | ||
| Slope of LCT | 0.952 (0.884–1.025) | 0.188 | ||
| Follow-up duration | 0.928 (0.770–1.118) | 0.432 | ||
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age at diagnosis | 0.962 (0.929–0.997) | 0.032 | 0.970 (0.926–1.017) | 0.210 |
| Female | 2.074 (0.795–5.412) | 0.136 | ||
| Medication of DM | 0 | 0.999 | ||
| Medication of HTN | 1.264 (0.470–3.401) | 0.643 | ||
| Vascular symptoms | 1.558 (0.543–4.477) | 0.410 | ||
| SDNN of heart rate variability test | 1.010 (0.989–1.031) | 0.351 | ||
| Axial length | 1.015 (0.939–1.098) | 0.703 | ||
| Central corneal thickness | 0.998 (0.985–1.011) | 0.752 | ||
| Baseline IOP | 0.921 (0.790–1.073) | 0.289 | ||
| Baseline rim area | 0.330 (0.057–1.891) | 0.213 | ||
| Baseline cup volume | 0.458 (0.090–2.326) | 0.346 | ||
| Baseline average pRNFL thickness | 0.993 (0.962–1.026) | 0.690 | ||
| Baseline average mGC/IPL thickness, | 0.956 (0.912–1.003) | 0.066 | 0.996 (0.925–1.072) | 0.906 |
| Baseline MD of VF | 0.967 (0.888–1.053) | 0.442 | ||
| Baseline PSD of VF | 1.094 (0.986–1.213) | 0.089 | 1.116 (0.946–1.317) | 0.192 |
| Disc area | 1.036 (0.410–2.620) | 0.940 | ||
| Cup shape measures | 0.506 (0.003–79.153) | 0.792 | ||
| Cup depth | 0.284 (0.011–7.235) | 0.446 | ||
| LCD | 1.001 (0.995–1.007) | 0.690 | ||
| LCT | 0.987 (0.971–1.002) | 0.089 | 0.985 (0.969–1.001) | 0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.J.; Oh, S.E.; Kim, S.A.; Park, C.K.; Park, H.-Y.L. Factors Contributing to the Development of Choroidal Microvasculature Dropout in Glaucoma Suspects and Patients with Glaucoma. J. Clin. Med. 2024, 13, 204. https://doi.org/10.3390/jcm13010204
Shin HJ, Oh SE, Kim SA, Park CK, Park H-YL. Factors Contributing to the Development of Choroidal Microvasculature Dropout in Glaucoma Suspects and Patients with Glaucoma. Journal of Clinical Medicine. 2024; 13(1):204. https://doi.org/10.3390/jcm13010204
Chicago/Turabian StyleShin, Hee Jong, Si Eun Oh, Seong Ah Kim, Chan Kee Park, and Hae-Young Lopilly Park. 2024. "Factors Contributing to the Development of Choroidal Microvasculature Dropout in Glaucoma Suspects and Patients with Glaucoma" Journal of Clinical Medicine 13, no. 1: 204. https://doi.org/10.3390/jcm13010204
APA StyleShin, H. J., Oh, S. E., Kim, S. A., Park, C. K., & Park, H.-Y. L. (2024). Factors Contributing to the Development of Choroidal Microvasculature Dropout in Glaucoma Suspects and Patients with Glaucoma. Journal of Clinical Medicine, 13(1), 204. https://doi.org/10.3390/jcm13010204






