A Critical Analysis of Pharyngeal Patterns of Collapse in Obstructive Sleep Apnea: Beyond the Endoscopic Classification Systems
Abstract
:1. Introduction
2. Methods
3. Results
3.1. DISE and UA Pattern of Collapse/Obstruction (Table 2)
| UA Site | UA Pattern of Collapse | |
|---|---|---|
| Patterns of Soft Palate Collapse | concentric collapse |
|
| antero-posterior collapse |
| |
| Patterns of Oropharyngeal Lateral Walls | latero-lateral collapse |
|
| Patterns of Base of Tongue Collapse | antero-posterior collapse |
|
| Patterns of Epiglottic Collapse | antero-posterior collapse latero-lateral collapse |
|
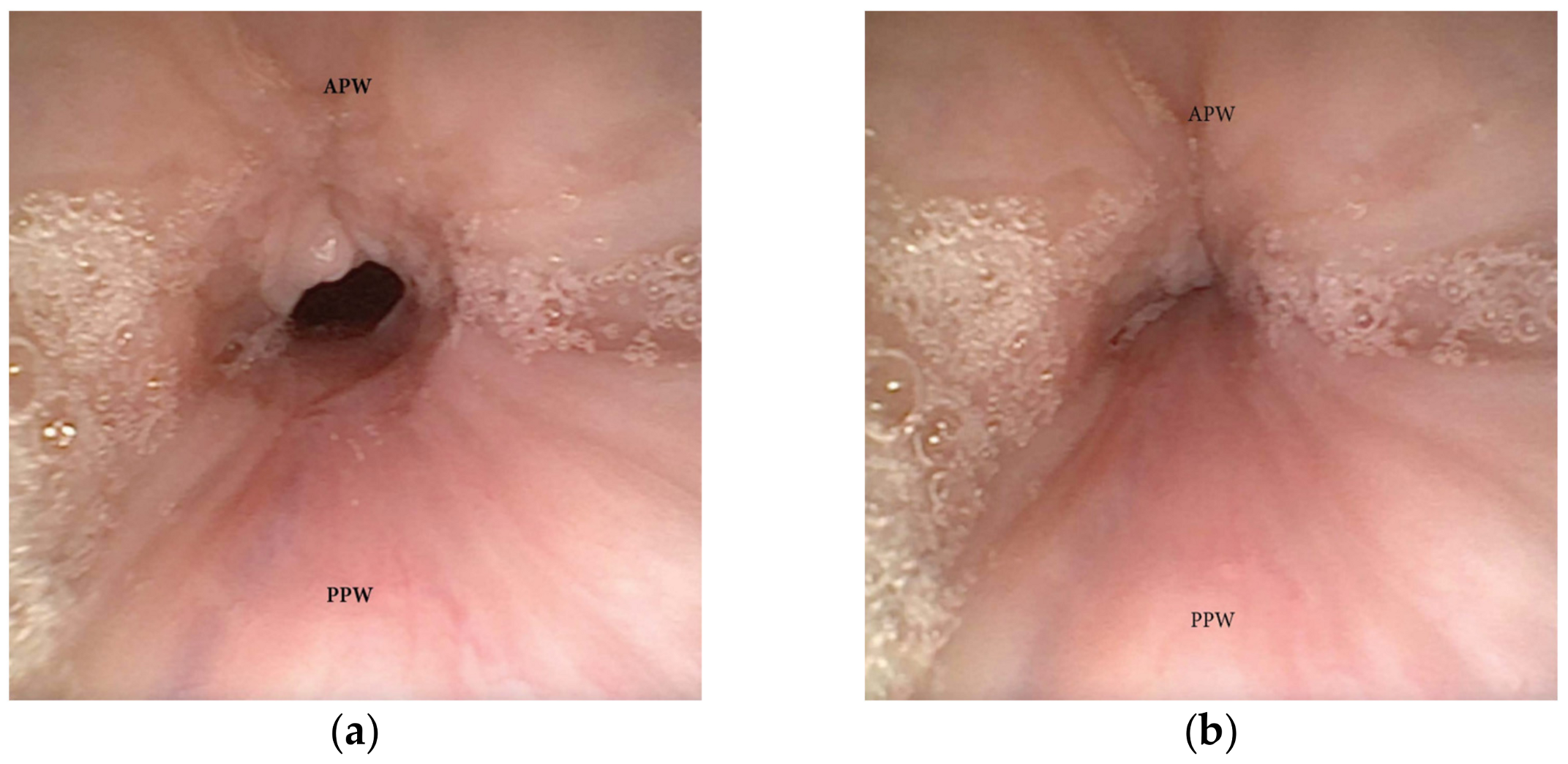
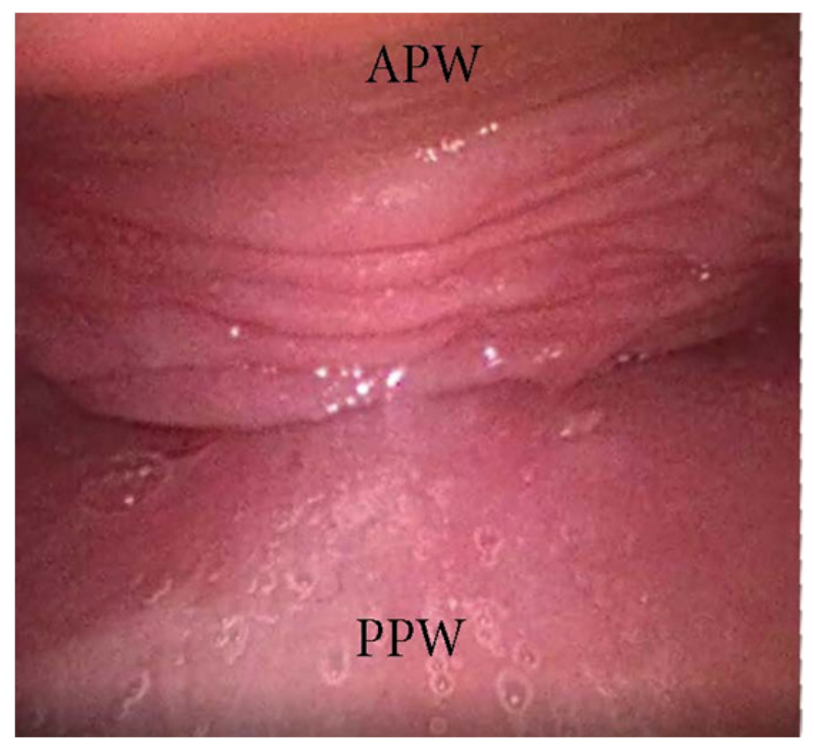
3.1.1. Patterns of Soft Palate Collapse
Concentric Pattern
Antero-Posterior (AP) Collapse
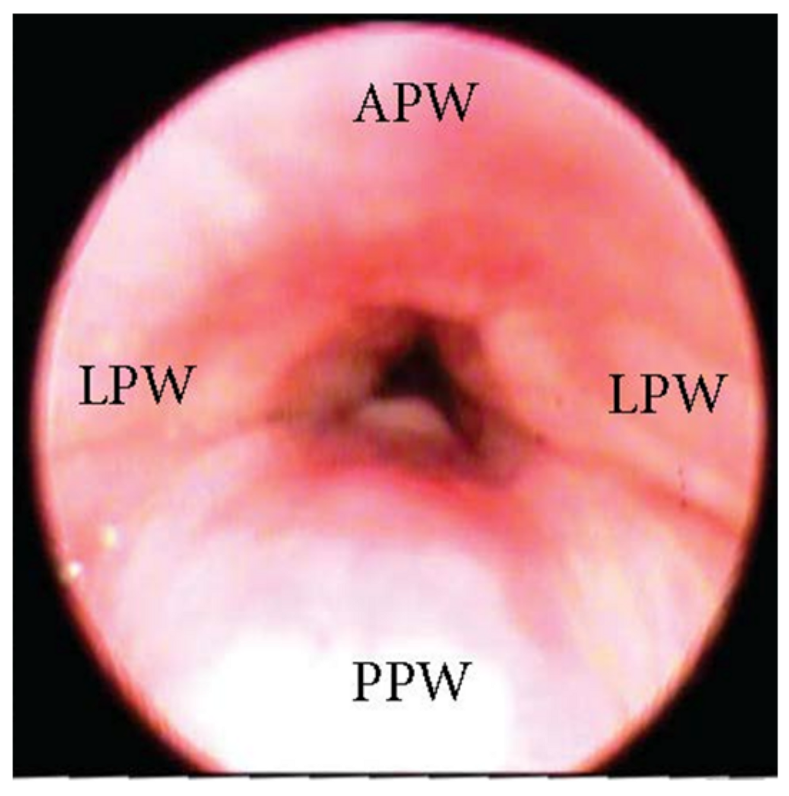
3.1.2. Patterns of Oropharyngeal Lateral Walls
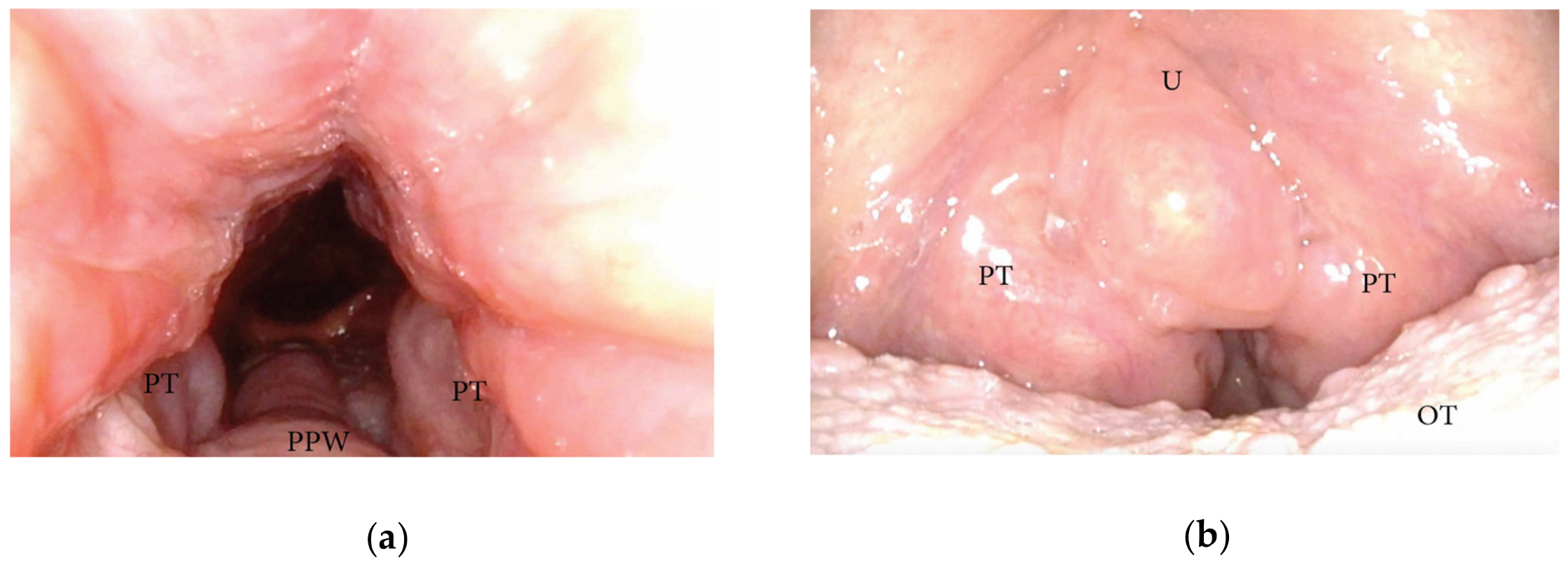
3.1.3. Patterns of the Base of Tongue Collapse
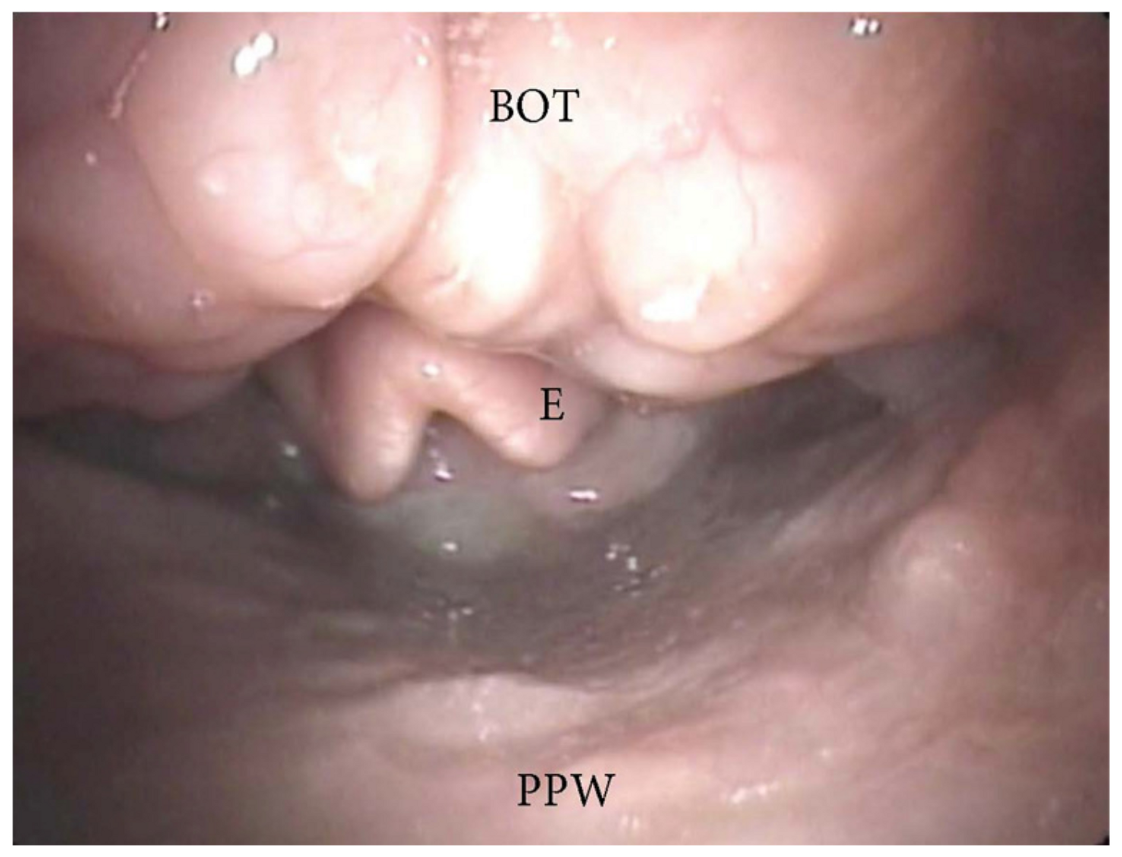
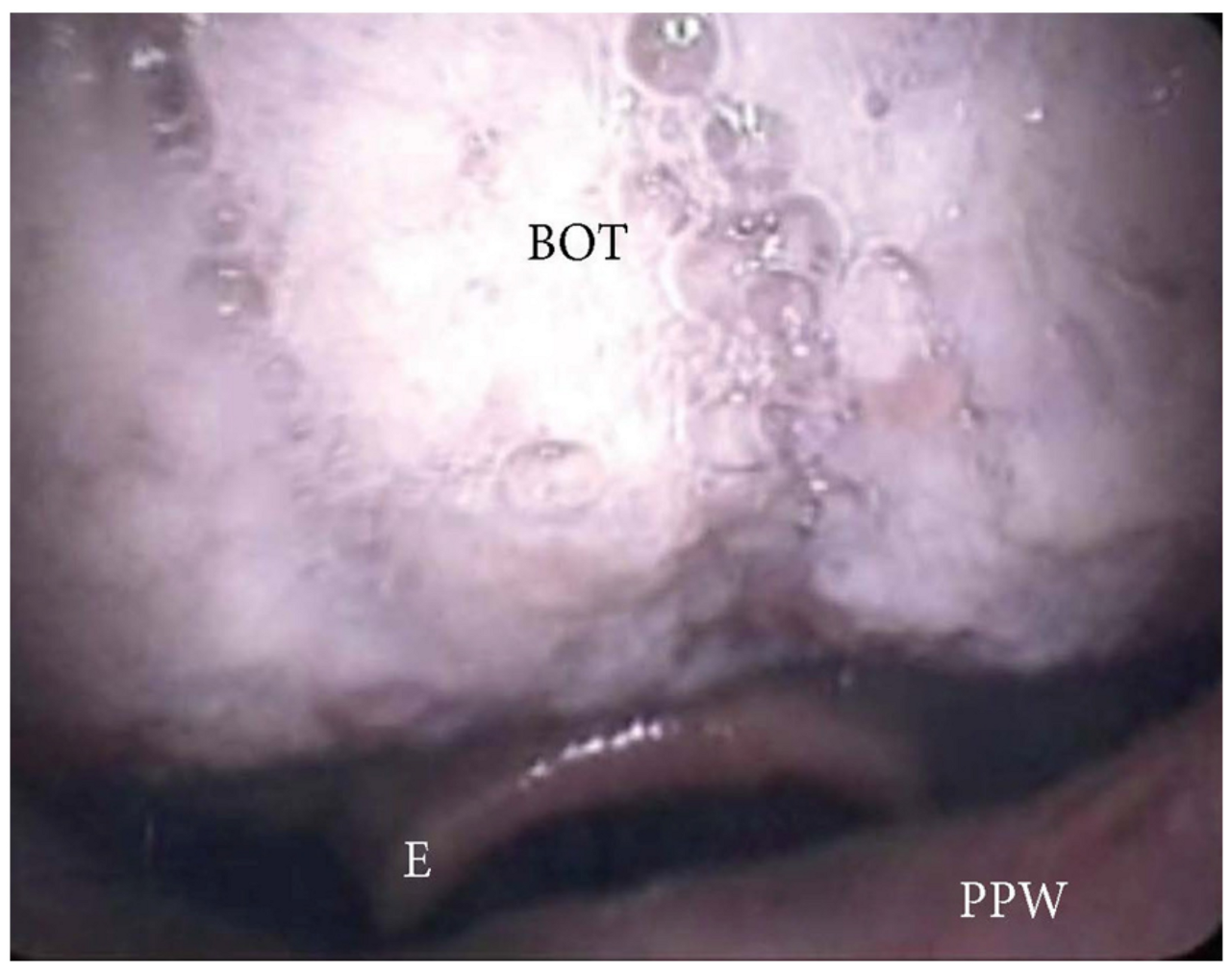
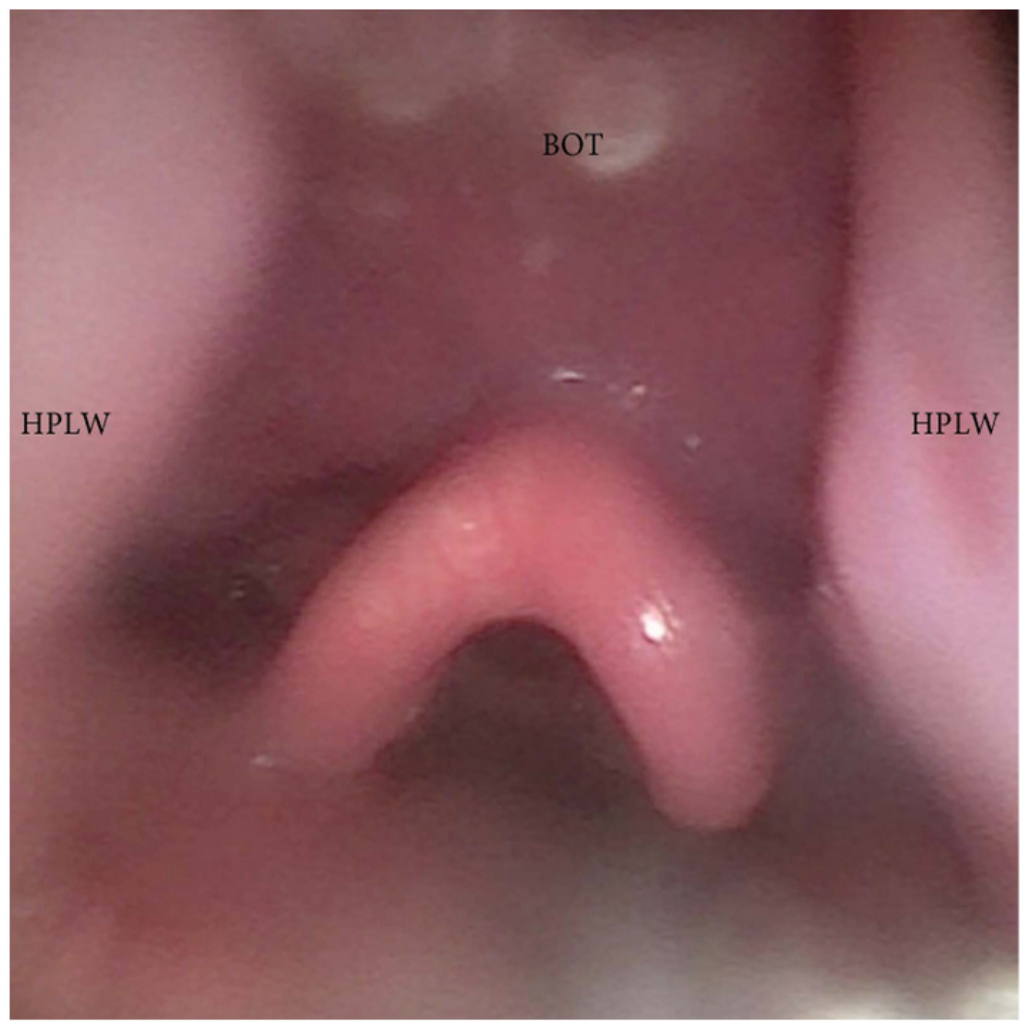
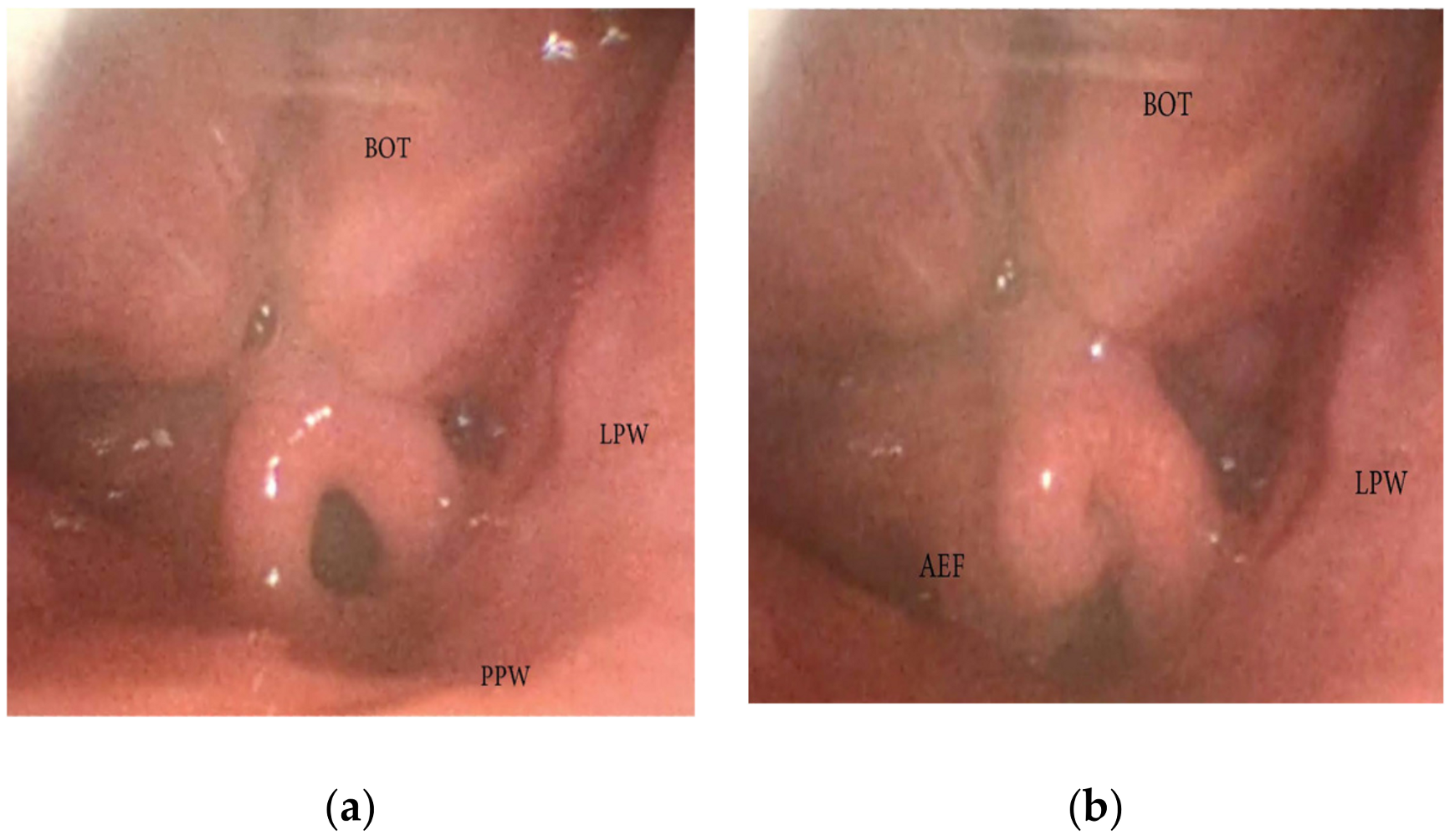
3.1.4. Patterns of Epiglottic Collapse
3.1.5. Oral Tongue Patterns of Collapse
3.1.6. Nasal Obstruction and Pattern of Collapse
4. Discussion
- Velum and Oropharyngeal patterns of collapse:
- Tongue patterns of collapse:
- Epiglottis patterns of collapse:
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, J.L.; Goldberg, A.N.; Alt, J.A.; Ashbrook, L.; Auckley, D.; Ayappa, I.; Bakhtiar, H.; Barrera, J.E.; Bartley, B.L.; Billings, M.E.; et al. International consensus statement on obstructive sleep apnea. Int. Forum. Allergy Rhinol. 2022, 13, 1061–1482. [Google Scholar] [CrossRef] [PubMed]
- Bosi, M.; De Vito, A.; Gobbi, R.; Poletti, V.; Vicini, C. The importance of obstructive sleep apnoea and hypopnea pathophysiology for customized therapy. Eur. Arch. Otorhinolaryngol. 2017, 274, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, B.W.; Murariu, D.; Pang, K.P. Trends in CPAP adherence over twenty years of data collection: A flattened curve. J. Otolaryngol. Head Neck Surg. 2016, 45, 43. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Woodson, B.T.; Koka, V.; Cammaroto, G.; Iannella, G.; Bosi, M.; Pelucchi, S.; Filograna-Pignatelli, G.R.; El Chater, P.; Vicini, C. OSA Upper Airways Surgery: A Targeted Approach. Medicina 2021, 57, 690. [Google Scholar] [CrossRef] [PubMed]
- Pringle, M.B.; Croft, C.B. A comparison of sleep nasendoscopy and the Muller manoeuvre. Clin. Otolaryngol. Allied. Sci. 1991, 16, 559–562. [Google Scholar] [CrossRef]
- Carrasco-Llatas, M.; Zerpa-Zerpa, V.; Dalmau-Galofre, J. Reliability of drug-induced sedation endoscopy: Interobserver agreement. Sleep Breath 2017, 21, 173–179. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Carrasco Llatas, M.; Ravesloot, M.J.; Kotecha, B.; De Vries, N.; Hamans, E.; Maurer, J.; Bosi, M.; Blumen, M.; Heiser, C.; et al. European position paper on drug-induced sleep endoscopy: 2017 Update. Clin. Otolaryngol. 2018, 43, 1541–1552. [Google Scholar] [CrossRef]
- Van den Bossche, K.; Van de Perck, E.; Kazemeini, E.; Willemen, M.; Van de Heyning, P.H.; Verbraecken, J.; Op de Beeck, S.; Vanderveken, O.M. Natural sleep endoscopy in obstructive sleep apnea: A systematic review. Sleep Med. Rev. 2021, 60, 101534. [Google Scholar] [CrossRef]
- Charakorn, N.; Kezirian, E.J. Drug-Induced Sleep Endoscopy. Otolaryngol. Clin. N. Am. 2016, 49, 1359–1372. [Google Scholar] [CrossRef]
- Vroegop, A.V.; Vanderveken, O.M.; Wouters, K.; Hamans, E.; Dieltjens, M.; Michels, N.R.; Hohenhorst, W.; Kezirian, E.J.; Kotecha, B.T.; de Vries, N.; et al. Observer variation in drug-induced sleep endoscopy: Experienced versus nonexperienced ear, nose, and throat surgeons. Sleep 2013, 36, 947–953. [Google Scholar] [CrossRef]
- Kezirian, E.J.; Hohenhorst, W.; de Vries, N. Drug-induced sleep endoscopy: The VOTE classification. Eur. Arch. Otorhinolaryngol. 2011, 268, 1233–1236. [Google Scholar] [CrossRef]
- Van de Perck, E.; Heiser, C.; Vanderveken, O.M. Concentric vs Anteroposterior-Laterolateral Collapse of the Soft Palate in Patients with Obstructive Sleep Apnea. Otolaryngol. Head Neck Surg. 2022, 166, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, E.; Woodson, B.T. Palatal anatomy for sleep apnea surgery. Laryngoscope Investig. Otolaryngol. 2019, 4, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Migueis, D.P.; Thuler, L.C.; Lemes, L.N.; Moreira, C.S.; Joffily, L.; Araujo-Melo, M.H. Systematic review: The influence of nasal obstruction on sleep apnea. Braz. J. Otorhinolaryngol. 2016, 82, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef]
- Bosi, M.; De Vito, A.; Eckert, D.; Steier, J.; Kotecha, B.; Vicini, C.; Poletti, V. Qualitative Phenotyping of Obstructive Sleep Apnea and Its Clinical Usefulness for the Sleep Specialist. Int. J. Environ. Res. Public Health 2020, 17, 2058. [Google Scholar] [CrossRef]
- Vanderveken, O.M. Drug-induced sleep endoscopy (DISE) for non-CPAP treatment selection in patients with sleep-disordered breathing. Sleep Breath 2013, 17, 13–14. [Google Scholar] [CrossRef]
- Kezirian, E.J. Nonresponders to pharyngeal surgery for obstructive sleep apnea: Insights from druginduced sleep endoscopy. Laryngoscope 2011, 121, 1320–1326. [Google Scholar] [CrossRef]
- Amos, J.M.; Durr, M.L.; Nardone, H.C.; Baldassari, C.M.; Duggins, A.; Ishman, S.L. Systematic Review of Drug-Induced Sleep Endoscopy Scoring Systems. Otolaryngol. Head Neck Surg. 2018, 158, 240–248. [Google Scholar] [CrossRef]
- Dijemeni, E.; D’Amone, G.; Gbati, I. Drug-induced sedation endoscopy (DISE) classification systems: A systematic review and meta-analysis. Sleep Breath 2017, 21, 983–994. [Google Scholar] [CrossRef]
- Dedhia, R.C.; Seay, E.G.; Schwartz, A.R. Beyond VOTE: The New Frontier of Drug-Induced Sleep Endoscopy. ORL J. Otorhinolaryngol. Relat. Spec. 2022, 84, 296–301. [Google Scholar] [CrossRef]
- Vanderveken, O.M.; Maurer, J.T.; Hohenhorst, W.; Hamans, E.; Lin, H.S.; Vroegop, A.V.; Anders, C.; de Vries, N.; Van de Heyning, P.H. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J. Clin. Sleep Med. 2013, 9, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Huyett, P.; Kent, D.T.; D’Agostino, M.A.; Green, K.K.; Soose, R.J.; Kaffenberger, T.M.; Woodson, B.T.; Huntley, C.; Boon, M.S.; Heiser, C.; et al. Drug-Induced Sleep Endoscopy and Hypoglossal Nerve Stimulation Outcomes: A Multicenter Cohort Study. Laryngoscope 2021, 131, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Woodson, B.T.; Robison, S.; Lim, H.J. Transpalatal advancement pharyngoplasty outcomes compared with uvulopalatopharyngoplasty. Otolaryngol. Head Neck Surg. 2005, 133, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.K.; Kodur, S.; Jha, R.H. A Novel Grading System for Salpingopharyngeal Fold Hypertrophy in Obstructive Sleep Apnoea. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Justin, G.A.; Chang, E.T.; Camacho, M.; Brietzke, S.E. Transoral Robotic Surgery for Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Otolaryngol. Head Neck Surg. 2016, 154, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Ilea, A.; Timuș, D.; Höpken, J.; Andrei, V.; Băbțan, A.M.; Petrescu, N.B.; Câmpian, R.S.; Boșca, A.B.; Șovrea, A.S.; Negucioiu, M.; et al. Oral appliance therapy in obstructive sleep apnea and snoring-systematic review and new directions of development. CRANIO 2021, 39, 472–483. [Google Scholar] [CrossRef]
- Bae, M.R.; Chung, Y.S. Epiglottic collapse in obstructive sleep apnea. Sleep Med. Res. 2021, 12, 15–19. [Google Scholar] [CrossRef]
- He, J.; Wang, C.; Li, W. Laryngopharyngeal Reflux in Obstructive Sleep Apnea-Hypopnea Syndrome: An Updated Meta-Analysis. Nat. Sci. Sleep. 2022, 14, 2189–2201. [Google Scholar] [CrossRef]
- Ayuse, T.; Inazawa, T.; Kurata, S.; Okayasu, I.; Sakamoto, E.; Oi, K.; Schneider, H.; Schwartz, A.R. Mouth-Opening Increases Upper-Airway Collapsibility without Changing Resistance during Midazolam Sedation. J. Dent. Res. 2004, 83, 718–722. [Google Scholar] [CrossRef]
- Fitzpatrick, M.F.; McLean, H.; Urton, A.M.; Tan a O’Donnell, D.; Driver, H.S. Effect of nasal or oral breathing route on upper airway resistance during sleep. Eur. Respir. J. 2003, 22, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Tregear, S.; Reston, J.; Schoelles, K.; Phillips, B. Obstructive sleep apnea and risk of motor vehicle crash: Systematic review and meta-analysis. J. Clin. Sleep Med. 2009, 5, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Kushida, C.A.; Littner, M.R.; Hirshkowitz, M.; Morgenthaler, T.I.; Alessi, C.A.; Bailey, D.; Boehlecke, B.; Brown, T.M.; Coleman, J., Jr.; Friedman, L.; et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep 2006, 29, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Pérez-Martín, N.; Morato, M.; Racionero, M.A.; Plaza, G. Nasal Surgery May Improve Upper Airway Collapse in Patients with Obstructive Sleep Apnea: A Drug-Induced Sleep Endoscopy Study. J. Craniofac. Surg. 2020, 31, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Green, K.K.; Kent, D.T.; D’Agostino, M.A.; Hoff, P.T.; Lin, H.S.; Soose, R.J.; Boyd Gillespie, M.; Yaremchuk, K.L.; Carrasco-Llatas, M.; Tucker Woodson, B.; et al. Drug-Induced Sleep Endoscopy and Surgical Outcomes: A Multicenter Cohort Study. Laryngoscope 2019, 129, 761–770. [Google Scholar] [CrossRef]
- Certal, V.F.; Pratas, R.; Guimarães, L.; Lugo, R.; Tsou, Y.; Camacho, M.; Capasso, R. Awake examination versus DISE for surgical decision making in patients with OSA: A systematic review. Laryngoscope 2016, 126, 768–774. [Google Scholar] [CrossRef]
- Lisan, Q.; Baudouin, R.; Lechien, J.R.; Hans, S.; Blumen, M. Is drug-induced sleep endoscopy associated with better outcomes after soft tissue surgery for sleep apnea? A systematic review and meta-analysis. Clin. Otolaryngol. 2023, 48, 122–129. [Google Scholar] [CrossRef]
- Albdah, A.A.; Alkusayer, M.M.; Al-Kadi, M.; Almofada, H.; Alnofal, E.A.; Almutairi, S. The Impact of Drug-induced Sleep Endoscopy on Therapeutic Decisions in Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Cureus 2019, 11, e6041. [Google Scholar] [CrossRef]
- Iannella, G.; Magliulo, G.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Pelucchi, S.; Ciorba, A.; Maniaci, A.; Cocuzza, S.; Gulotta, G.; et al. Effectiveness of drug-induced sleep endoscopy in improving outcomes of barbed pharyngoplasty for obstructive sleep apnea surgery: A prospective randomized trial. Sleep Breath 2022, 26, 1621–1632. [Google Scholar] [CrossRef]
- Virk, J.S.; Kotecha, B. When continuous positive airway pressure (CPAP) fails. J. Thorac. Dis. 2016, 8, E1112–E1121. [Google Scholar] [CrossRef]
- De Vito, A.; Agnoletti, V.; Zani, G.; Corso, R.M.; D’Agostino, G.; Firinu, E.; Marchi, C.; Hsu, Y.S.; Maitan, S.; Vicini, C. The importance of drug-induced sedation endoscopy (D.I.S.E.) techniques in surgical decision making: Conventional versus target controlled infusion techniques-a prospective randomized controlled study and a retrospective surgical outcomes analysis. Eur. Arch Otorhinolaryngol. 2017, 274, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Wilkins, D.; Kotecha, B. A review on drug-induced sedation endoscopy—Technique, grading systems and controversies. Sleep Med. Rev. 2018, 41, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Rudzki, M.; Plößl, S.; Plontke, S.; Kellner, P. Depth of sedation during drug induced sedation endoscopy monitored by BiSpectral Index® and Cerebral State Index®. Sleep Breath 2021, 25, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Nunes, H.d.S.S.; Vaz de Castro, J.; Favier, V.; Carsuzaa, F.; Kim, M.H.R.; Mira, F.A.; Meccariello, G.; Vicini, C.; De Vito, A.; Lechien, J.R.; et al. Predictors of Success of Pharyngeal Surgery in the Treatment of Obstructive Sleep Apnea: A Narrative Review. J. Clin. Med. 2023, 12, 6773. [Google Scholar] [CrossRef]
- Saenwandee, P.; Neruntarat, C.; Saengthong, P.; Wiriyaamornchai, P.; Khuancharee, K.; Sirisomboonwech, S.; Chuoykwamdee, N. Barbed pharyngoplasty for obstructive sleep apnea: A meta-analysis. Am. J. Otolaryngol. 2022, 43, 103306. [Google Scholar] [CrossRef]
- Yu, J.L.; Thuler, E.; Seay, E.G.; Schwartz, A.R.; Dedhia, R.C. The Accuracy and Reliability of Visually Assessed Pharyngeal Opening Pressures during Drug-Induced Sleep Endoscopy. Otolaryngol. Head Neck Surg. 2022, 168, 1945998221120793. [Google Scholar] [CrossRef]



| Total Papers Found on PubMed | Systematic Reviews, Meta-Analysis, RCTs | Papers Included in the Manuscript |
|---|---|---|
| 105 | 54 | 46 |
| 1. OSA patients are unable to accept or tolerate the pressure of CPAP titration [17] |
| 2. OSA patients who failed previous UA surgery [18] |
| 3. OSA patients considering non–CPAP treatment options for OSA, such as UA surgery, MAD, hypoglossal nerve stimulation, positional therapy, or combination therapy [9] |
| 4. OSA patients with socially problematic primary snoring [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vito, A.; Olszewska, E.; Kotecha, B.; Thuler, E.; Casale, M.; Cammaroto, G.; Vicini, C.; Vanderveken, O.M. A Critical Analysis of Pharyngeal Patterns of Collapse in Obstructive Sleep Apnea: Beyond the Endoscopic Classification Systems. J. Clin. Med. 2024, 13, 165. https://doi.org/10.3390/jcm13010165
De Vito A, Olszewska E, Kotecha B, Thuler E, Casale M, Cammaroto G, Vicini C, Vanderveken OM. A Critical Analysis of Pharyngeal Patterns of Collapse in Obstructive Sleep Apnea: Beyond the Endoscopic Classification Systems. Journal of Clinical Medicine. 2024; 13(1):165. https://doi.org/10.3390/jcm13010165
Chicago/Turabian StyleDe Vito, Andrea, Ewa Olszewska, Bhik Kotecha, Eric Thuler, Manuele Casale, Giovanni Cammaroto, Claudio Vicini, and Olivier M. Vanderveken. 2024. "A Critical Analysis of Pharyngeal Patterns of Collapse in Obstructive Sleep Apnea: Beyond the Endoscopic Classification Systems" Journal of Clinical Medicine 13, no. 1: 165. https://doi.org/10.3390/jcm13010165
APA StyleDe Vito, A., Olszewska, E., Kotecha, B., Thuler, E., Casale, M., Cammaroto, G., Vicini, C., & Vanderveken, O. M. (2024). A Critical Analysis of Pharyngeal Patterns of Collapse in Obstructive Sleep Apnea: Beyond the Endoscopic Classification Systems. Journal of Clinical Medicine, 13(1), 165. https://doi.org/10.3390/jcm13010165










