Anxiety and Perception of Disease Control in Multiple Sclerosis Subjects Treated with Natalizumab
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Inclusion Criteria
2.2. Study Design and Setting
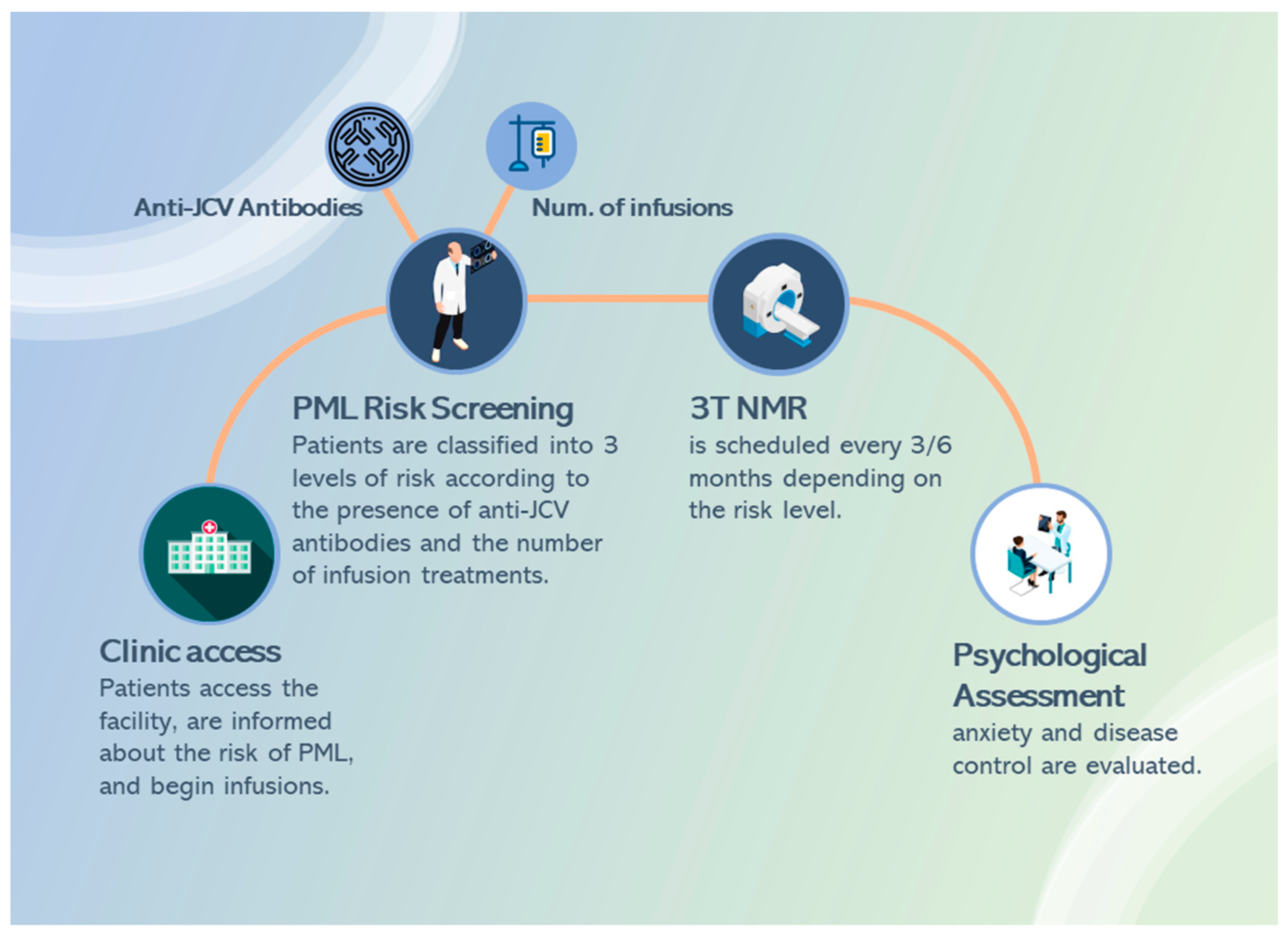
2.3. Assessment of Risk of PML
2.4. Assessment of Anxiety and Perception of Disease Control
2.5. Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.2. Anxiety and Perception of Disease Control
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hauser, S.L.; Goodin, D.S. Multiple sclerosis and other demyelinating diseases. Harrisons Princ. Intern. Med. 2005, 16, 2461. [Google Scholar]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; Van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Eskyte, I.; Manzano, A.; Pepper, G.; Pavitt, S.; Ford, H.; Bekker, H.; Chataway, J.; Schmierer, K.; Meads, D.; Webb, E.; et al. Understanding treatment decisions from the perspective of people with relapsing remitting multiple Sclerosis: A critical interpretive synthesis. Mult. Scler. Relat. Disord. 2019, 27, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; Berkovich, R.; Gudesblatt, M.; Luce, E.; Schneider, B.; de Moor, C.; Liao, S.; Lee, L.; Bodhinathan, K.; Avila, R. Characterizing the ‘feel-good experience’ in multiple sclerosis patients treated with natalizumab or other therapies. Neurodegener. Dis. Manag. 2023, 13, 23–34. [Google Scholar] [CrossRef]
- Perumal, J.; Balabanov, R.; Su, R.; Chang, R.; Balcer, L.; Galetta, S.; Fox, R.J. Natalizumab in early relaps-ing-remitting multiple sclerosis: A 4-year, open-label study. Adv. Ther. 2021, 38, 3724–3742. [Google Scholar] [CrossRef]
- Stephenson, J.J.; Kern, D.M.; Agarwal, S.S.; Zeidman, R.; Rajagopalan, K.; Kamat, S.A.; Foley, J. Impact of natali-zumab on patient-reported outcomes in multiple sclerosis: A longitudinal study. Health Qual. Life Outcomes 2012, 10, 1–9. [Google Scholar] [CrossRef]
- Lublin, F.D.; Cutter, G.; Giovannoni, G.; Pace, A.; Campbell, N.R.; Belachew, S. Natalizumab reduces relapse clinical severity and improves relapse recovery in MS. Mult. Scler. Relat. Disord. 2014, 3, 705–711. [Google Scholar] [CrossRef]
- Voloshyna, N.; Havrdova, E.; Hutchinson, M.; Nehrych, T.; You, X.; Belachew, S.; Paes, D. Natalizumab improves ambulation in relapsing− remitting multiple scle-rosis: Results from the prospective TIMER study and a retrospective analysis of AFFIRM. Eur. J. Neurol. 2015, 22, 570–577. [Google Scholar] [CrossRef]
- Chaudhuri, A. Lessons for clinical trials from natalizumab in multiple sclerosis. BMJ 2006, 332, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.P., Jr. Progressive multifocal leukoencephalopathy. N. Engl. J. Med. 1961, 265, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wright, C.; Flores, A. Asymptomatic progressive multifocal leukoencephalopathy: A case report and review of the literature. J. Med. Case Rep. 2018, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- O’connor, P.W.; Kremenchutzky, M. Use of Natalizumab in Patients with Multiple Sclerosis: 2015 Update. Can. J. Neurol. Sci./J. Can. des Sci. Neurol. 2015, 42, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Clerico, M.; Artusi, C.A.; Di Liberto, A.; Rolla, S.; Bardina, V.; Barbero, P.; Durelli, L. Natalizumab in multiple sclerosis: Long-term management. Int. J. Mol. Sci. 2017, 18, 940. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Radue, E.-W.; Comi, G.; Montalban, X.; Butzkueven, H.; Wiendl, H.; Giovannoni, G.; Hartung, H.-P.; Derfuss, T.; Naegelin, Y.; et al. Switching from natalizumab to fingolimod. Neurology 2015, 85, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Cohan, S.L.; Moses, H.; Calkwood, J.; Tornatore, C.; LaGanke, C.; Smoot, K.E.; Meka, V.; Okwuokenye, M.; Hotermans, C.; Mendoza, J.P.; et al. Clinical outcomes in patients with relapsing-remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: A multicenter retrospective observational study (STRATEGY). Mult. Scler. Relat. Disord. 2018, 22, 27–34. [Google Scholar] [CrossRef]
- Romanazzo, S.; Mansueto, G.; Cosci, F. Anxiety in the Medically Ill: A Systematic Review of the Literature. Front. Psychiatry 2022, 13, 873126. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA Confirms Recommendations to Minimise Risk of Brain Infection PML with Tysabri. More Frequent MRI Scans Should be Considered for Patients at Higher Risk; 25/04/2016 EMA/266665/201; EMA: Amsterdam, The Netherlands, 2016.
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Toboso, I.; Tejeda-Velarde, A.; Alvarez-Lafuente, R.; Arroyo, R.; Hegen, H.; Deisenhammer, F.; de la Maza, S.S.; Alvarez-Cermeño, J.C.; Izquierdo, G.; Paramo, D.; et al. New Algorithms Improving PML Risk Stratification in MS Patients Treated with Natalizumab. Front. Neurol. 2020, 11, 579438. [Google Scholar] [CrossRef] [PubMed]
- Tugemann, B.; Berger, J.R. Improving risk-stratification of natalizumab-associated PML. Ann. Clin. Transl. Neurol. 2021, 8, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.S.; Bertolotto, A.; Edan, G.; Giovannoni, G.; Gold, R.; Havrdova, E.; Kappos, L.; Kieseier, B.C.; Montalban, X.; Olsson, T. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult. Scler. J. 2012, 18, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.S.; Bertolotto, A.; Edan, G.; Giovannoni, G.; Gold, R.; Havrdova, E.; Olsson, T. Risk stratification for progressive multifocal leukoencephalopathy (PML) in MS patients: Role of prior immunosuppressant use, natalizumab-treatment duration, and anti-JCV antibody status. Neurology 2011, 76, 248. [Google Scholar]
- Stratify JCV. Available online: https://stratifyjcv.unilabsweb.com/ (accessed on 15 July 2023).
- Hamilton MAX. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Brandstadter, R.; Sand, I.K. The use of natalizumab for multiple sclerosis. Neuropsychiatr. Dis. Treat. 2017, 13, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Morrow, S.A.; Clift, F.; Devonshire, V.; Lapointe, E.; Schneider, R.; Stefanelli, M.; Vosoughi, R. Use of natalizumab in persons with multiple sclerosis: 2022 update. Mult. Scler. Relat. Disord. 2022, 65, 103995. [Google Scholar] [CrossRef] [PubMed]
- Bloomgren, G.; Richman, S.; Hotermans, C.; Subramanyam, M.; Goelz, S.; Natarajan, A.; Bozic, C. Risk of natali-zumab-associated progressive multifocal leukoencephalopathy. N. Engl. J. Med. 2012, 366, 1870–1880. [Google Scholar] [CrossRef]
- López-Reyes, L.; Guío-Sánchez, C.; González-Uribe, C.; Cárdenas-Robledo, S. Fertility preferences and unmet need for family planning in women with multiple sclerosis. Front. Neurol. 2022, 13, 1035596. [Google Scholar] [CrossRef]
- Mazzotti, E.; Sebastiani, C.; Marchetti, P. Patient perception of disease control and psychological distress. Cancer Manag. Res. 2012, 4, 335–340. [Google Scholar] [CrossRef]
- Petrie, K.J.; Jago, L.A.; Devcich, D.A. The role of illness perceptions in patients with medical conditions. Curr. Opin. Psychiatry 2007, 20, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Šilić, P.; Motl, R.W.; Duffecy, J. Multiple sclerosis and anxiety: Is there an untapped opportunity for exercise? Mult. Scler. Relat. Disord. 2023, 73, 104698. [Google Scholar] [CrossRef] [PubMed]

| Risk Levels | JCV | N. Infusions | Time Interval | |||
|---|---|---|---|---|---|---|
| Neg | pos | ≤24 | >24 | 3T NMR | Infusions | |
| Level 1 | • | 1 year | 4 weeks | |||
| Level 2 | • | • | 6 months | 4 weeks | ||
| Level 3 | • | • | 3 months | 6 weeks | ||
| Risk Classification | |||
|---|---|---|---|
| Level 1 | Level 2 | Level 3 | |
| Participants, n (%) | 36 (58.1) | 12 (19.3) | 14 (22.6) |
| Males, n (%) | 5 (38.4) | 5 (38.5) | 3 (23.1) |
| Age (years), mean ± SD | 43.5 ± 10.5 | 45.7 ± 8.4 | 42.1 ± 13.4 |
| Risk rate, mean ± SD | 0.8 ± 2.4 | 2.7 ± 3.4 | 5.9 ± 4.0 |
| Scores at Each Examination (Median [First–Third Quartile]) | One-Way Repeated Measures Analysis | Post Hoc Analysis | |||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | Test (df) | p-Value | Significant Differences | p-Value | |
| HAM-A Risk L1 | 19.5 [17.0–21.0] | 11.0 [10.0–14.0] | 6.5 [5.0–8.0] | 71.5 (2) | <0.001 | T1–T0 | <0.001 |
| T2–T0 | <0.001 | ||||||
| T2–T1 | <0.001 | ||||||
| HAM-A Risk L2 | 14.5 [14.0–15.7] | 18.0 [9.7–12.2] | 6.0 [5.7–6.2] | 23.5 (2) | <0.001 | T1–T0 | 0.003 |
| T2–T0 | 0.002 | ||||||
| T2–T1 | 0.002 | ||||||
| HAM-A Risk L3 | 17.0 [15.2–18.7] | 10.0 [10.0–13.2] | 6.0 [5.2–8.0] | 27.5 (2) | <0.001 | T1–T0 | 0.002 |
| T2–T0 | 0.001 | ||||||
| T2–T1 | 0.001 | ||||||
| pdc Risk L1 | 3.0 [2.7–4.0] | 6.0 [5.0–6.0] | 8.0 [7.0–9.0] | 72.0 (2) | <0.001 | T1–T0 | <0.001 |
| T2–T0 | <0.001 | ||||||
| T2–T1 | <0.001 | ||||||
| pcd Risk L2 | 3.5 [3.0–4.0] | 5.5 [5.0–7.2] | 8.0 [7.7–8.2] | 24.0 (2) | <0.001 | T1–T0 | 0.002 |
| T2–T0 | 0.002 | ||||||
| T2–T1 | 0.002 | ||||||
| pcd Risk L3 | 3.0 [3.0–4.0] | 6.0 [6.0–6.0] | 8.0 [7.2–9.0] | 27.5 (2) | <0.001 | T1–T0 | <0.001 |
| T2–T0 | <0.001 | ||||||
| T2–T1 | 0.001 | ||||||
| Sum Sq | Mean Sq | Test (df) | p-Value | ||
|---|---|---|---|---|---|
| HAM-A | Risk level | 19 | 19 | 1.97 (1) | 0.162 |
| Assessment time | 4321 | 4321 | 444.66 (1) | <0.001 | |
| Risk–time | 14 | 14 | 1.43 (1) | 0.233 | |
| Residuals | 1769 | 10 | (182) | - | |
| Perceived disease control | Risk level | 2.7 | 2.7 | 2.22 (1) | 0.138 |
| Assessment time | 800.2 | 800.2 | 645.91 (1) | <0.001 | |
| Residuals | 226.7 | 1.2 | (183) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corallo, F.; Sessa, E.; Rifici, C.; De Cola, M.C.; Di Cara, M.; Cardile, D.; Venuti, G.; Bonfiglio, N.; D’Aleo, G.; Quartarone, A.; et al. Anxiety and Perception of Disease Control in Multiple Sclerosis Subjects Treated with Natalizumab. J. Clin. Med. 2024, 13, 13. https://doi.org/10.3390/jcm13010013
Corallo F, Sessa E, Rifici C, De Cola MC, Di Cara M, Cardile D, Venuti G, Bonfiglio N, D’Aleo G, Quartarone A, et al. Anxiety and Perception of Disease Control in Multiple Sclerosis Subjects Treated with Natalizumab. Journal of Clinical Medicine. 2024; 13(1):13. https://doi.org/10.3390/jcm13010013
Chicago/Turabian StyleCorallo, Francesco, Edoardo Sessa, Carmela Rifici, Maria Cristina De Cola, Marcella Di Cara, Davide Cardile, Giuseppe Venuti, Noemi Bonfiglio, Giangaetano D’Aleo, Angelo Quartarone, and et al. 2024. "Anxiety and Perception of Disease Control in Multiple Sclerosis Subjects Treated with Natalizumab" Journal of Clinical Medicine 13, no. 1: 13. https://doi.org/10.3390/jcm13010013






