Abstract
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths globally. BRAF mutation is present in about 10% of CRC patients and is associated with a poor response to chemotherapy. These patients have a relatively poor prognosis. This review aims to assess the efficacy and safety of BRAF inhibitors in BRAF-mutated CRC patients. A literature search was performed on PubMed and Embase, and clinical trials relevant to BRAF inhibitors in CRC were included. Data were extracted for efficacy and safety variables. Two randomized clinical trials (n = 765) and eight non-randomized trials (n = 281) were included based on inclusion criteria. In RCTs, an overall response was reported in 23% of the patients treated with BRAF inhibitor-based regimens compared to 2.5% with control regimens. The hazard ratio of overall survival was also significantly better with triplet encorafenib therapy at 0.52 (95% CI = 0.39–0.70). In single-arm trials, ORR was 17% and 34% in two-drug and three-drug regimens, respectively. BRAF inhibitor-based regimens were safe and effective in the treatment of BRAF-mutated CRC. Large-scale randomized trials are needed to find a suitable population for each regimen. PROSPERO registration No. CRD42023471627.
1. Introduction
Colorectal cancer (CRC) is a universal public health issue. It is the third leading cause of cancer and the second most common cause of cancer-related deaths globally [1]. According to the statistics from the Global Cancer Observatory, colorectal cancer led to 9.4% of all deaths caused by cancer in 2020 and had an incidence rate of 10% compared to all other new cases of cancer in 2020 [2]. CRC, being a molecularly heterogeneous disease, has subtypes characterized by genetic alterations, with major efforts being made to reveal the molecular landscape of metastatic colorectal cancer (mCRC) [3]. CRC has a high prevalence of mutations in the mitogen-activated protein kinase pathway (MAPK pathway), including neuroblastoma ras viral oncogene homolog (NRAS) and Kirsten rat sarcoma 2 viral oncogene homolog (KRAS) sequence variations in roughly 50% of cancers and the v-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutation, which is seen in 10%. BRAF, which is a biomarker in CRC, is a protein related to signal transduction for cell division and growth through the MAPKP. The BRAF proto-oncogene mutation leads to mutant CRC, a very distinct subtype of CRC with substitution in valine amino acid (V600E) due to transversion T1799A in exon 15, the most common mutation in BRAF [4]. BRAFV600E mutation is more prevalent in right-sided colon cancer, females, the elderly, Caucasians, mucinous histology, mismatch repair defects, advanced-stage cancers, and those with a high tumor burden [4]. The BRAF mutation causes constitutive activation of the MAPKP, which leads to the high activating capacity of the MAPK pathway after a cascade of phosphorylation in the transcription of genes enhancing the differentiation, proliferation, inhibition of apoptosis, and survival of tumor cells [3].
Standard chemotherapy and trials to intensify therapy have shown poor outcomes for BRAFV600E-mutated CRC. Even though the presence of BRAFV600E mutations is a hallmark of a bad prognosis, it is a therapeutic target for treatment optimization and targeted therapy against BRAF mutation in BRAF-positive CRC. BRAF inhibitors have shown significant clinical activity against BRAFV600E-mutated tumor types like non-small cell lung cancer and melanoma. BRAF inhibition alone in BRAF-mutated CRC enhances feedback activation in other pathways, like MAPKP and epidermal growth factor receptor (EGFR) [5,6] (Figure 1).
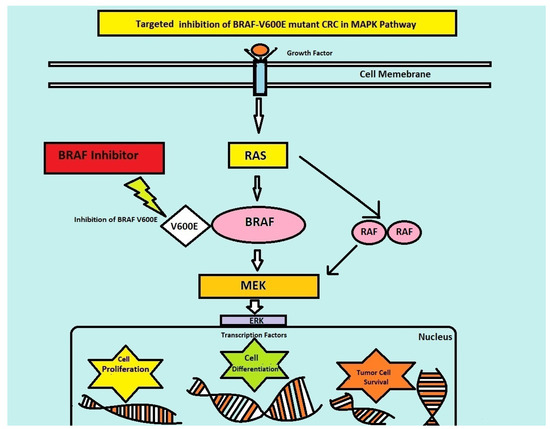
Figure 1.
Targeted inhibition of BRAF pathway.
Therefore, multiple target inhibitions including EGFR and MEK inhibitors are required to inhibit the proliferation of this type of cancer. Preclinical studies have shown the benefits of a combination of BRAF inhibitors with MEK inhibitors and anti-EGFR antibodies, which has led to clinical trials on these combinations.
This review aims to assess, acknowledge, and compile data from clinical trials regarding the safety and efficacy of BRAF inhibitor-based combinations in BRAF-mutated CRC for clinicians and to give directions for future clinical trials.
2. Materials and Methods
Cochrane Handbook [7] and PRISMA guidelines [8] were used to conduct this systematic review. The methodology for this systematic review was registered on PROSPERO with registration No. CRD42023471627.
2.1. Search Strategy
For this systematic review, we conducted a comprehensive literature search on PubMed (Medline) and Embase for clinical trials. We used keywords “colorectal cancer’’ and “B-raf protooncogene” from the inception of data to 15 August 2023. PICOS framework was used for the literature search [9] (Table S1).
2.2. Inclusion and Exclusion Criteria
We included all clinical trials conducted on adult patients (18+) with BRAF-mutated CRC treated with BRAF inhibitors. We included treatment regimens that showed clinical activity in BRAF-mutated CRC patients and were tolerated by most of the patients.
We excluded all preclinical studies, case reports, meta-analyses, review articles, and observational studies. We excluded clinical studies with more than 20% of patients with other types of cancer. We also excluded trials that could not be completed due to clinically ineffective regimens or high levels of toxicity.
2.3. Data Extraction
The primary efficacy outcomes of interest were overall response rate (ORR), median progression-free survival (mPFS), and median overall survival (mOS). Other secondary outcomes of interest were stable disease (SD), progressive disease (PD), complete response (CR), and partial response (PR). Safety data of interest were ≥ grade 3 adverse effects.
2.4. Risk of Bias Assessment
The risk of bias in selected clinical studies was assessed by two independent researchers using the Rob-2 tool for risk of bias assessment in RCTs [10].
3. Results
A thorough literature search on PubMed and Embase found 4635 articles. After careful screening based on predefined inclusion and exclusion criteria, two randomized clinical trials (RCT, n = 765) [11,12], and nine non-randomized trials (nRCTs, n = 423) [3,13,14,15,16,17,18,19,20] were included, as in Figure 2.
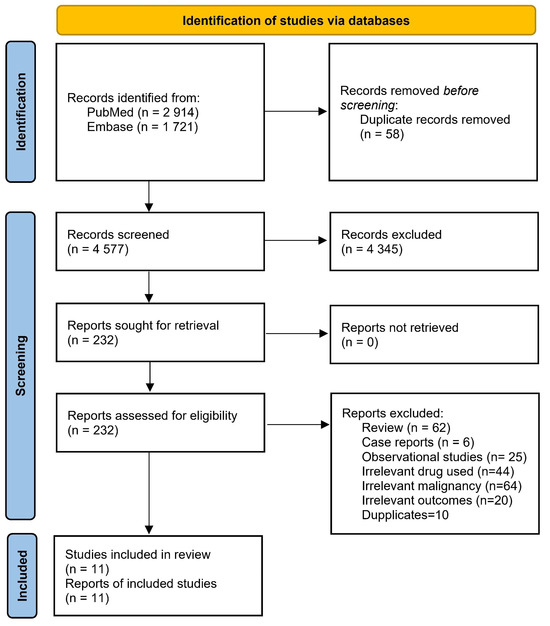
Figure 2.
PRISMA flow chart of included studies.
3.1. Risk of Bias
The risk of bias was high in all studies included due to the lack of a placebo group and blinding. In two RCTs [11,12], the risk of bias was low for the randomization process, missing outcome data, measurement of the outcome, and selection of reported results. However, the risk of bias was high due to the open-label nature of these RCTs. The risk of bias was high in nRCTs [3,13,14,15,16,17,18,19,20], due to the lack of randomization and blinding, as shown in Figure 3.
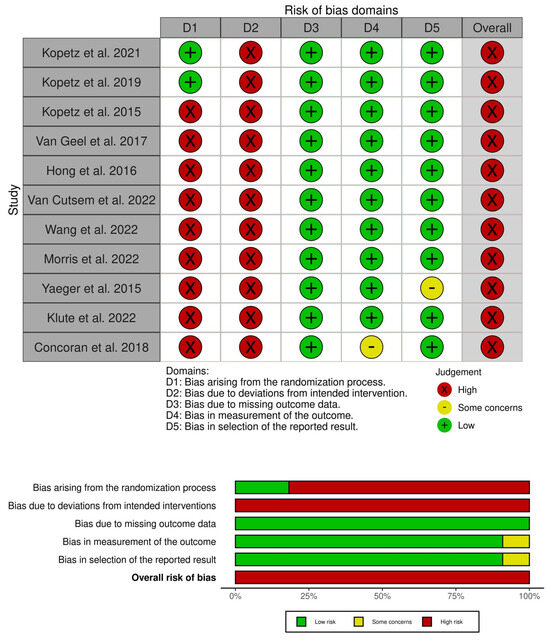
Figure 3.
Risk of bias with ROB 2 tool [3,11,12,13,14,15,16,17,18,19,20].
3.2. BRAF Inhibitors in Relapsed/Refractory (R/R) mCRC
Ten clinical trials (n = 1093) were conducted on patients with R/R mCRC. A total of 21 patients were treated with BRAF inhibitors as monotherapy, 311 patients were treated with BRAF inhibitors in two-drug regimens, 447 patients were treated with BRAF inhibitors in three-drug regimens, and 302 patients were treated with chemotherapy regimens without BRAF inhibitors as a comparison group. Among the BRAF-treated patients, 619 patients were treated with encorafenib, 111 with dabrafenib, and 156 patients were treated with vemurafenib, as shown in Table 1.

Table 1.
Baseline characteristics of patients included in clinical trials on BRAF inhibitors in colorectal cancer.
3.2.1. Efficacy
In two RCTs (n = 765) [11,12], CR, PR, and OR were 4/111 (3.6%), 25/111 (22.5%), and 37/161 (23%), respectively, in patients treated with a three-drug BRAF combination. CR, PR, and OR were 6/113 (5.3%), 17/113 (15%), and 23/113 (20.3%), respectively, in patients treated with two-drug BRAF regimens. CR, PR, and OR were 0/157 (0), 4/157 (2.5%), and 4/157 (2.5%), respectively, in patients treated with two-drug chemotherapy control regimens without BRAF inhibitors. In the RCT by Kopetz et al., 2021 [12], the hazard ratio (HR) for progression-free survival (PFS) was 0.5 (95% CI = 0.32–0.76) in favor of vemurafenib + cetuximab + irinotecan as compared to cetuximab + irinotecan. However, overall survival (OS) was similar among the two groups (HR = 0.77, 95% CI = 0.5–1.18). In the RCT by Kopetz et al., 2019 [11], HR for PFS was significantly better with triplet encorafenib therapy (encorafenib + binimetinib + cetuximab) 0.38 (95% CI = 0.29–0.49) as compared to the control group and 0.4 (95% CI = 0.31–0.52) as compared to doublet encorafenib therapy. HR for OS was also significantly better with triplet encorafenib therapy at 0.52 (95% CI = 0.39–0.70) as compared to control therapy, as shown in Table 2.

Table 2.
Efficacy of BRAF inhibitor-based regimens in BRAF-mutated CRC.
In nRCTs on two-drug regimens (n = 72) [18], CR, PR, and OR were 1/72 (1%), 11/72 (15%), and 12/72 (17%), respectively, on treatment with two-drug BRAF-based regimens. In nRCTs on three-drug regimens (n = 152), CR, PR, and OR were 3/152 (2%), 49/152 (32%), and 52/152 (34%), respectively, on treatment with three-drug regimens, as shown in Table 2.
3.2.2. Safety
In two RCTs (n = 765) [11,12], ≥ grade 3 diarrhea was reported in 22/222 (10%), nausea in 10/222 (5%), vomiting in 9/222 (4%), abdominal pain in 13/222 (5.5%), anemia in 24/222 (11%), fatigue in 5/222 (2.2%), and rash in 1/222 (<1%) of patients treated with encorafenib + binimetinib + cetuximab. Adverse effects leading to discontinuation were reported in 7% of the patients. Higher than grade 3 diarrhea was reported in 4/216 (2%), nausea in 1/216 (0.5%), vomiting in 3/216 (1.4%), abdominal pain in 5/216 (2%), anemia in 9/216 (4%), fatigue in 9/216 (4%), and rash in 0/216 (0%) of patients treated with encorafenib + cetuximab. Treatment discontinuation due to adverse effects was reported in 8% of the patients. Higher than grade 3 diarrhea was reported in 19/193 (10%), nausea in 2/193 (1%), vomiting in 5/193 (3%), abdominal pain in 9/193 (5%), anemia in 8/193 (4%), fatigue in 8/193 (4%), and rash in 3/193 (2%) of patients treated with cetuximab and irinotecan OR cetuximab and FOLFIRI. Treatment discontinuation due to adverse effects in this arm was reported in 11% of the patients. In an RCT by Kopetz et al., 2021 [12], ≥grade 3 diarrhea was reported in 11/47 (23%), nausea in 9/47 (19%), vomiting in 5/47 (11%), abdominal pain in 2/47 (4%), anemia in 6/47 (13%), fatigue in 8/47 (17%), and rash in 1/47 (<1%) of patients treated with cetuximab + irinotecan. Treatment discontinuation due to adverse effects was reported in 8% of the patients. Higher than grade 3 diarrhea was reported in 6/46 (13%), nausea in 1/46 (2%), vomiting in 1/46 (2%), abdominal pain in 1/46 (2%), anemia in 0, fatigue in 7/46 (14%), and rash in 3/46 (6%) of patients treated with vemurafenib + cetuximab + irinotecan. Treatment discontinuation due to adverse effects was reported in 22% of the patients in this group. In other small-scale nRCTs, ≥ grade 3 diarrhea, rash, anemia, and treatment discontinuation due to toxicity were reported in 4%, 18%, 4%, and 7% of the patients, respectively, in patients treated with cobimetinib + vemurafenib. Higher than grade 3 diarrhea, rash, neutropenia, anemia, and adverse effects related to discontinuation were reported in 10%, 14%, 38%, 14%, and 0% of the patients, respectively, treated with vemurafenib + cetuximab + FOLFIRI, as shown in Table S2.
3.3. BRAF Inhibitor as First-Line Therapy in mCRC
In a phase II clinical trial (n = 95), all patients were treated with a three-drug regimen of encorafenib + cetuximab + binimetinib. CR, PR, and ORR were reported in 44/95 (46%), 41/95 (42%), and 3/95 (4%), respectively. The median PFS and OS were 5.8 (4.6–6.6) and 18.3 (14.1–21.1), respectively, as shown in Table 2. Severe side effects were reported in 49 (51.6%) of the patients. Higher than grade 3 diarrhea, nausea, vomiting, anemia, and intestinal obstruction were reported in 9 (9.5%), 8 (8.4%), 3 (3.2%), 10 (10.5%), and 14 (14.7%) patients, respectively, and 24% of the patients discontinued treatment due to adverse effects, as shown in Table S2.
3.4. Ongoing Clinical Trials
Randomized clinical trials are in progress for combinations of vemurafenib and irinotecan and cetuximab, and a combination of encurafenib and cetuximab. Other newer combination regimens of BRAF inhibitors include combinations of cetuximab, camrelizumab, HL-085, pembrolizumab, ZN-c3, hydroxychloroquine, binimetinib, and nivolumab, and non-randomized clinical trials are in progress, as shown in Table S3.
4. Discussion
The management of metastatic colon cancer with BRAF mutations includes utilizing aggressive treatment options like doublet and triplet chemotherapy regimens, as these patients have poor survival rates. Patients with BRAF mutations had a limited response to chemotherapy. Treatment strategies have evolved significantly in recent years; however, a clear standard of care has not been established. Ongoing research efforts have led to the emergence of BRAF inhibitors such as vemurafenib and encorafenib.
BRAF inhibitors initially showed significant survival benefits as monotherapy in BRAF-mutated solid tumors like melanoma [21]. However, the results were not repeated in the pilot study by Kopetz et al., 2015 [13], on the monotherapy of vemurafenib in BRAF-mutated colorectal cancer. Only minimal benefits were reported with the use of vemurafenib as a monotherapy. The likely reason was due to the over-activation of other pathways like MEK, EGFR, and phosphoinositide 3-kinase (PI3K). Similarly, EGFR inhibitors had activity in BRAF-mutated CRC patients; however, based on the results of a meta-analysis, they did not significantly improve outcomes in this population [22]. In a recent RCT by Stintzing et al., the EGFR inhibitor remains inferior to anti-VEGF (bevacizumab) in combination with chemotherapy in BRAF-mutated CRC patients [23]. Therefore, the addition of BRAF inhibitors was crucial to the treatment of BRAF-mutated CRC patients.
One of the earliest trials was conducted by Yaeger et al., 2015 [17], to study the efficacy and safety profile of vemurafenib and panitumumab (EGFR inhibitors) on patients who had previously progressed on at least one line of chemotherapy. Panitumumab was added to inhibit the reactivation of the EGFR signaling pathway. The combination showed clinical benefit in this patient population, but the magnitude was smaller, with a possible reason due to insufficient inhibition of other pathways like extracellular signal-regulated kinase (ERK) by the drug [24]. The paradoxical activation of other pathways including RAS and ERK remains a major challenge in BRAF inhibitors, which was addressed by combinations with other drugs. In this study, a safety assessment was conducted after six patients were enrolled due to concerns about potential overlapping toxicity between vemurafenib and panitumumab, particularly dermatologic toxicity. The results did not show any significant safety concerns and were well tolerated by most of the patients. Skin rashes generally correspond to the EGFR-targeted therapy efficacy, which does not apply to this case due to the opposing effects of these two agents in the skin used in this study. Liver enzymes were significantly elevated in 20% of the patients and should be followed up regularly in patients treated with this regimen.
Corcoran et al. initially tested a three-drug combination to inhibit EGFR, MEK, and BRAF pathways by combining dabrafenib, trametinib, and panitumumab [19]. The trial showed good response rates (21%) and an mPFS of 4.2 months. However, the benefit was limited due to safety concerns, especially serious dermatological toxicity. Van Geel et al. conducted a phase Ib trial on a three-drug regimen of encorafenib + cetuximab ± alpelisib. Dose-limiting toxicities were observed in three of the patients receiving dual therapy and two of the patients receiving triple therapy. Both the dual- and triple-combination therapies showed acceptable safety profiles. The clinical efficacy was better in the three-drug regimen as compared to the two-drug regimen. The trial showed that the combination of BRAF with EGFR and PI3Kα was a viable treatment option for this difficult-to-treat patient population. However, the study was non-randomized, the baseline characteristics of the patients were different among the two groups, and the ECOG score was higher in the two-drug regimen group. Therefore, the comparison between the two groups should be interpreted with caution.
Another combination of a BRAF inhibitor, an EGFR inhibitor, and chemotherapy was tested by Hong et al. combining vemurafenib, irinotecan, and cetuximab. At all dose levels of vemurafenib, anti-tumor activity was exhibited and mPFS was prolonged. Vemurafenib in combination with irinotecan and cetuximab was tolerated favorably, with only three patients experiencing dose-limiting toxicity. Fewer severe hepatotoxic events were reported in this three-drug regimen. The SWOG 1406 study conducted by Kopetz et al. expanded upon the same combination of vemurafenib, cetuximab, and irinotecan in the RCT. The trial demonstrated significant PFS improvement with the addition of a BRAF inhibitor to an EGFR inhibitor and a single-drug chemotherapy regimen. On subgroup analysis, the prior exposure to irinotecan and cetuximab did not significantly decrease the potential benefits of the above-mentioned regimen.
The BEACON trial was a large RCT that investigated the comparison of BRAF and EGFR inhibitors ± MEK inhibitor vs. the investigators’ choice of either cetuximab and FOLFIRI or cetuximab and irinotecan. The trial was not intended to compare the two experimental groups directly, but the overall survival analysis showed a hazard ratio for death that favored the triplet regimen (encorafenib + cetuximab + binimetinib). Adverse events were slightly higher in the three-drug regimen as compared to the two-drug regimen and were similar to side effects seen with previous trials on BRAF, EGFR, and MEK inhibitors. A real-world study by Boccaccino et al. returned the same response results for the three-drug regimen vs. the two-drug regimen as seen in the BEACON trial [25]. However, in the long-term updated results of the BEACON trial by Tabernero et al., response rates were slightly better with the three-drug regimen vs. two-drug regimen (27% vs. 20%), but there was no difference in median OS among the two groups (9.3 months vs. 9.3 months). The overall survival was significantly better than in the control group (9.3 vs. 5.9 months). Therefore, the two-drug regimen (encorafenib + cetuximab) can be used instead of the three-drug regimen or previous standard-of-care regimens.
Another dual combination of a BRAF inhibitor, vemurafenib, and a MEK inhibitor, cobimetinib, was not tested in the SWOG or BEACON trials. This combination showed anti-tumor activity in pretreated patients with no new safety concerns. However, this was a small-scale study, and the combination needs to be tested in RCTs to compare with the BRAF and EGFR inhibitor combination.
After promising results of the BRAF + EGFR inhibitor combination in the BEACON trial, the IMPROVEMENT trial combined these drugs with FOLFIRI [3]. A total of 85% of patients achieved an objective response with the combination therapy, while 100% achieved disease control that was significantly better than prior tolerated regimens. The trial also included patients with ECOG 2, who were underrepresented in prior studies. The regimen was well tolerated by most of the patients and required dose reductions in 9/21 patients.
ANCHOR CRC assessed the triple combination of encorafenib, binimetinib, and cetuximab in a first-line setting for the first time without chemotherapy [20]. The results were similar to chemotherapy + bevacizumab in the FIRE-4.5 study [23]. This trial showed a feasible chemotherapy-free option for first-line therapy in BRAF-mutated patients. However, more large-scale studies are needed to find a subgroup of patients better suited for chemotherapy-free regimens and compare it with chemotherapy-based regimens.
The BRAF mutation is generally considered a poor prognostic factor; however, different subsets of BRAF-mutated patients might have different prognoses and responses to treatment. Therefore, the development of biomarkers to predict response and prognosis is important, as is identifying patients who might benefit from the intensification of therapy. In a retrospective study by Loupakis et al., a scoring system “BeCool” was devised for BRAF-mutated patients to predict prognosis. The scoring was majorly based on CA19-9, lactate dehydrogenase levels, ECOG performance, neutrophil to lymphocyte ratio, metastatic sites, and grading of cancer, as they were independently associated with significant changes in OS. Patients with a low score (0–4) had an mOS of 29.6, intermediate (5–8) had 15.5, and high (9–16) had an mOS of 6.6 months. However, most of the patients in this study were not treated with BRAF inhibitors [26]. An exploratory study by Elez et al. suggested that the RNF43 mutation in BRAF-mutated patients is a favorable predictor of response to BRAF inhibitors in BRAF-mutated microsatellite stable (MSS) CRC patients. The ORR was 73% in RNF43-mutated vs. 31% in RNF43-wild-type CRC patients treated with BRAF inhibitors [27]. A prospective study by Ros et al. found that a high plasmatic allele fraction (AF) of BRAF was associated with worse PFS and OS as compared to low AF in patients with BRAF-mutated CRC treated with BRAF and anti-EGFR inhibitors. Thus, patients with high AF might benefit from the intensification of BRAF inhibitor therapy [28]. In a retrospective study by Kopetz et al., BRAF-mutated CRC patients with the consensus molecular subtype (CMS-4) and BRAF mutant (BM1) subtypes had better responses to triplet therapy as compared to doublet therapy. Thus, this highlighted the potential of using CMS and BM classification as predictors of response to different regimens [29].
Microsatellite instability (MSI) and BRAF gene mutations are closely related to each other and coexist in about 52% of the patients with a BRAF mutation [30]. Checkpoint inhibitors like nivolumab, ipilimumab, and pembrolizumab have shown promising results in advanced MSI colorectal cancers. A combination of checkpoint inhibitors with BRAF inhibitors is also a feasible combination to test in BRAF-mutated patients. In clinical studies, patients with microsatellite stability (MSS) treated with encorafenib + cetuximab had transient microsatellite instability (MSI) [4]; therefore, Morris et al. conducted a phase I/II study (n = 26) on the addition of nivolumab to encorafenib + cetuximab in MSS patients. Based on interim results presented in the ASCO annual meeting of 2022, the combination was well tolerated and was clinically active. Large-scale RCTs are needed to assess the significance of nivolumab or other PD-1/PD-L1 inhibitors in these patients. There is another phase III clinical trial, NCT05217446, in progress to assess the efficacy and safety of BRAF inhibitors ± pembrolizumab in BRAF and MSI-H colorectal cancer patients.
5. Conclusions
BRAF inhibitors, encorafenib, and vemurafenib, were well tolerated by most of the patients in combination with EGFR inhibitors, MEK inhibitors, and FOLFIRI drugs. In RCTs, BRAF-based three-drug and two-drug regimens were more effective than prior standard-of-care chemotherapy regimens in R/R patients with BRAF-mutated CRC. In the long-term results, survival rates of BRAF-based two-drug regimens (BRAF + EGFR) were similar to those of BRAF-based three-drug regimens with better safety profiles. Therefore, two drug regimens can be used as a standard of care in BRAF-mutated R/R patients. Among nRCTs, a combination of BRAF with EGFR and FOLFIRI drugs showed the highest response and survival rates in R/R BRAF-mutated CRC patients. The BRAF-based, three-drug, chemotherapy-free regimen also showed clinical activity with acceptable toxicity as a first-line therapy. Large-scale RCTs are needed to compare BRAF-based regimens and find appropriate populations for BRAF-based regimens.
6. Limitation
There were no double-blinded large-scale clinical studies available to include in this systematic review; therefore, the risk of bias was high in the included studies. Most of the BRAF-based regimens, including BRAF combinations with chemotherapy and checkpoint inhibitors, were only tested in small-scale, single-arm studies, and randomized studies are in progress. Since most of the treatment regimens were only tested in small-scale, single-arm studies, a meaningful meta-analysis could not be conducted. Despite these limitations, the article was able to provide a comprehensive review of the current evidence available on BRAF inhibitors in BRAF-mutated CRC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13010113/s1, Table S1: PICOS table and database search.; Table S2: Safety of BRAF inhibitor-based regimens in CRC patients; Table S3. Ongoing clinical trials on BRAF inhibitor-based regimens in CRC.
Author Contributions
Conceptualization, W.A. and M.A.A.; methodology, W.A. and M.A.A.; software, S.J., M.Q. and U.A.; validation, M.M., G.G., B.A.O., V.S.C. and H.S.; formal analysis, W.A., J.G., O.A. and M.A.A.; investigation, J.G., M.Q. and A.A.; resources, M.N.R. and O.A.; data curation, M.N.R., B.A.O. and V.S.C.; writing—original draft preparation, M.A.A., S.J., U.A., M.Q., M.N.R., O.A., B.A.O., V.S.C. and W.A.; writing—review and editing, M.M., G.G. and H.S.; supervision, H.S., G.G. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. Jama 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qin, B.D.; Ye, C.Y.; Wang, M.M.; Yuan, L.Y.; Dai, W.P.; Sun, L.; Liu, K.; Qin, W.X.; Jiao, X.D.; et al. Cetuximab and vemurafenib plus FOLFIRI (5-fluorouracil/leucovorin/irinotecan) for BRAF V600E-mutated advanced colorectal cancer (IMPROVEMENT): An open-label, single-arm, phase II trial. Eur. J. Cancer 2022, 163, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.M.; Labajos, V.A.; Ballena, S.L.; Macha, C.A.; Lezama, M.S.; Roman, C.P.; Beltran, P.M.; Torrejon, A.F. Targeting BRAF V600E in metastatic colorectal cancer: Where are we today? Ecancermedicalscience 2022, 16, 1489. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Tian, F.; Mariadason, J.M.; Tsao, C.C.; Lemos, R., Jr.; Dayyani, F.; Gopal, Y.N.; Jiang, Z.Q.; Wistuba, I.I.; Tang, X.M.; et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B.; Atreya, C.E.; Falchook, G.S.; Kwak, E.L.; Ryan, D.P.; Bendell, J.C.; Hamid, O.; Messersmith, W.A.; Daud, A.; Kurzrock, R.; et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 4023–4031. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2008; Available online: https://training.cochrane.org/handbook/archive/v5.0.0/ (accessed on 7 October 2023).
- Sarkis-Onofre, R.; Catalá-López, F.; Aromataris, E.; Lockwood, C. How to properly use the PRISMA Statement. Syst. Rev. 2021, 10, 117. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 386. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. bmj 2019, 366, 14898. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Kopetz, S.; Guthrie, K.A.; Morris, V.K.; Lenz, H.J.; Magliocco, A.M.; Maru, D.; Yan, Y.; Lanman, R.; Manyam, G.; Hong, D.S.; et al. Randomized Trial of Irinotecan and Cetuximab With or Without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG S1406). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Desai, J.; Chan, E.; Hecht, J.R.; O’Dwyer, P.J.; Maru, D.; Morris, V.; Janku, F.; Dasari, A.; Chung, W.; et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 4032–4038. [Google Scholar] [CrossRef] [PubMed]
- van Geel, R.; Tabernero, J.; Elez, E.; Bendell, J.C.; Spreafico, A.; Schuler, M.; Yoshino, T.; Delord, J.P.; Yamada, Y.; Lolkema, M.P.; et al. A Phase Ib Dose-Escalation Study of Encorafenib and Cetuximab with or without Alpelisib in Metastatic BRAF-Mutant Colorectal Cancer. Cancer Discov. 2017, 7, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Morris, V.K.; El Osta, B.; Sorokin, A.V.; Janku, F.; Fu, S.; Overman, M.J.; Piha-Paul, S.; Subbiah, V.; Kee, B.; et al. Phase IB Study of Vemurafenib in Combination with Irinotecan and Cetuximab in Patients with Metastatic Colorectal Cancer with BRAFV600E Mutation. Cancer Discov. 2016, 6, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Parseghian, C.M.; Escano, M.; Johnson, B.; Raghav, K.P.S.; Dasari, A.; Huey, R.; Overman, M.J.; Willis, J.; Lee, M.S.; et al. Phase I/II trial of encorafenib, cetuximab, and nivolumab in patients with microsatellite stable, BRAFV600E metastatic colorectal cancer. J. Clin. Oncol. 2022, 40, 12. [Google Scholar] [CrossRef]
- Yaeger, R.; Cercek, A.; O’Reilly, E.M.; Reidy, D.L.; Kemeny, N.; Wolinsky, T.; Capanu, M.; Gollub, M.J.; Rosen, N.; Berger, M.F.; et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin. Cancer Res. 2015, 21, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Klute, K.A.; Rothe, M.; Garrett-Mayer, E.; Mangat, P.K.; Nazemzadeh, R.; Yost, K.J.; Duvivier, H.L.; Ahn, E.R.; Cannon, T.L.; Alese, O.B.; et al. Cobimetinib Plus Vemurafenib in Patients With Colorectal Cancer With BRAF Mutations: Results From the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. JCO Precis. Oncol. 2022, 6, e2200191. [Google Scholar] [CrossRef]
- Corcoran, R.B.; André, T.; Atreya, C.E.; Schellens, J.H.M.; Yoshino, T.; Bendell, J.C.; Hollebecque, A.; McRee, A.J.; Siena, S.; Middleton, G.; et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAFV600E-Mutant Colorectal Cancer. Cancer Discov. 2018, 8, 428–443. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Taieb, J.; Yaeger, R.; Yoshino, T.; Grothey, A.; Maiello, E.; Elez, E.; Dekervel, J.; Ross, P.; Ruiz-Casado, A.; et al. ANCHOR CRC: Results From a Single-Arm, Phase II Study of Encorafenib Plus Binimetinib and Cetuximab in Previously Untreated BRAF(V600E)-Mutant Metastatic Colorectal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 2628–2637. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Engl. J. Med. 2014, 372, 30–39. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Petrelli, F.; Coinu, A.; Di Bartolomeo, M.; Borgonovo, K.; Maggi, C.; Cabiddu, M.; Iacovelli, R.; Bossi, I.; Lonati, V.; et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur. J. Cancer 2015, 51, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, S.; Heinrich, K.; Tougeron, D.; Modest, D.P.; Schwaner, I.; Eucker, J.; Pihusch, R.; Stauch, M.; Kaiser, F.; Kahl, C.; et al. FOLFOXIRI Plus Cetuximab or Bevacizumab as First-Line Treatment of BRAF(V600E)-Mutant Metastatic Colorectal Cancer: The Randomized Phase II FIRE-4.5 (AIO KRK0116) Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Lito, P.; Rosen, N.; Solit, D.B. Tumor adaptation and resistance to RAF inhibitors. Nat. Med. 2013, 19, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Boccaccino, A.; Borelli, B.; Intini, R.; Antista, M.; Bensi, M.; Rossini, D.; Passardi, A.; Tamberi, S.; Giampieri, R.; Antonuzzo, L.; et al. Encorafenib plus cetuximab with or without binimetinib in patients with BRAF V600E-mutated metastatic colorectal cancer: Real-life data from an Italian multicenter experience. ESMO Open 2022, 7, 100506. [Google Scholar] [CrossRef] [PubMed]
- Loupakis, F.; Intini, R.; Cremolini, C.; Orlandi, A.; Sartore-Bianchi, A.; Pietrantonio, F.; Pella, N.; Spallanzani, A.; Dell’Aquila, E.; Scartozzi, M.; et al. A validated prognostic classifier for V600EBRAF-mutated metastatic colorectal cancer: The ‘BRAF BeCool’ study. Eur. J. Cancer 2019, 118, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Elez, E.; Ros, J.; Fernández, J.; Villacampa, G.; Moreno-Cárdenas, A.B.; Arenillas, C.; Bernatowicz, K.; Comas, R.; Li, S.; Kodack, D.P.; et al. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAFV600E metastatic colorectal cancer. Nat. Med. 2022, 28, 2162–2170. [Google Scholar] [CrossRef]
- Ros, J.; Matito, J.; Villacampa, G.; Comas, R.; Garcia, A.; Martini, G.; Baraibar, I.; Saoudi, N.; Salvà, F.; Martin, Á.; et al. Plasmatic BRAF-V600E allele fraction as a prognostic factor in metastatic colorectal cancer treated with BRAF combinatorial treatments. Ann. Oncol. 2023, 34, 543–552. [Google Scholar] [CrossRef]
- Kopetz, S.; Murphy, D.A.; Pu, J.; Ciardiello, F.; Desai, J.; Grothey, A.; Van Cutsem, E.; Wasan, H.S.; Yaeger, R.; Yoshino, T.; et al. Molecular correlates of clinical benefit in previously treated patients (pts) with BRAF V600E-mutant metastatic colorectal cancer (mCRC) from the BEACON study. J. Clin. Oncol. 2021, 39, 3513. [Google Scholar] [CrossRef]
- Michael, T.P.; Daniel, D.B.; Bryony, T.; Joanne, P.Y.; Amanda, B.S. Correlation of tumour BRAF mutations and methylation with germline mismatch repair (MMR) gene mutation status: A literature review assessing utility of tumour features for MMR variant classification. J. Med. Genet. 2012, 49, 151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).