Efficacy of Sleeve Gastrectomy with Concomitant Hiatal Hernia Repair versus Sleeve–Fundoplication on Gastroesophageal Reflux Disease Resolution: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection of Trials
2.2. Outcome Measures
2.3. Inclusion and Exclusion Criteria
2.4. Critical Assessment of Trials and Collection of Data
2.5. Data Extraction
2.6. Statistical Analysis
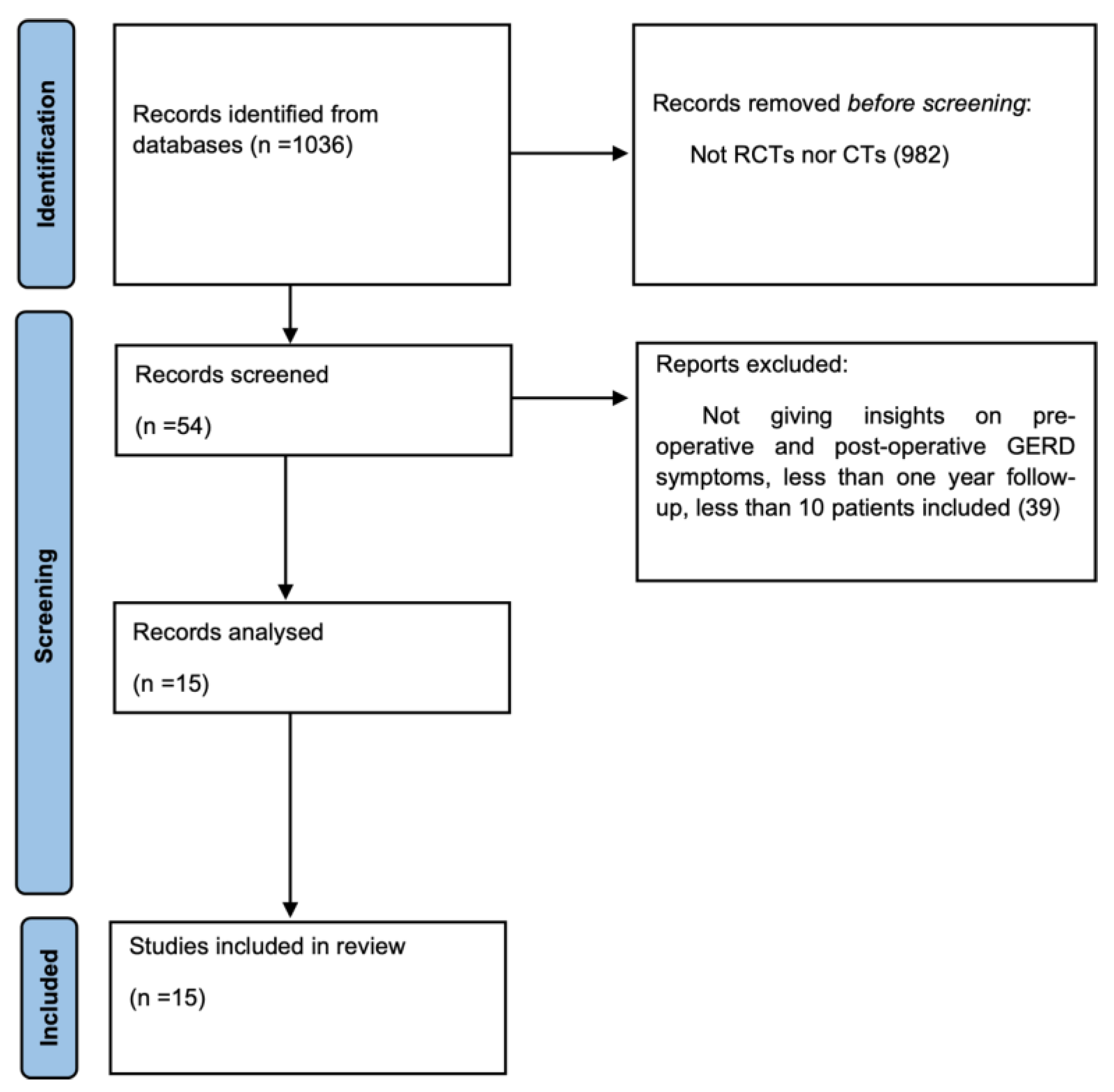
3. Results
3.1. Methodological Quality Assessment and Risk of Bias
3.2. Primary Outcomes
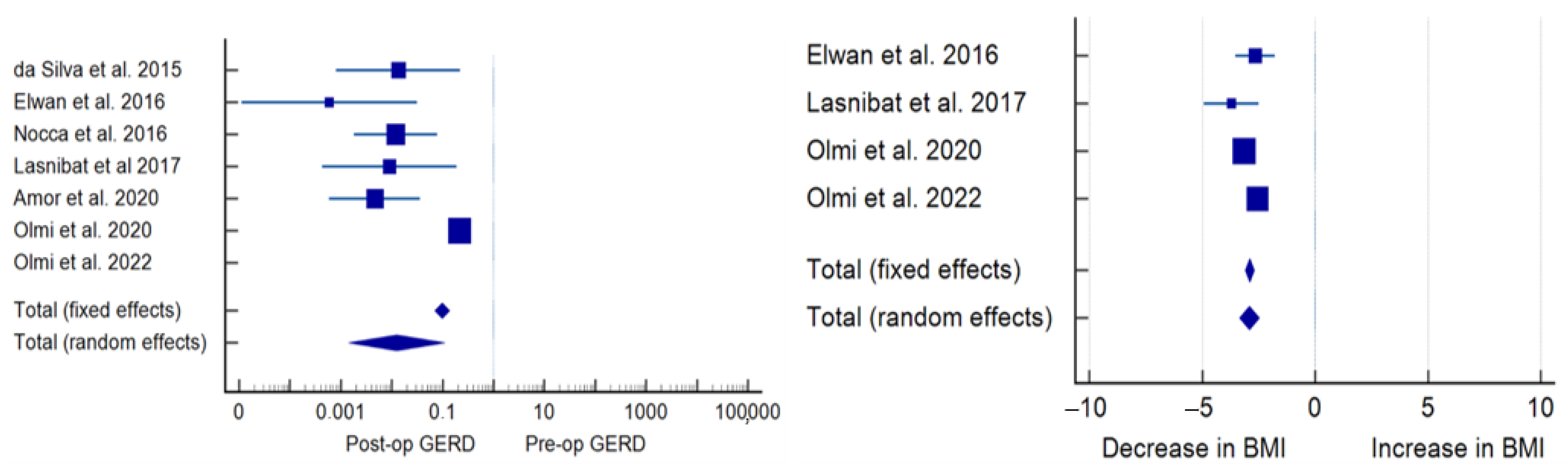
3.3. Secondary Outcomes
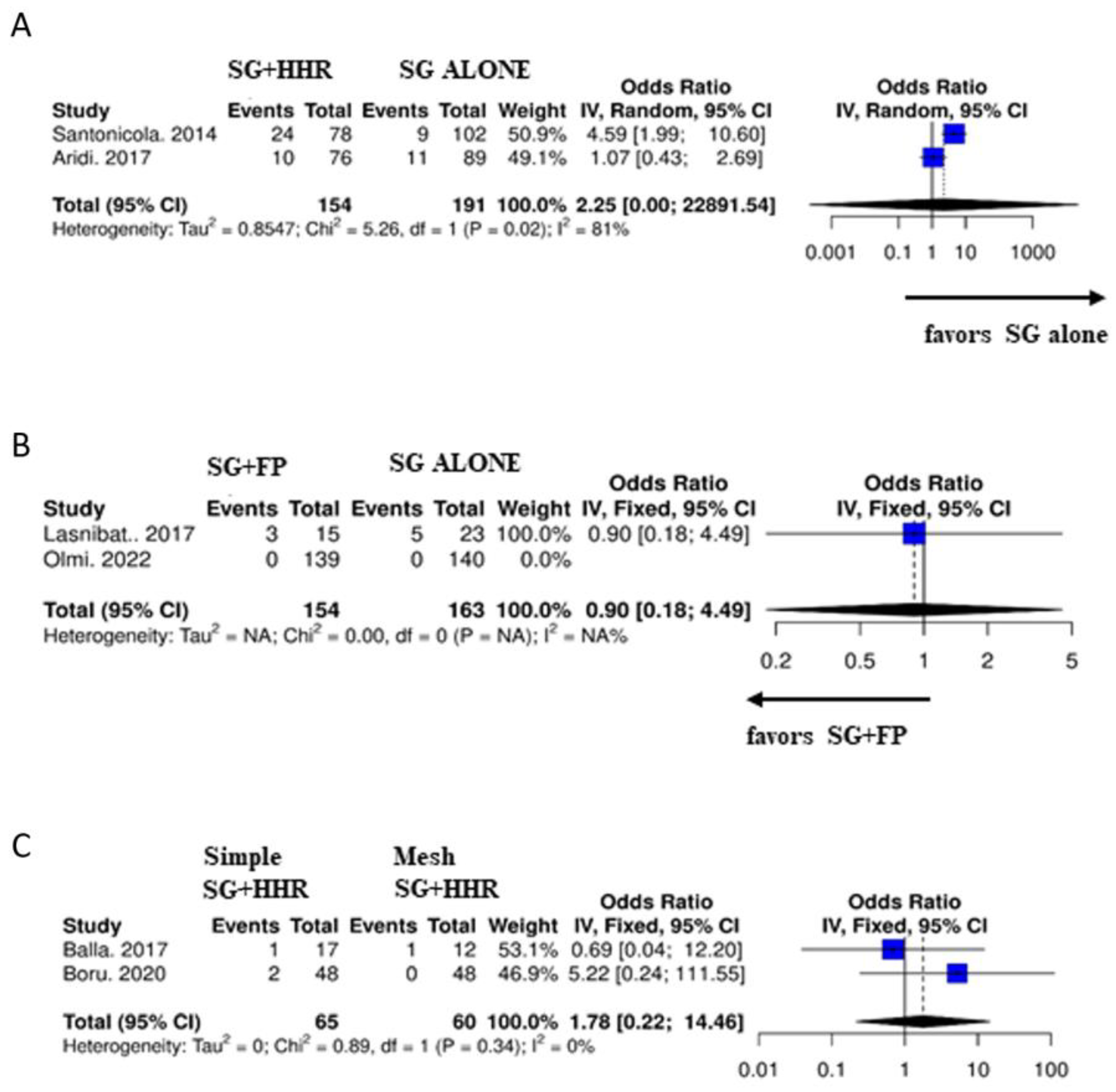
3.4. Subgroup Analysis
4. Discussion
Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seventh IFSO Global Registry Report. 2022. Available online: https://www.ifso.com/pdf/ifso-7th-registry-report-2022.pdf (accessed on 1 March 2023).
- Han, Y.; Jia, Y.; Wang, H.; Cao, L.; Zhao, Y. Comparative analysis of weight loss and resolution of comorbidities between laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: A systematic review and meta-analysis based on 18 studies. Int. J. Surg. 2020, 76, 101–110. [Google Scholar] [CrossRef]
- Castagneto-Gissey, L.; Mingrone, G. Insulin sensitivity and secretion modifications after bariatric surgery. J. Endocrinol. Investig. 2012, 35, 692–698. [Google Scholar] [CrossRef]
- Puzziferri, N.; Roshek, T.B., 3rd; Mayo, H.G.; Gallagher, R.; Belle, S.H.; Livingston, E.H. Long-term follow-up after bariatric surgery: A systematic review. JAMA 2014, 312, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Castagneto-Gissey, L. Type 2 diabetes mellitus in 2013: A central role of the gut in glucose homeostasis. Nat. Rev. Endocrinol. 2014, 10, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Lai, D.D.; Lin, Z.H.; Jiang, T.Y.; Zhang, A.M.; Dai, J.F. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: A systematic review and meta-analysis of randomized and nonrandomized trials. Surg. Laparosc. Endosc. Percutan. Tech. 2014, 24, 1–11. [Google Scholar] [CrossRef]
- Peterli, R.; Borbély, Y.; Kern, B.; Gass, M.; Peters, T.; Thurnheer, M.; Schultes, B.; Laederach, K.; Bueter, M.; Schiesser, M. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): A prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann. Surg. 2013, 258, 690–694; discussion 695. [Google Scholar] [CrossRef]
- Pallati, P.K.; Shaligram, A.; Shostrom, V.K.; Oleynikov, D.; McBride, C.L.; Goede, M.R. Improvement in gastroesophageal reflux disease symptoms after various bariatric procedures: Review of the Bariatric Outcomes Longitudinal Database. Surg. Obes. Relat. Dis. 2014, 10, 502–507. [Google Scholar] [CrossRef]
- Genco, A.; Castagneto-Gissey, L.; Gualtieri, L.; Lucchese, M.; Leuratti, L.; Soricelli, E.; Casella, G. GORD and Barrett’s oesophagus after bariatric procedures: Multicentre prospective study. Br. J. Surg. 2021, 108, 1498–1505. [Google Scholar] [CrossRef]
- DuPree, C.E.; Blair, K.; Steele, S.R.; Martin, M.J. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: A national analysis. JAMA Surg. 2014, 149, 328–334. [Google Scholar] [CrossRef]
- Castagneto-Gissey, L.; Genco, A.; Del Corpo, G.; Badiali, D.; Pronio, A.M.; Casella, G. Sleeve gastrectomy and gastroesophageal reflux: A comprehensive endoscopic and pH-manometric prospective study. Surg. Obes. Relat. Dis. 2020, 16, 1629–1637. [Google Scholar] [CrossRef]
- Che, F.; Nguyen, B.; Cohen, A.; Nguyen, N.T. Prevalence of hiatal hernia in the morbidly obese. Surg. Obes. Relat. Dis. 2013, 9, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.M.; Huang, C.K.; Lee, Y.C.; Chang, C.Y.; Lee, C.T.; Lin, J.T. Increase in gastroesophageal reflux disease symptoms and erosive esophagitis 1 year after laparoscopic sleeve gastrectomy among obese adults. Surg. Endosc. 2013, 27, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Angrisani, L.; Cutolo, P.; Formisano, G.; Iovino, P. The effect of laparoscopic sleeve gastrectomy with or without hiatal hernia repair on gastroesophageal reflux disease in obese patients. Surg. Obes. Relat. Dis. 2014, 10, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Dakour Aridi, H.N.; Tamim, H.; Mailhac, A.; Safadi, B.Y. Concomitant hiatal hernia repair with laparoscopic sleeve gastrectomy is safe: Analysis of the ACS-NSQIP database. Surg. Obes. Relat. Dis. 2017, 13, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; The Cochrane Collaboration: London, UK, 2011.
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 25 April 2016).
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- MedCalc® Statistical Software, Version 20.211; MedCalc Software Ltd.: Ostend, Belgium, 2023. Available online: https://www.medcalc.org (accessed on 20 February 2023).
- Lau, J.; Ioannidis, J.P.; Schmid, C. Quantitative Synthesis in Systematic Reviews. Ann. Intern. Med. 1997, 127, 820–826. [Google Scholar] [CrossRef]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 26.0; IBM Corp.: Armonk, NY, USA, 2019.
- Angrisani, L.; Santonicola, A.; Borrelli, V.; Iovino, P. Sleeve gastrectomy with concomitant hiatal hernia repair in obese patients: Long-term results on gastroesophageal reflux disease. Surg. Obes. Relat. Dis. 2020, 16, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Boru, C.E.; Coluzzi, M.G.; de Angelis, F.; Silecchia, G. Long-Term Results After Laparoscopic Sleeve Gastrectomy with Concomitant Posterior Cruroplasty: 5-Year Follow-up. J. Gastrointest. Surg. 2020, 24, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Gero, D.; Ribeiro-Parenti, L.; Arapis, K.; Marmuse, J.P. Sleeve Gastrectomy Combined with the Simplified Hill Repair in the Treatment of Morbid Obesity and Gastro-esophageal Reflux Disease: Preliminary Results in 14 Patients. World J. Surg. 2017, 41, 1035–1039. [Google Scholar] [CrossRef]
- Soricelli, E.; Iossa, A.; Casella, G.; Abbatini, F.; Calì, B.; Basso, N. Sleeve gastrectomy and crural repair in obese patients with gastroesophageal reflux disease and/or hiatal hernia. Surg. Obes. Relat. Dis. 2013, 9, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Dakour Aridi, H.; Asali, M.; Fouani, T.; Alami, R.S.; Safadi, B.Y. Gastroesophageal Reflux Disease After Laparoscopic Sleeve Gastrectomy with Concomitant Hiatal Hernia Repair: An Unresolved Question. Obes Surg. 2017, 27, 2898–2904. [Google Scholar] [CrossRef]
- Ayman; Elwan, M.; Abomera, M.A.; Ibrahim, A.R.; Atwa, N.S.; Bakheet, G.M.; Slah; Ziada, G.; Alsamahy, O.; Al Makarem, M.A.A. Feasibility of Laparoscopic Management of Hiatal Hernia and/or Gastroesophageal Reflux Disease with Laparoscopic Sleeve Gastrectomy or Greater Curvature Plication in Morbidly Obese Patients. Trends Med. Res. 2016, 11, 54–61. [Google Scholar]
- Nocca, D.; Skalli, E.M.; Boulay, E.; Nedelcu, M.; Michel Fabre, J.; Loureiro, M. Nissen Sleeve (N-Sleeve) operation: Preliminary results of a pilot study. Surg. Obes. Relat. Dis. 2016, 12, 1832–1837. [Google Scholar] [CrossRef]
- Olmi, S.; Uccelli, M.; Cesana, G.C.; Ciccarese, F.; Oldani, A.; Giorgi, R.; De Carli, S.M.; Villa, R. Modified laparoscopic sleeve gastrectomy with Rossetti antireflux fundoplication: Results after 220 procedures with 24-month follow-up. Surg. Obes. Relat. Dis. 2020, 16, 1202–1211. [Google Scholar] [CrossRef]
- Olmi, S.; Cesana, G.; Gambioli, A.; Bonaldi, M.; Ferrari, D.; Uccelli, M.; Ciccarese, F.; Stefano, C.; Riccardo, G.; Lorenzo, M. Effect of laparoscopic sleeve gastrectomy vs laparoscopic sleeve + Rossetti fundoplication on weight loss and de novo GERD in patients affected by morbid obesity: A randomized clinical study. Obes. Surg. 2022, 32, 1451–1458, Erratum in Obes. Surg. 2022, 32, 2102. [Google Scholar] [CrossRef]
- Cottam, S.; Cottam, D.; Cottam, A. Sleeve Gastrectomy Weight Loss and the Preoperative and Postoperative Predictors: A Systematic Review. Obes. Surg. 2019, 29, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Termine, P.; Boru, C.E.; Iossa, A.; Ciccioriccio, M.C.; Campanelli, M.; Bianciardi, E.; Gentileschi, P.; Silecchia, G. Transhiatal Migration After Laparoscopic Sleeve Gastrectomy: Myth or Reality? A Multicenter, Retrospective Study on the Incidence and Clinical Impact. Obes. Surg. 2021, 31, 3419–3426. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, K.K.; Omar, I.; Singhal, R.; Aggarwal, S.; Allouch, M.I.; Alsabah, S.K.; Angrisani, L.; Badiuddin, F.M.; Balibrea, J.M.; Bashir, A.; et al. The first modified Delphi consensus statement on sleeve gastrectomy. Surg. Endosc. 2021, 35, 7027–7033. [Google Scholar] [CrossRef]
- Peterli, R.; Wölnerhanssen, B.K.; Peters, T.; Vetter, D.; Kröll, D.; Borbély, Y.; Schultes, B.; Beglinger, C.; Drewe, J.; Schiesser, M.; et al. Effect of Laparoscopic Sleeve Gastrectomy vs. Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients with Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA 2018, 319, 255–265. [Google Scholar] [CrossRef]
- Lim, C.H.; Lee, P.C.; Lim, E.; Tan, J.; Chan, W.H.; Tan, H.C.; Ganguly, S.; Tham, K.W.; Eng, A. Correlation Between Symptomatic Gastro-Esophageal Reflux Disease (GERD) and Erosive Esophagitis (EE) Post-vertical Sleeve Gastrectomy (VSG). Obes. Surg. 2019, 29, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Del Genio, G.; Tolone, S.; Gambardella, C.; Brusciano, L.; Volpe, M.L.; Gualtieri, G.; Del Genio, F.; Docimo, L. Sleeve Gastrectomy and Anterior Fundoplication (D-SLEEVE) Prevents Gastroesophageal Reflux in Symptomatic GERD. Obes. Surg. 2020, 30, 1642–1652, Erratum in Obes. Surg. 2021, 31, 1902. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H.; Mahdy, T.; Schou, C.; Kramer, M.; Shikora, S. Systematic review of the outcome of single-anastomosis sleeve ileal (SASI) bypass in treatment of morbid obesity with proportion meta-analysis of improvement in diabetes mellitus. Int. J. Surg. 2021, 92, 106024. [Google Scholar] [CrossRef]

| Authors | Year of Publication | Study Type | Control Group | Number of Patients | Follow-Up (Months) | Surgical Technique | Quality Assessment |
|---|---|---|---|---|---|---|---|
| Soricelli et al. [9] | 2013 | Prospective | No | 97 | 18 | Posterior repair using non-absorbable sutures | 9 |
| Santonicola et al. [10] | 2014 | Retrospective | Group A (SG + HHR) vs. Group B (SG alone) | 78 vs. 102 | 14.6 | Posterior repair using 0-Ethibond | 9 |
| Elwan et al. [11] | 2016 | Retrospective | Group A (SG + HHR) vs. Group B (SG + FP) | 20 vs. 20 | 14.1 | Posterior repair using 2–0 non-absorbable sutures | 8 |
| Aridi et al. [12] | 2017 | Retrospective | Group A (SG + HHR) vs. Group B (SG alone) | 76 vs. 89 | 12 | Posterior repair using 2–0 Ethibond sutures | 8 |
| Attia et al. [13] | 2017 | Prospective | No | 53 | 18 | Posterior repair using 0-Ethibond | 8 |
| Balla et al. [14] | 2017 | Retrospective | Group A (SG + simple HHR) vs. Group B (SG + mesh HHR) | 12 vs. 17 | 33.2 ± 16.3 | Posterior repair using 2–0 non-absorbable sutures vs. cruroplasty using absorbable synthetic mesh | 7 |
| Gero et al. [15] | 2017 | Retrospective | No | 14 | 12.5 | Posterior closure with EGJ fixed to the median arcuate ligament using 0-non-absorbable sutures | 8 |
| Angrisani et al. [16] | 2020 | Retrospective | No | 91 | 94 ± 10 | Posterior repair using 2–0 non-absorbable sutures | 8 |
| Boru et al. [17] | 2020 | Prospective | Group A (SG + simple HHR) vs. Group B (SG + mesh HHR) | 48 vs. 48 | 59.1 ± 9.1 | Posterior repair using non-absorbable sutures vs. cruroplasty using biologic mesh | 7 |
| Authors | Year of Publication | Study Type | Control Group | Number of Patients | Follow-Up (Months) | Surgical Technique | Quality Assessment |
|---|---|---|---|---|---|---|---|
| da Silva et al. [18] | 2015 | Retrospective | No | 122 | 36 | Sleeve Collis–Nissen Hiatoplasty | 7 |
| Elwan et al. [11] | 2016 | Retrospective | Group A (SG + HHR) vs. Group B (SG + FP) | 20 vs. 20 | 14.1 | Nissen sleeve | 8 |
| Nocca et al. [19] | 2016 | Prospective | No | 25 | 12 | Nissen sleeve | 8 |
| Lasnibat et al. [20] | 2017 | Retrospective | Group A (SG + FP) vs. Group B (SG alone) | 15 vs. 23 | 12 | Nissen sleeve | 7 |
| Amor et al. [21] | 2020 | Prospective | No | 70 | 12 | Nissen sleeve | 8 |
| Olmi et al. [22] | 2020 | Retrospective | No | 220 | 24 | Sleeve Rossetti fundoplication | 9 |
| Olmi et al. [23] | 2022 | RCT | Group A (SG alone) vs. Group B (SG + FP) | 140 vs. 138 | 12 | Sleeve Rossetti fundoplication | 5 |
| Authors | Year of Publication | Number of Patients | Pre-op BMI (kg/m2) | Post-op BMI (kg/m2) | %EWL | Pre-op GERD n (%) | Post-op GERD n (%) | Pre-op HH n (%) | Post-op HH n (%) | Pre-op Esophagitis n (%) | Post-op Esophagitis n (%) | Bleeding n (%) | Perforation n (%) | Leaks n (%) | Mortality n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soricelli et al. [9] | 2013 | 97 | 44 ± 3.5 | 32.8 ± 5.5 | NR | 60 (61.9) | 19 (19.5) | 97 (100) | NR | 56 (58) | NR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Santonicola et al. [10] | 2014 | 78 | 44.6 ± 7 | 31.7 ± 8 | 62.8 ± 3.53 | 30 (38.4) | 34 (43.3) | 23 (28.9) | NR | NR | NR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Elwan et al. [11] | 2016 | 20 | 45.05 ± 2.96 | 35.0 ± 2.99 | 57 | 20 (100) | 4 (20) | 5 (25) | 8 (40) | NR | NR | 0 (0) | 0 (0) | 1 (5) | 0 (0) |
| Aridi et al. [12] | 2017 | 76 | 42.7 ± 15.3 | 28 ± 6.6 | 87 ± 23.7 | 29 (38.2) | 24 (31.9) | 76 (100) | 2 (2.6) | NR | 19 (25) | 5 (6.5) | 0 (0) | 0 (0) | 0 (0) |
| Attia et al. [13] | 2017 | 53 | 50.1 | NR | 61 | 47 (88.6) | 30 (56.6) | NR | NR | NR | NR | 1 (1.8) | 0 (0) | 0 (0) | 0 (0) |
| Balla et al. [14] | 2017 | 12 | 42.1 ± 8.3 | 29.7 ± 4.1 | NR | 8 (66.6) | 1 (8.3) | 12 (100) | 2 (16.6) | 4 (33.3) | NR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Balla et al. [14] | 2017 | 17 | 43.5 ± 4.7 | 32.8 ± 3.2 | NR | 9 (52.9) | 1 (5.8) | 17 (100) | 0 (0) | 4 (23.5) | NR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gero et al. [15] | 2017 | 14 | 41 | 30.9 | NR | 14 (100) | 3 (21.4) | 12 (85.7) | NR | 4 (28.5) | NR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Angrisani et al. [16] | 2020 | 91 | 44.8 ± 6.1 | 34.9 ± 4.9 | 58.4 ± 15.6 | 36 (39.6) | 12 (13.6) | 37 (40.6) | 15 (16.5) | 22 (24) | 15 (16.5) | NR | NR | NR | NR |
| Boru et al. [17] | 2020 | 48 | NR | NR | 65–7 ± 17.1 | 17 (35.4) | 0 (0) | 11 (22.3) | 0 (0) | 6 (12.5) | 2 (5.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Boru et al. [17] | 2020 | 48 | NR | NR | 55.9 ± 15.1 | 20 (41.6) | 2 (4.1) | 14 (29.1) | 0 (0) | 4 (8.3) | 2 (4.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Authors | Year of Publication | Number of Patients | Pre-op BMI (kg/m2) | Post-op BMI (kg/m2) | %EWL | Pre-op GERD n (%) | Post-op GERD n (%) | Pre-op HH n (%) | Post-op HH n (%) | Pre-op Esophagitis n (%) | Post-op Esophagitis n (%) | Bleeding n (%) | Perforation n (%) | Leaks n (%) | Mortality n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| da Silva et al. [18] | 2015 | 122 | 42.5 ± 5.6 | NR | 64.4 ± 7.2 | 28 (23) | 0 (0) | 82 (67) | 4 (3.3) | NR | NR | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) |
| Elwan et al. [11] | 2016 | 20 | 44.10 ± 2.48 | 37.95 ± 2.1 | NR | 20 (100) | 0 (0) | 6 (30) | 0 (0) | NR | NR | 6 (30) | 0 (0) | 0 (0) | 1 (5) |
| Nocca et al. [19] | 2016 | 25 | 42 ± 4.8 | NR | 58 ± 23 | 23 (92) | 3 (12) | 22 (88) | NR | 10 (40) | 0 (0) | 1 (4) | 1 (4) | 0 (0) | 0 (0) |
| Lasnibat et al. [20] | 2017 | 15 | 33.9 ± 2.11 | 26.6 ± 1.7 | 82.02 | 15 (100) | 3 (20) | NR | NR | 12 (80) | 3 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Amor et al. [21] | 2020 | 70 | 40 ± 5 | NR | 69 ±20 | 53 (76) | 1 (0.7) | 63 (90) | NR | 44 (63) | 14 (28.6) | 1 (0.7) | 0 (0) | 1 (0.7) | 0 (0) |
| Olmi et al. [22] | 2020 | 220 | 42.58 ± 5.93 | 29.4 | 70.1 | 137 (62.3) | 2 (0.9) | 62 (28.2) | NR | 65 (29.5) | 2 (0.9) | 6 (2.7) | 12 (5.5) | 1 (0.5) | 1 (0.45) |
| Olmi et al. [23] | 2022 | 138 | 43.4 ± 5.9 | 29.4 ± 5.0 | NR | 0 (0) | 0 (0) | 18 (13.4) | 23 (16.7) | NR | 3 (2.2) | 1 (0.7) | 6 (4.3) | 0 (0) | 1 (0.72) |
| SG + HHR (n = 554) | SG + FP (n = 610) | p Value | |
|---|---|---|---|
| Bleeding, n (%) | 6 (1.08) | 10 (1.63) | 0.07 |
| Gastric perforation, n (%) | 0 (0) | 19 (3.1) | 0.002 |
| Staple-line leak, n (%) | 1 (0.18) | 2 (0.33) | 0.657 |
| Mortality, n (%) | 0 (0) | 3 (0.5) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagneto-Gissey, L.; Russo, M.F.; D’Andrea, V.; Genco, A.; Casella, G. Efficacy of Sleeve Gastrectomy with Concomitant Hiatal Hernia Repair versus Sleeve–Fundoplication on Gastroesophageal Reflux Disease Resolution: Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3323. https://doi.org/10.3390/jcm12093323
Castagneto-Gissey L, Russo MF, D’Andrea V, Genco A, Casella G. Efficacy of Sleeve Gastrectomy with Concomitant Hiatal Hernia Repair versus Sleeve–Fundoplication on Gastroesophageal Reflux Disease Resolution: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(9):3323. https://doi.org/10.3390/jcm12093323
Chicago/Turabian StyleCastagneto-Gissey, Lidia, Maria Francesca Russo, Vito D’Andrea, Alfredo Genco, and Giovanni Casella. 2023. "Efficacy of Sleeve Gastrectomy with Concomitant Hiatal Hernia Repair versus Sleeve–Fundoplication on Gastroesophageal Reflux Disease Resolution: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 9: 3323. https://doi.org/10.3390/jcm12093323
APA StyleCastagneto-Gissey, L., Russo, M. F., D’Andrea, V., Genco, A., & Casella, G. (2023). Efficacy of Sleeve Gastrectomy with Concomitant Hiatal Hernia Repair versus Sleeve–Fundoplication on Gastroesophageal Reflux Disease Resolution: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(9), 3323. https://doi.org/10.3390/jcm12093323








