Ventilatory Effects of Isoflurane Sedation via the Sedaconda ACD-S versus ACD-L: A Substudy of a Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Drug Administration
2.3. Mechanical Ventilation
2.4. Measurements
2.5. Outcomes
2.6. Statistical Analysis
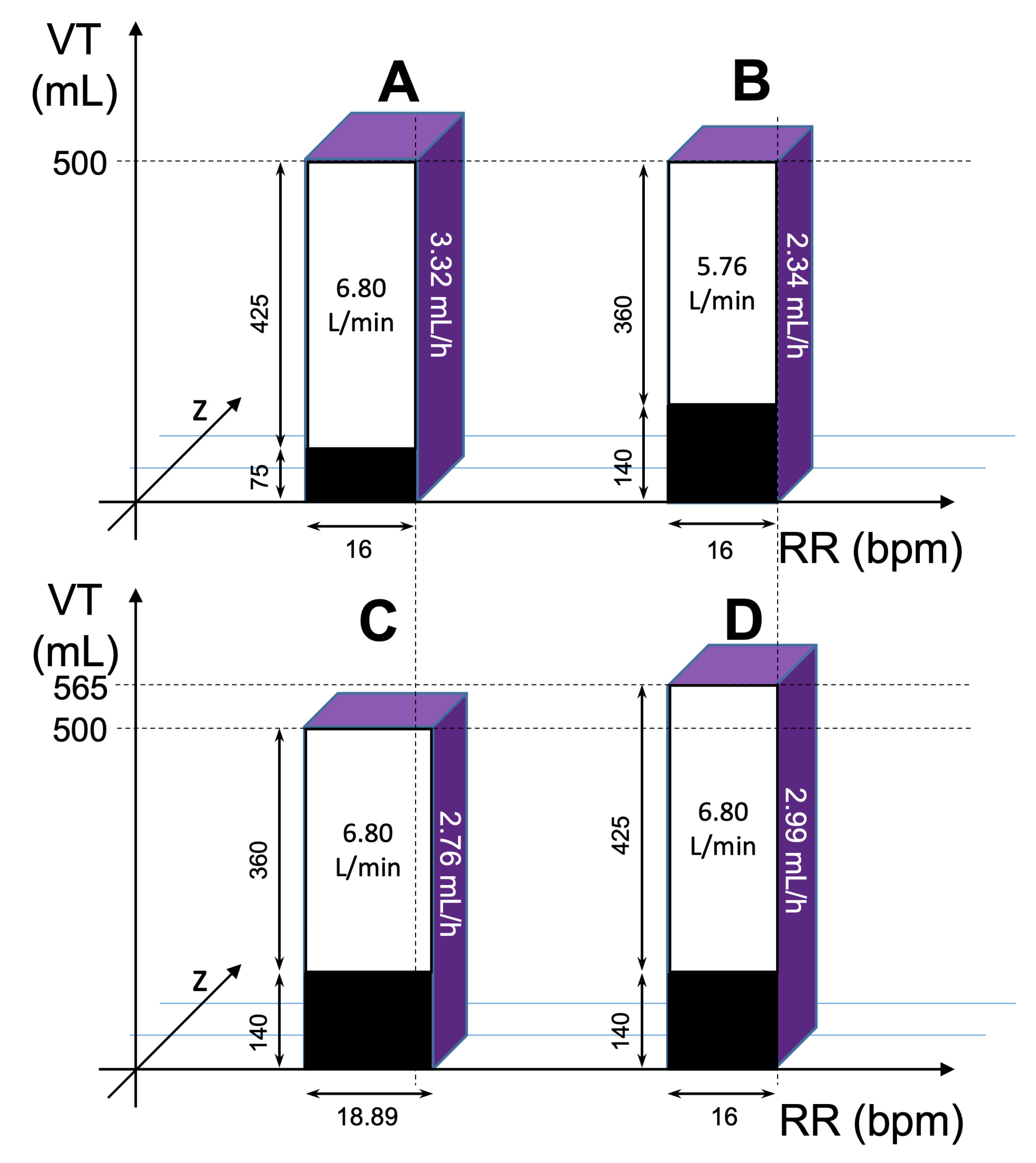
IR/MV = slope × VT × c-Iso × (100 Vol%)−1
IR = slope × VT × c-Iso × (100 Vol%)−1 × RR × VT × (1000 mL/L)−1
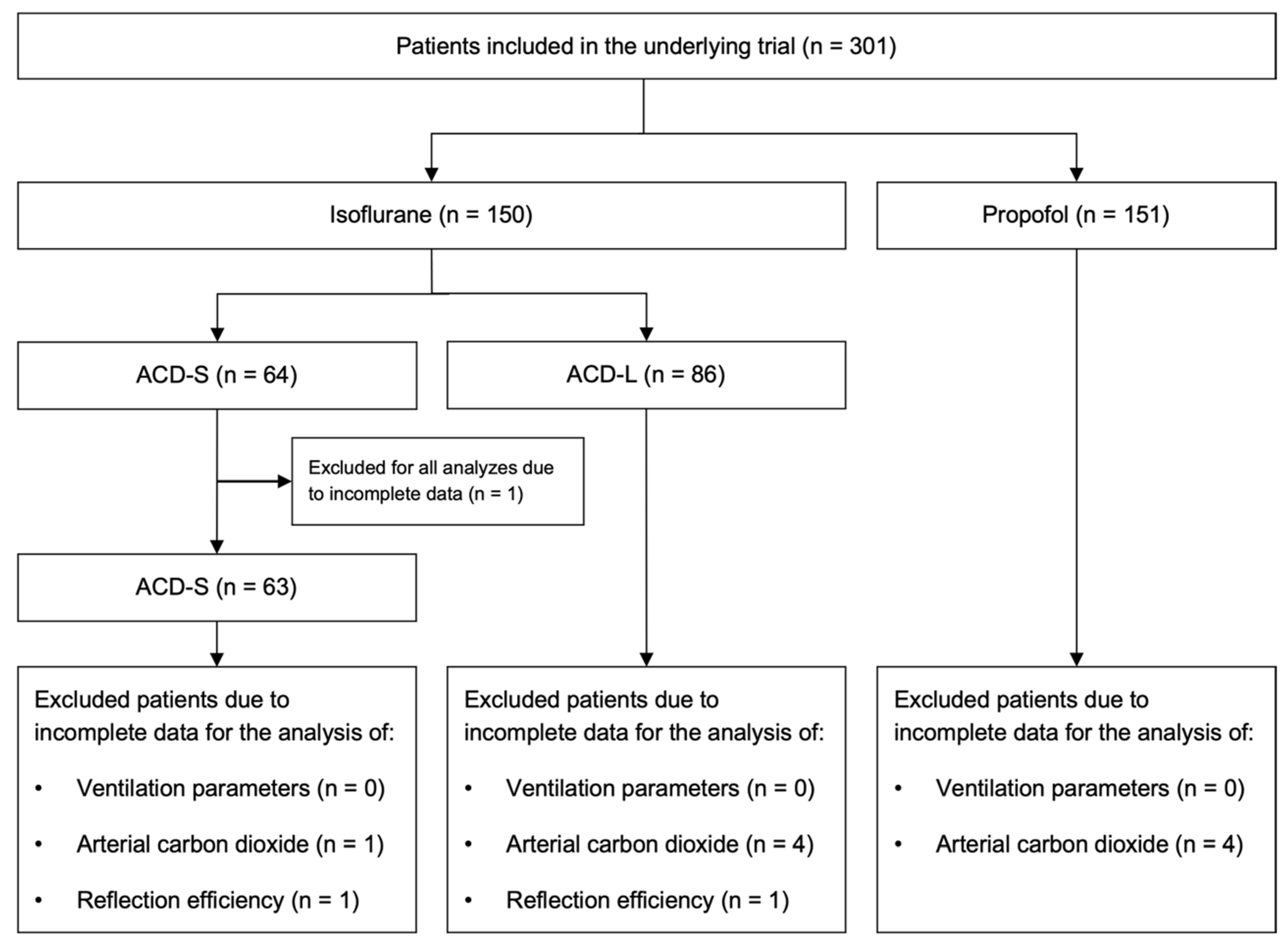
3. Results
3.1. Study Population Characteristics
3.2. Primary Outcome—Minute Ventilation
3.3. Ventilation Parameters
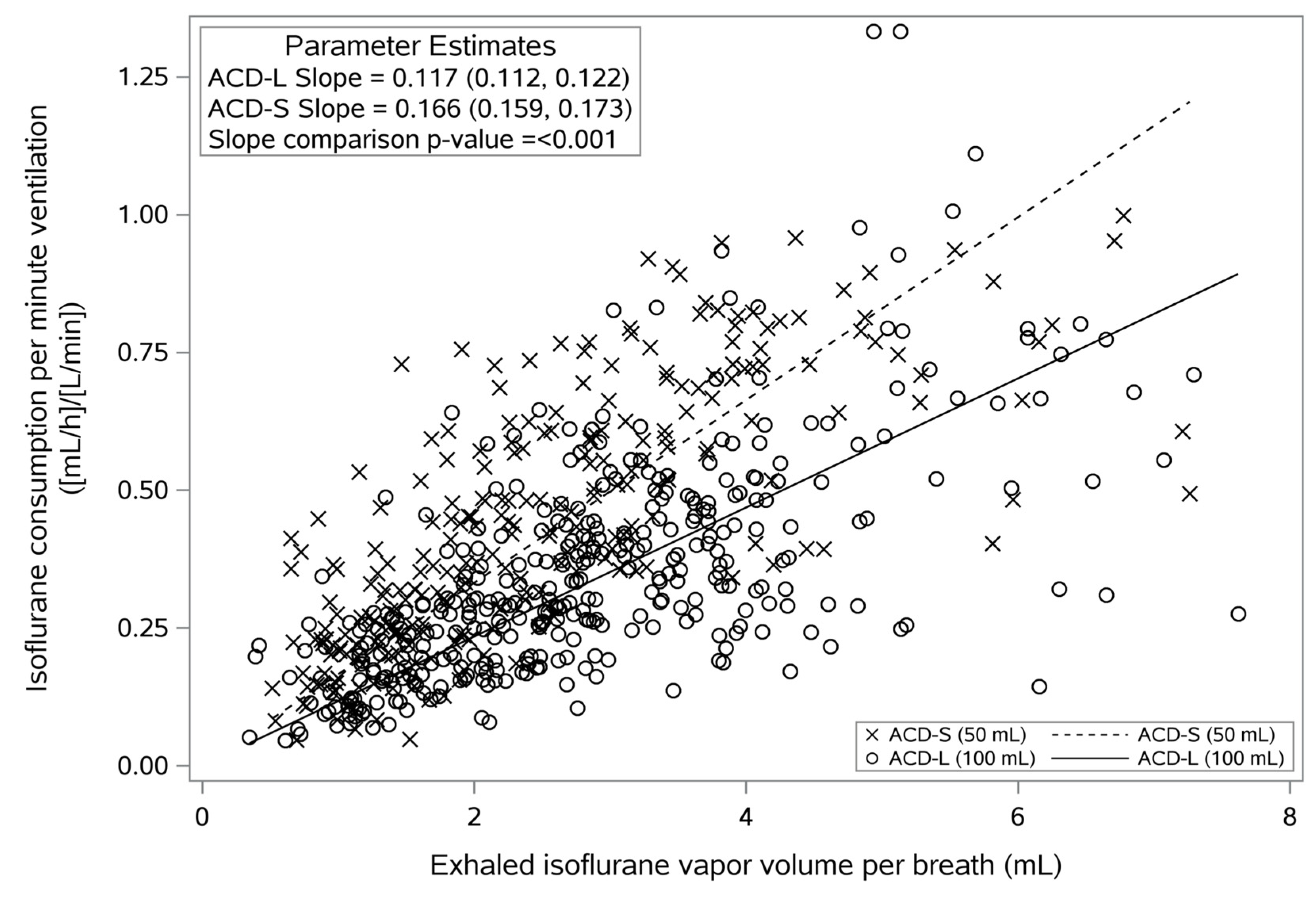
3.4. Volatile Anesthetic Consumption and Reflection Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemphill, S.; McMenamin, L.; Bellamy, M.C.; Hopkins, P.M. Propofol Infusion Syndrome: A Structured Literature Review and Analysis of Published Case Reports. Br. J. Anaesth. 2019, 122, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.; Khoo, K.; Soni, P.; Patel, I. Increased Volume of Distribution Prolongs Midazolam Half-life. Br. J. Clin. Pharmacol. 1990, 29, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.; Shintani, A.; Peterson, J.; Pun, B.T.; Wilkinson, G.R.; Dittus, R.S.; Bernard, G.R.; Ely, E.W. Lorazepam Is an Independent Risk Factor for Transitioning to Delirium in Intensive Care Unit Patients. Anesthesiology 2006, 104, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Levy, N.T.; Ahrens, T.S.; Schaiff, R.; Prentice, D.; Sherman, G. The Use of Continuous IV Sedation Is Associated with Prolongation of Mechanical Ventilation. Chest 1998, 114, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Jerath, A.; Wong, K.; Wasowicz, M.; Fowler, T.; Steel, A.; Grewal, D.; Huszti, E.; Parotto, M.; Zhang, H.; Wilcox, M.E.; et al. Use of Inhaled Volatile Anesthetics for Longer Term Critical Care Sedation: A Pilot Randomized Controlled Trial. Crit. Care Explor. 2020, 2, e0281. [Google Scholar] [CrossRef]
- Bellgardt, M.; Bomberg, H.; Herzog-Niescery, J.; Dasch, B.; Vogelsang, H.; Weber, T.P.; Steinfort, C.; Uhl, W.; Wagenpfeil, S.; Volk, T.; et al. Survival after Long-Term Isoflurane Sedation as Opposed to Intravenous Sedation in Critically Ill Surgical Patients. Eur. J. Anaesthesiol. 2016, 33, 6–13. [Google Scholar] [CrossRef]
- Meiser, A.; Volk, T.; Wallenborn, J.; Guenther, U.; Becher, T.; Bracht, H.; Schwarzkopf, K.; Knafelj, R.; Faltlhauser, A.; Thal, S.C.; et al. Inhaled Isoflurane via the Anaesthetic Conserving Device versus Propofol for Sedation of Invasively Ventilated Patients in Intensive Care Units in Germany and Slovenia: An Open-Label, Phase 3, Randomised Controlled, Non-Inferiority Trial. Lancet Respir. Med. 2021, 9, 1231–1240. [Google Scholar] [CrossRef]
- Beitler, J.R.; Talmor, D. Volatile Anesthetics for ICU Sedation: The Future of Critical Care or Niche Therapy? Intensiv. Care Med. 2022, 48, 1413–1417. [Google Scholar] [CrossRef]
- Farrell, R.; Oomen, G.; Carey, P. A Technical Review of the History, Development and Performance of the Anaesthetic Conserving Device “AnaConDa” for Delivering Volatile Anaesthetic in Intensive and Post-Operative Critical Care. J. Clin. Monit. Comput. 2018, 32, 595–604. [Google Scholar] [CrossRef]
- Bomberg, H.; Glas, M.; Groesdonk, V.H.; Bellgardt, M.; Schwarz, J.; Volk, T.; Meiser, A. A Novel Device for Target Controlled Administration and Reflection of Desflurane—The MirusTM. Anaesthesia 2014, 69, 1241–1250. [Google Scholar] [CrossRef]
- Sturesson, L.W.; Bodelsson, M.; Jonson, B.; Malmkvist, G. Anaesthetic Conserving Device AnaConDa®: Dead Space Effect and Significance for Lung Protective Ventilation. Br. J. Anaesth. 2014, 113, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Bomberg, H.; Meiser, F.; Daume, P.; Bellgardt, M.; Volk, T.; Sessler, D.I.; Groesdonk, H.V.; Meiser, A. Halving the Volume of AnaConDa®: Evaluation of a New Small-Volume Anesthetic Reflector in a Test Lung Model. Anesth. Analg. 2019, 129, 371–379. [Google Scholar] [CrossRef]
- Bomberg, H.; Meiser, F.; Zimmer, S.; Bellgardt, M.; Volk, T.; Sessler, D.I.; Groesdonk, H.V.; Meiser, A. Halving the Volume of AnaConDa®: Initial Clinical Experience with a New Small-Volume Anaesthetic Reflector in Critically Ill Patients-a Quality Improvement Project. J. Clin. Monit. Comput. 2018, 32, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Kermad, A.; Speltz, J.; Daume, P.; Volk, T.; Meiser, A. Reflection Efficiencies of AnaConDa-S and AnaConDa-100 for Isoflurane under Dry Laboratory and Simulated Clinical Conditions: A Bench Study Using a Test Lung. Expert Rev. Med. Devices 2021, 18, 189–195. [Google Scholar] [CrossRef]
- Le Gall, J.-R. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA 1993, 270, 2957. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Vidal, J.M.; Merino, M.; González, R.; García, C.; Rey, S.; Pérez, I. Comparison of the Use of AnaConDa® versus AnaConDa-S® during the Post-Operative Period of Cardiac Surgery under Standard Conditions of Practice. J. Clin. Monit. Comput. 2020, 34, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sturesson, L.W.; Malmkvist, G.; Bodelsson, M.; Niklason, L.; Jonson, B. Carbon Dioxide Rebreathing with the Anaesthetic Conserving Device, AnaConDa®. Br. J. Anaesth. 2012, 109, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Bomberg, H.; Veddeler, M.; Volk, T.; Groesdonk, H.V.; Meiser, A. Volumetric and Reflective Device Dead Space of Anaesthetic Reflectors under Different Conditions. J. Clin. Monit. Comput. 2018, 32, 1073–1080. [Google Scholar] [CrossRef]
- Sturesson, L.W.; Bodelsson, M.; Johansson, A.; Jonson, B.; Malmkvist, G. Apparent Dead Space with the Anesthetic Conserving Device, AnaConDa®: A Clinical and Laboratory Investigation. Anesth. Analg. 2013, 117, 1319–1324. [Google Scholar] [CrossRef]
- Dikmen, Y.; Eminoglu, E.; Salihoglu, Z.; Demiroluk, S. Pulmonary Mechanics during Isoflurane, Sevoflurane and Desflurane Anaesthesia. Anaesthesia 2003, 58, 745–748. [Google Scholar] [CrossRef]
- Meiser, A.; Groesdonk, H.V.; Bonnekessel, S.; Volk, T.; Bomberg, H. Inhalation Sedation in Subjects with ARDS Undergoing Continuous Lateral Rotational Therapy. Respir. Care 2018, 63, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Heider, J.; Bansbach, J.; Kaufmann, K.; Heinrich, S.; Loop, T.; Kalbhenn, J. Does Volatile Sedation with Sevoflurane Allow Spontaneous Breathing during Prolonged Prone Positioning in Intubated ARDS Patients? A Retrospective Observational Feasibility Trial. Ann. Intensiv. Care 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Bansbach, J.; Wenz, J.; Kaufmann, K.; Heinrich, S.; Kalbhenn, J. Sevoflurane in Combination with Esketamine Is an Effective Sedation Regimen in COVID-19 Patients Enabling Assisted Spontaneous Breathing Even during Prone Positioning. Anaesthesiol. Intensiv. Ther. 2022, 54, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Müller-Wirtz, L.M.; Behne, F.; Kermad, A.; Wagenpfeil, G.; Schroeder, M.; Sessler, D.I.; Volk, T.; Meiser, A. Isoflurane Promotes Early Spontaneous Breathing in Ventilated Intensive Care Patients: A Post Hoc Subgroup Analysis of a Randomized Trial. Acta. Anaesthesiol. Scand. 2022, 66, 354–364. [Google Scholar] [CrossRef]
- Müller-Wirtz, L.M.; Grimm, D.; Albrecht, F.W.; Fink, T.; Volk, T.; Meiser, A. Increased Respiratory Drive after Prolonged Isoflurane Sedation: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 5422. [Google Scholar] [CrossRef]
- Ferrando, C.; Aguilar, G.; Piqueras, L.; Soro, M.; Moreno, J.; Belda, F.J. Sevoflurane, but Not Propofol, Reduces the Lung Inflammatory Response and Improves Oxygenation in an Acute Respiratory Distress Syndrome Model: A Randomised Laboratory Study. Eur. J. Anaesthesiol. 2013, 30, 455–463. [Google Scholar] [CrossRef]
- de Conno, E.; Steurer, M.P.; Wittlinger, M.; Zalunardo, M.P.; Weder, W.; Schneiter, D.; Schimmer, R.C.; Klaghofer, R.; Neff, T.A.; Schmid, E.R.; et al. Anesthetic-Induced Improvement of the Inflammatory Response to One-Lung Ventilation. Anesthesiology 2009, 110, 1316–1326. [Google Scholar] [CrossRef]
- Jabaudon, M.; Boucher, P.; Imhoff, E.; Chabanne, R.; Faure, J.-S.; Roszyk, L.; Thibault, S.; Blondonnet, R.; Clairefond, G.; Guérin, R.; et al. Sevoflurane for Sedation in Acute Respiratory Distress Syndrome A Randomized Controlled Pilot Study. Am. J. Respir. Crit. Care Med. 2017, 195, 792–800. [Google Scholar] [CrossRef]
- Meiser, A.; Bellgardt, M.; Belda, J.; Röhm, K.; Laubenthal, H.; Sirtl, C. Technical Performance and Reflection Capacity of the Anaesthetic Conserving Device—A Bench Study with Isoflurane and Sevoflurane. J. Clin. Monit. Comput. 2009, 23, 11–19. [Google Scholar] [CrossRef]

| Variable | Unit | Description |
|---|---|---|
| IR | (mL/h) | Isoflurane pump rate (in steady state this will reflect isoflurane consumption) |
| VT | (mL) | Tidal volume |
| RR | (min−1) | Respiratory rate |
| MV | (L/min) | Minute ventilation (MV can be calculated by multiplying RR with VT × (1000 mL/L)−1) |
| Slope | (mL−1) | Slope of the regression line (0.166 Vol%−1 × mL−1 for ACD-S or 0.117 Vol%−1 × mL−1 for ACD-L) |
| c-Iso | (Vol%) | End-tidal isoflurane concentration |
| VV | (mL) | Isoflurane vapor volume contained in one breath (VV is calculated by multiplying VT and c-Iso × (100 Vol%)−1) |
| Parameter | Propofol | ACD-S | ACD-L | SMD |
|---|---|---|---|---|
| n | 151 | 63 | 86 | - |
| Sex (male) | 98 (65) | 41 (65) | 62 (72) | 0.16 |
| Age (years) | 64 ± 13 | 66 ± 12 | 66 ± 12 | 0.12 |
| Height (cm) | 173 ± 9 | 172 ± 9 | 174 ± 8 | 0.08 |
| Weight (kg) | 84 ± 23 | 84 ± 21 | 84 ± 17 | 0.01 |
| BMI (kg/m2) | 28 ± 8 | 29 ± 7 | 28 ± 5 | 0.03 |
| SAPS II | 41 ± 18 | 38.3 ± 17 | 40 ± 17 | 0.17 |
| COPD (n) | 14 (9) | 7 (11) | 17 (20) | 0.30 |
| Variable | Propofol | ACD-S | ACD-L | Average Difference | p |
|---|---|---|---|---|---|
| End-tidal isoflurane concentration [%] | - | n = 64 0.44 (0.40, 0.49) | n = 85 0.46 (0.42, 0.50) | 0.04 (−0.03, 0.10) | 0.287 |
| Isoflurane pump rate [mL/h] | - | n = 64 3.7 (2.9, 4.5) | n = 86 3.0 (2.3, 3.8) | −0.7 (−1.3, 0.1) | 0.022 |
| Propofol infusion rate [mg/kg/h] | n = 149 2.4 (2.2, 2.6) | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller-Wirtz, L.M.; Becher, T.; Günther, U.; Bellgardt, M.; Sackey, P.; Volk, T.; Meiser, A. Ventilatory Effects of Isoflurane Sedation via the Sedaconda ACD-S versus ACD-L: A Substudy of a Randomized Trial. J. Clin. Med. 2023, 12, 3314. https://doi.org/10.3390/jcm12093314
Müller-Wirtz LM, Becher T, Günther U, Bellgardt M, Sackey P, Volk T, Meiser A. Ventilatory Effects of Isoflurane Sedation via the Sedaconda ACD-S versus ACD-L: A Substudy of a Randomized Trial. Journal of Clinical Medicine. 2023; 12(9):3314. https://doi.org/10.3390/jcm12093314
Chicago/Turabian StyleMüller-Wirtz, Lukas M., Tobias Becher, Ulf Günther, Martin Bellgardt, Peter Sackey, Thomas Volk, and Andreas Meiser. 2023. "Ventilatory Effects of Isoflurane Sedation via the Sedaconda ACD-S versus ACD-L: A Substudy of a Randomized Trial" Journal of Clinical Medicine 12, no. 9: 3314. https://doi.org/10.3390/jcm12093314
APA StyleMüller-Wirtz, L. M., Becher, T., Günther, U., Bellgardt, M., Sackey, P., Volk, T., & Meiser, A. (2023). Ventilatory Effects of Isoflurane Sedation via the Sedaconda ACD-S versus ACD-L: A Substudy of a Randomized Trial. Journal of Clinical Medicine, 12(9), 3314. https://doi.org/10.3390/jcm12093314








