Long-Term Safety and Efficacy of Pars Plana Vitrectomy for Uveitis: Experience of a Tertiary Referral Centre in the United Kingdom
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Data Collection
2.2. Surgical Procedure
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hung, J.H.; Rao, N.A.; Chiu, W.C.; Sheu, S.J. Vitreoretinal surgery in the management of infectious and non-infectious uveitis—A narrative review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; Eggenschwiler, L.; Stephenson, A.; Montieth, A.; Nakhoul, N.; Araùjo-Miranda, R.; Foster, C.S. The Challenge of Pediatric Uveitis: Tertiary Referral Center Experience in the United States. Ocul. Immunol. Inflamm. 2019, 27, 410–417. [Google Scholar] [CrossRef]
- Tomkins-Netzer, O.; Talat, L.; Bar, A.; Lula, A.; Taylor, S.R.; Joshi, L.; Lightman, S. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology 2014, 121, 2387–2392. [Google Scholar] [CrossRef]
- Miserocchi, E.; Fogliato, G.; Modorati, G.; Bandello, F. Review on the worldwide epidemiology of uveitis. Eur. J. Ophthalmol. 2013, 23, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Jeroudi, A.; Yeh, S. Diagnostic vitrectomy for infectious uveitis. Int. Ophthalmol. Clin. 2014, 54, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Tanawade, R.G.; Tsierkezou, L.; Bindra, M.S.; Patton, N.A.; Jones, N.P. Visual outcomes of pars plana vitrectomy with epiretinal membrane peel in patients with uveitis. Retina 2015, 35, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, I.-E.; Ivanova, T.; Moussa, G.; Ferrara, M.; Patton, N.; Dhawahir-Scala, F.; Ch’ng, S.W.; Mitra, A.; Tyagi, A.K.; Lett, K.S.; et al. Functional and Anatomical Outcomes of Pars Plana Vitrectomy for Epiretinal Membrane in Patients with Uveitis. Diagnostics 2022, 12, 3044. [Google Scholar] [CrossRef]
- Becker, M.; Davis, J. Vitrectomy in the treatment of uveitis. Am. J. Ophthalmol. 2005, 140, 1096–1105. [Google Scholar] [CrossRef]
- Shin, Y.U.; Shin, J.Y.; Ma, D.J.; Cho, H.; Yu, H.G. Preoperative Inflammatory Control and Surgical Outcome of Vitrectomy in Intermediate Uveitis. J. Ophthalmol. 2017, 2017, 5946240. [Google Scholar] [CrossRef]
- Vithalani, N.M.; Basu, S. Therapeutic Vitrectomy in the Management of Uveitis: Opportunities and Challenges. Semin. Ophthalmol. 2022, 37, 820–829. [Google Scholar] [CrossRef]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [PubMed]
- Nussenblatt, R.B.; Palestine, A.G.; Chan, C.C.; Roberge, F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 1985, 92, 467–471. [Google Scholar] [CrossRef]
- Branson, S.V.; McClafferty, B.R.; Kurup, S.K. Vitrectomy for Epiretinal Membranes and Macular Holes in Uveitis Patients. J. Ocul. Pharmacol. Ther. 2017, 33, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Heiligenhaus, A.; Bornfeld, N.; Wessing, A. Long-term results of pars plana vitrectomy in the management of intermediate uveitis. Curr. Opin. Ophthalmol. 1996, 7, 77–79. [Google Scholar] [CrossRef]
- Scott, R.A.; Haynes, R.J.; Orr, G.M.; Cooling, R.J.; Pavésio, C.E.; Charteris, D.G. Vitreous surgery in the management of chronic endogenous posterior uveitis. Eye 2003, 17, 221–227. [Google Scholar] [CrossRef]
- Eckardt, C.; Bacskulin, A. Vitrectomy in intermediate uveitis. Dev. Ophthalmol. 1992, 23, 232–238. [Google Scholar] [PubMed]
- Dev, S.; Mieler, W.F.; Pulido, J.S.; Mittra, R.A. Visual outcomes after pars plana vitrectomy for epiretinal membranes associated with pars planitis. Ophthalmology 1999, 106, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Giuliari, G.P.; Chang, P.Y.; Thakuria, P.; Hinkle, D.M.; Foster, C.S. Pars plana vitrectomy in the management of paediatric uveitis: The Massachusetts Eye Research and Surgery Institution experience. Eye 2010, 24, 7–13. [Google Scholar] [CrossRef]
- Verbraeken, H. Therapeutic pars plana vitrectomy for chronic uveitis: A retrospective study of the long-term results. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234, 288–293. [Google Scholar] [CrossRef]
- Takayama, K.; Tanaka, A.; Ishikawa, S.; Mochizuki, M.; Takeuchi, M. Comparison between Outcomes of Vitrectomy in Granulomatous and Nongranulomatous Uveitis. Ophthalmologica 2016, 235, 18–25. [Google Scholar] [CrossRef]
- Svozilkova, P.; Heissigerova, J.; Brichova, M.; Kalvodova, B.; Dvorak, J.; Rihova, E. The role of pars plana vitrectomy in the diagnosis and treatment of uveitis. Eur. J. Ophthalmol. 2011, 21, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Quinones, K.; Choi, J.Y.; Yilmaz, T.; Kafkala, C.; Letko, E.; Foster, C.S. Pars plana vitrectomy versus immunomodulatory therapy for intermediate uveitis: A prospective, randomized pilot study. Ocul. Immunol. Inflamm. 2010, 18, 411–417. [Google Scholar] [CrossRef]
- Romano, M.R.; Stocchino, A.; Ferrara, M.; Lagazzo, A.; Repetto, R. Fluidics of Single and Double Blade Guillotine Vitrectomy Probes in Balanced Salt Solution and Artificial Vitreous. Transl. Vis. Sci. Technol. 2018, 7, 19. [Google Scholar] [CrossRef]
- Bansal, R.; Dogra, M.; Chawla, R.; Kumar, A. Pars plana vitrectomy in uveitis in the era of microincision vitreous surgery. Indian J. Ophthalmol. 2020, 68, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Apple, D.; Hu, J.; Tewari, A. Advancements of vitreoretinal surgical machines. Curr. Opin. Ophthalmol. 2017, 28, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Fardeau, C.; Champion, E.; Massamba, N.; LeHoang, P. Uveitic macular edema. Eye 2016, 30, 1277–1292. [Google Scholar] [CrossRef]
- Lardenoye, C.W.T.A.; van Kooij, B.; Rothova, A. Impact of macular edema on visual acuity in uveitis. Ophthalmology 2006, 113, 1446–1449. [Google Scholar] [CrossRef]
- Accorinti, M.; Okada, A.A.; Smith, J.R.; Gilardi, M. Epidemiology of Macular Edema in Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 169–180. [Google Scholar] [CrossRef]
- Rothova, A.; Suttorp-van Schulten, M.S.; Frits Treffers, W.; Kijlstra, A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br. J. Ophthalmol. 1996, 80, 332–336. [Google Scholar] [CrossRef]
- Algvere, P.; Alanko, H.; Dickhoff, K.; Lähde, Y.; Saari, K.M. Pars plana vitrectomy in the management of intraocular inflammation. Acta Ophthalmol. 1981, 59, 727–736. [Google Scholar] [CrossRef]
- Radetzky, S.; Walter, P.; Fauser, S.; Koizumi, K.; Kirchhof, B.; Joussen, A.M. Visual outcome of patients with macular edema after pars plana vitrectomy and indocyanine green-assisted peeling of the internal limiting membrane. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 273–278. [Google Scholar] [CrossRef]
- Cho, M.; D’Amico, D.J. Transconjunctival 25-gauge pars plana vitrectomy and internal limiting membrane peeling for chronic macular edema. Clin. Ophthalmol. 2012, 6, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Schaal, S.; Tezel, T.H.; Kaplan, H.J. Surgical intervention in refractory CME--role of posterior hyaloid separation and internal limiting membrane peeling. Ocul. Immunol. Inflamm. 2008, 16, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.R.; Allegrini, D.; Della Guardia, C.; Schiemer, S.; Baronissi, I.; Ferrara, M.; Cennamo, G. Vitreous and intraretinal macular changes in diabetic macular edema with and without tractional components. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1–8. [Google Scholar] [CrossRef]
- Dugel, P.U.; Rao, N.A.; Ozler, S.; Liggett, P.E.; Smith, R.E. Pars plana vitrectomy for intraocular inflammation-related cystoid macular edema unresponsive to corticosteroids. A preliminary study. Ophthalmology 1992, 99, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Kiryu, J.; Kita, M.; Tanabe, T.; Yamashiro, K.; Miyamoto, N.; Ieki, Y. Pars plana vitrectomy for cystoid macular edema secondary to sarcoid uveitis. Ophthalmology 2001, 108, 1140–1144. [Google Scholar] [CrossRef]
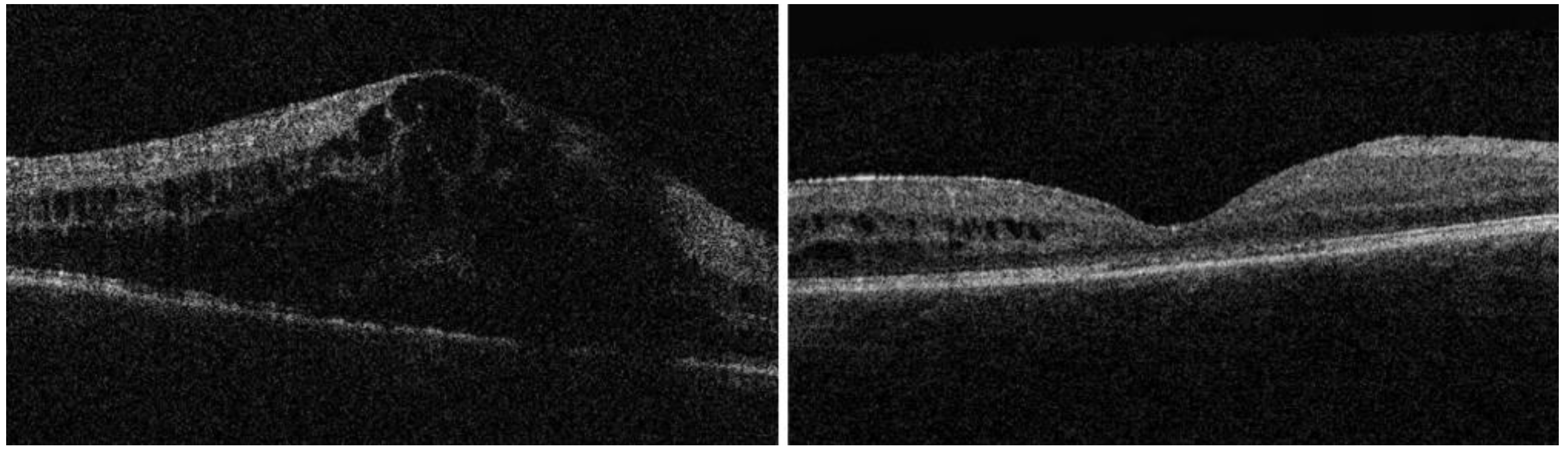
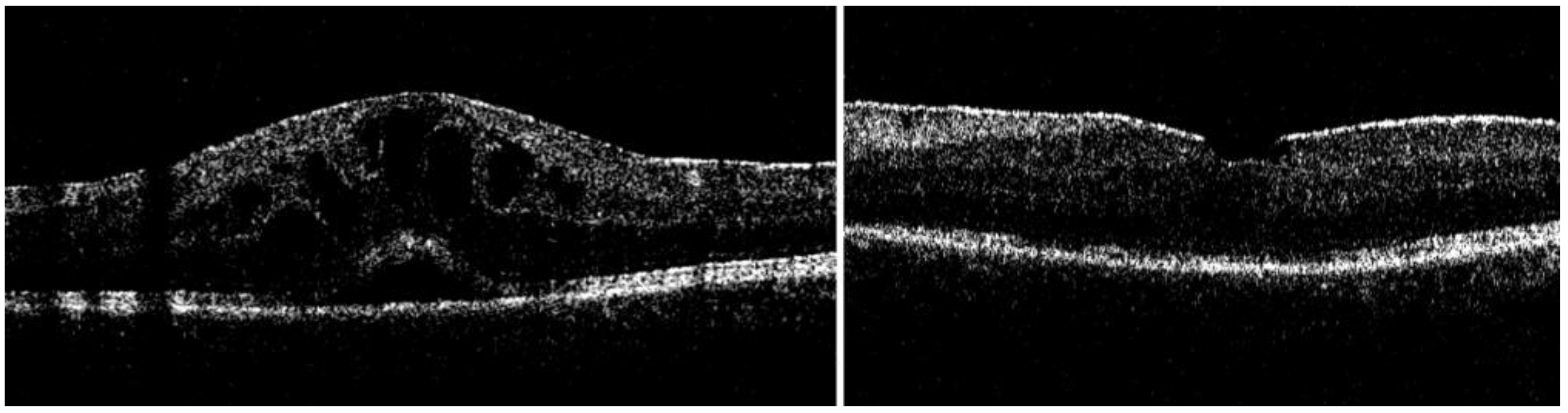
| Baseline Characteristic | |
|---|---|
| Age (years) mean (SD) | 45.8 (18.7) |
| Sex, male n (%) | 16 (59.3) |
| Lens status—n (%) | Phakic without cataract—11 (40.8) Phakic with cataract—8 (29.6) Pseudophakic—8 (29.6) |
| Uveitis etiology—n (%) | Idiopathic—4 (14.8) Sarcoidosis—9 (33) Toxoplasma—5 (18.5) Tuberculosis—1 (3.7) Fuchs Uveitis Syndrome—6 (22.2) Varicella-zoster virus—primary/active—1 (3.7) Toxocara—1 (3.7) |
| SUN classification—n (%) | Intermediate—9 (33) Posterior—9 (33) Panuveitis—9 (33) |
| Primary indication for vitrectomy—n (%) | Clearance of inflammatory debris—20 (74.1) Clearance of Hemorrhagic debris—3 (11.1) Cytological sampling—4 (14.8) |
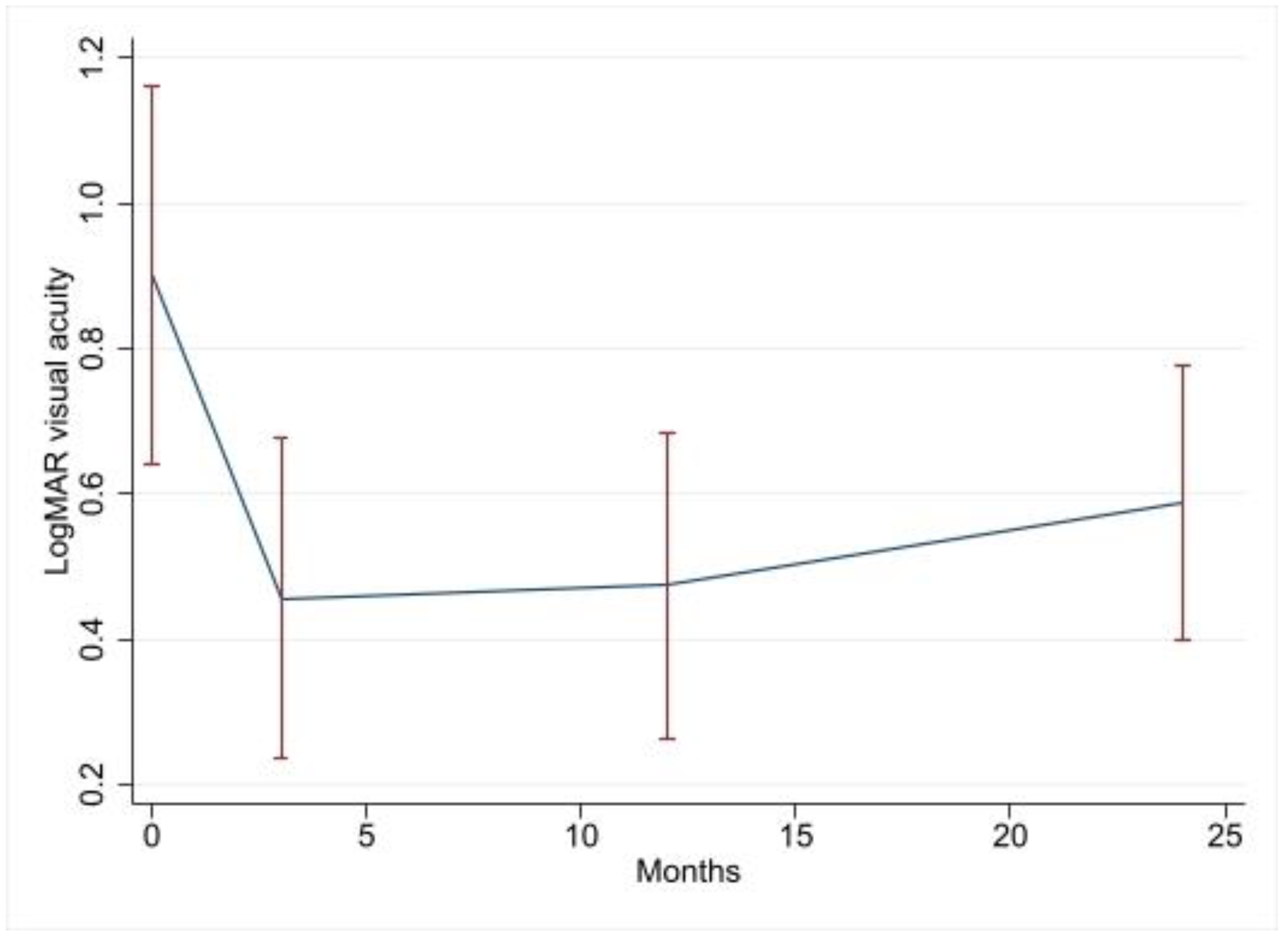
| Variable | Change in LogMAR Visual Acuity Compared to Baseline | 95% CI | p Value |
|---|---|---|---|
| Univariate | |||
| 3-month visit | −0.45 | −0.76–−0.13 | 0.006 |
| 12-month visit | −0.43 | −0.75–−0.11 | 0.008 |
| 24-month visit | −0.36 | −0.69–−0.04 | 0.027 |
| Age (Decade) | 0.07 | 0.001–0.013 | 0.027 |
| CME | 0.41 | 0.15–0.68 | 0.002 |
| Posterior compared to Intermediate | 0.56 | 0.29–0.84 | <0.001 |
| Panuveitis compared to Intermediate | 0.15 | −0.13–0.42 | 0.29 |
| Baseline Pseudophakic compared to phakic | −0.23 | −0.51–0.04 | 0.098 |
| Combined phacovitrectomy | 0.32 | 0.07–0.58 | 0.014 |
| Multivariate | |||
| 3-month visit | −0.46 | −0.74–−0.18 | 0.002 |
| 12-month visit | −0.39 | −0.67–−0.11 | 0.007 |
| 24-month visist | −0.30 | −0.59–−0.013 | 0.041 |
| Age (Decade) | 0.04 | −0.002–0.011 | 0.17 |
| CME | 0.36 | 0.11–0.62 | 0.005 |
| Posterior compared to Intermediate | 0.35 | 0.08–0.61 | 0.01 |
| Panuveitis compared to Intermediate | 0.21 | −0.04–0.45 | 0.097 |
| Baseline pseudophakic compared to phakic * | −0.21 | −0.45–0.04 | 0.093 |
| Combined phacovitrectomy * | 0.16 | −0.09–0.40 | 0.21 |
| Therapeutic Regimen | Baseline Eyes, n (%) | 3 m FU Eyes, n (%) | 12 m FU Eyes, n (%) | 24 m FU Eyes, n (%) |
|---|---|---|---|---|
| None | 7 (25.9) | 2 (7.4) | 5 (18.5) | 6 (22.2) |
| TS | 8 (29.6) | 19 (70.4) | 15 (55.6) | 15 (55.6) |
| OS | 2 (7.4) | 0 (0) | 0 (0) | 0 (0) |
| TS + OS | 7 (25.9) | 4 (14.8) | 5 (18.5) | 2 (7.4) |
| TS + DMARD | 0 | 1 (3.7) | 1 (3.7) | 1 (3.7) |
| TS + OS + DMARD | 3 (11.1) | 1 (3.7) | 1 (3.7) | 2 (7.4) |
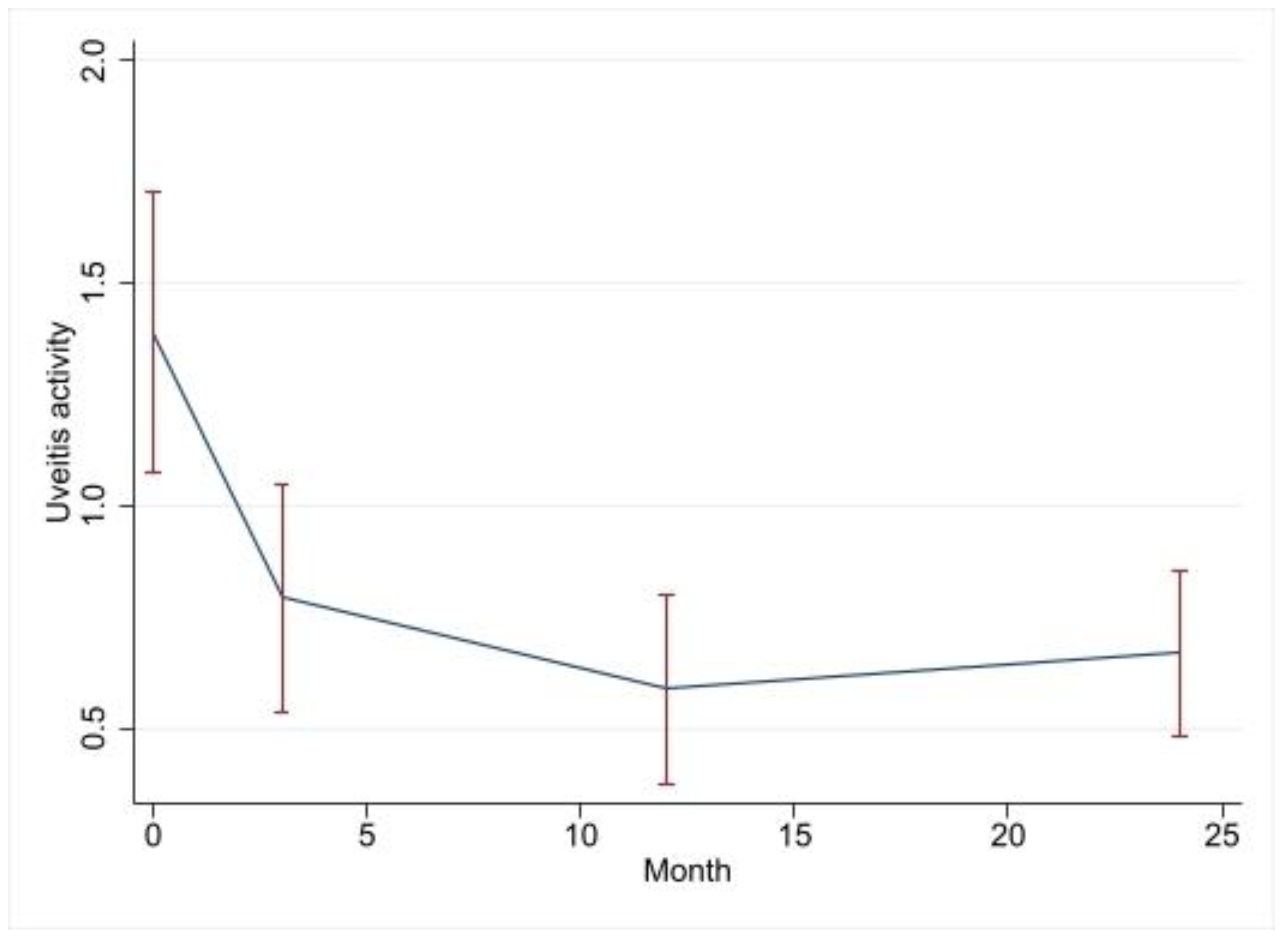
| Variable | Change in Uveitis Activity Compared to that of the Baseline | 95% CI | p Value |
|---|---|---|---|
| 3-month visit | −2.14 | −3.3–−1.00 | <0.001 |
| 12-month visit | −2.50 | −3.66–−1.33 | <0.001 |
| 24-month visit | −2.34 | −3.5–−1.16 | <0.001 |
| Age | 0.005 | −0.02–−0.03 | 0.625 |
| Posterior compared to Intermediate | −0.11 | −1.14–0.92 | 0.834 |
| Panuveitis compared to Intermediate | 1.53 | 0.56–2.51 | 0.002 |
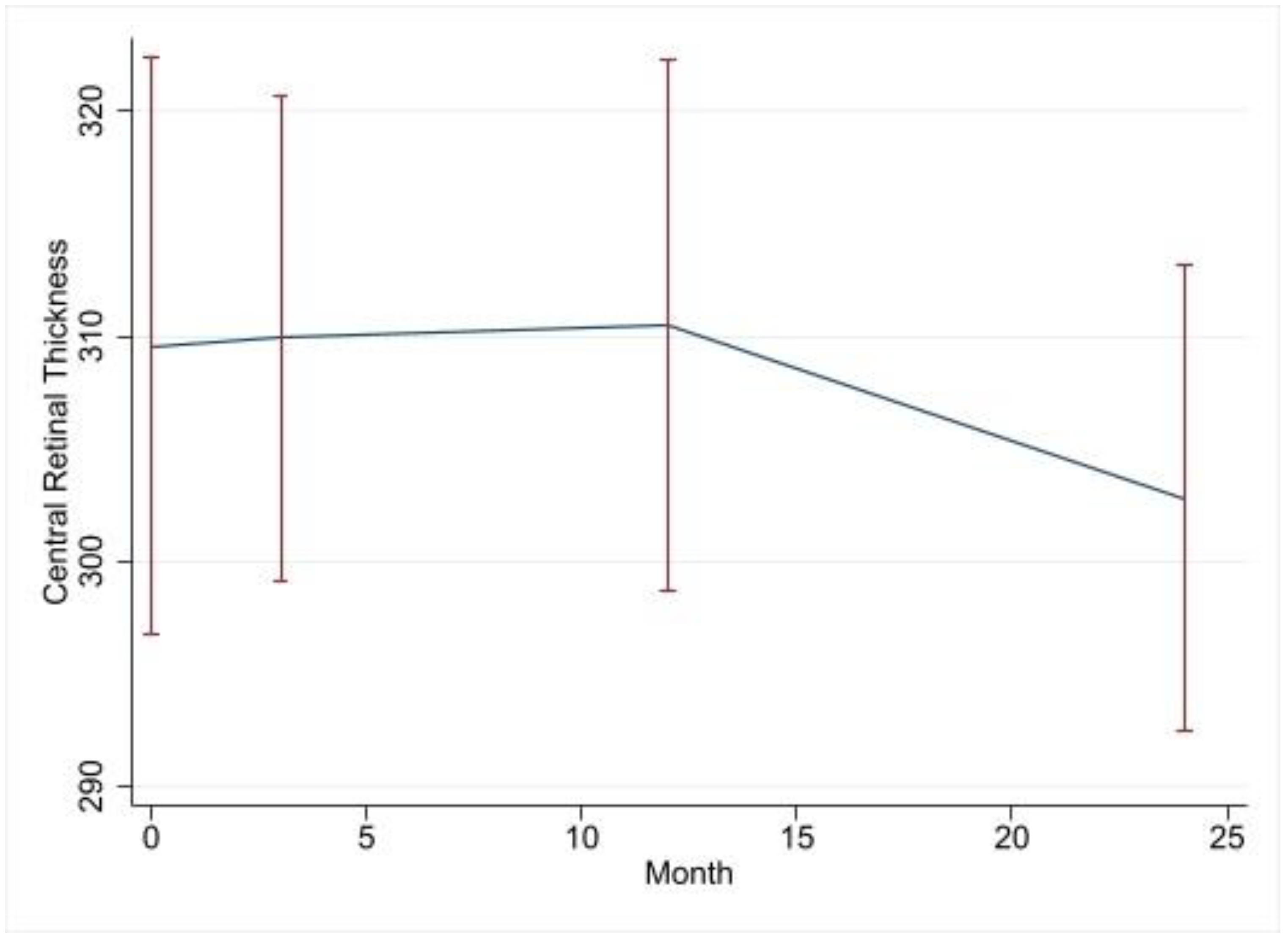
| Variable | Change in Central Retinal Thickness Compared to that of the Baseline | 95% CI | p Value |
|---|---|---|---|
| 3-month visit | −7.8 | −71.2–55.6 | 0.809 |
| 12-month visit | −34.4 | −101.5–32.6 | 0.312 |
| 24-month visit | −56.9 | −119.7–5.9 | 0.076 |
| Age | −0.15 | −1.7–−1.4 | 0.847 |
| CME | 78.5 | 26.0–131.0 | 0.003 |
| Variable | Odds Ratio of CME Compared to Baseline | 95% CI | p Value |
|---|---|---|---|
| 3-month visit | 0.66 | 0.2–2.9 | 0.576 |
| 12-month visit | 0.51 | 0.1–2.5 | 0.404 |
| 24-month visit | 0.50 | 0.98–2.5 | 0.394 |
| Age | 0.99 | 0.96–1.03 | 0.834 |
| CME | 1.03 | 0.07–3.06 | 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Faouri, M.; Ally, N.; Lippera, M.; Subramani, S.; Moussa, G.; Ivanova, T.; Patton, N.; Dhawahir-Scala, F.; Rocha-de-Lossada, C.; Ferrara, M.; et al. Long-Term Safety and Efficacy of Pars Plana Vitrectomy for Uveitis: Experience of a Tertiary Referral Centre in the United Kingdom. J. Clin. Med. 2023, 12, 3252. https://doi.org/10.3390/jcm12093252
El Faouri M, Ally N, Lippera M, Subramani S, Moussa G, Ivanova T, Patton N, Dhawahir-Scala F, Rocha-de-Lossada C, Ferrara M, et al. Long-Term Safety and Efficacy of Pars Plana Vitrectomy for Uveitis: Experience of a Tertiary Referral Centre in the United Kingdom. Journal of Clinical Medicine. 2023; 12(9):3252. https://doi.org/10.3390/jcm12093252
Chicago/Turabian StyleEl Faouri, Muhannd, Naseer Ally, Myrta Lippera, Siddharth Subramani, George Moussa, Tsveta Ivanova, Niall Patton, Felipe Dhawahir-Scala, Carlos Rocha-de-Lossada, Mariantonia Ferrara, and et al. 2023. "Long-Term Safety and Efficacy of Pars Plana Vitrectomy for Uveitis: Experience of a Tertiary Referral Centre in the United Kingdom" Journal of Clinical Medicine 12, no. 9: 3252. https://doi.org/10.3390/jcm12093252
APA StyleEl Faouri, M., Ally, N., Lippera, M., Subramani, S., Moussa, G., Ivanova, T., Patton, N., Dhawahir-Scala, F., Rocha-de-Lossada, C., Ferrara, M., & Jalil, A. (2023). Long-Term Safety and Efficacy of Pars Plana Vitrectomy for Uveitis: Experience of a Tertiary Referral Centre in the United Kingdom. Journal of Clinical Medicine, 12(9), 3252. https://doi.org/10.3390/jcm12093252






