Congenital Ventricular Diverticulum
Abstract
1. Introduction
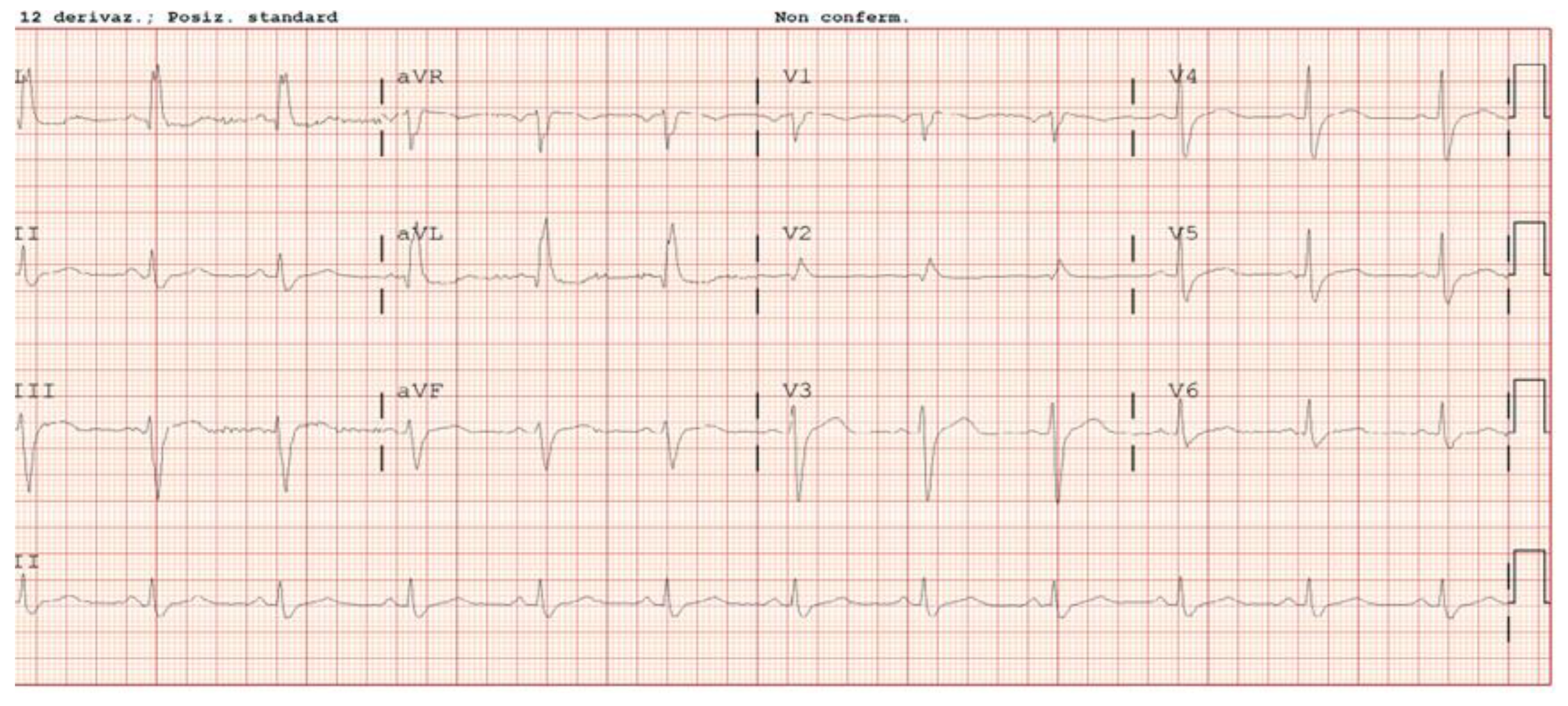
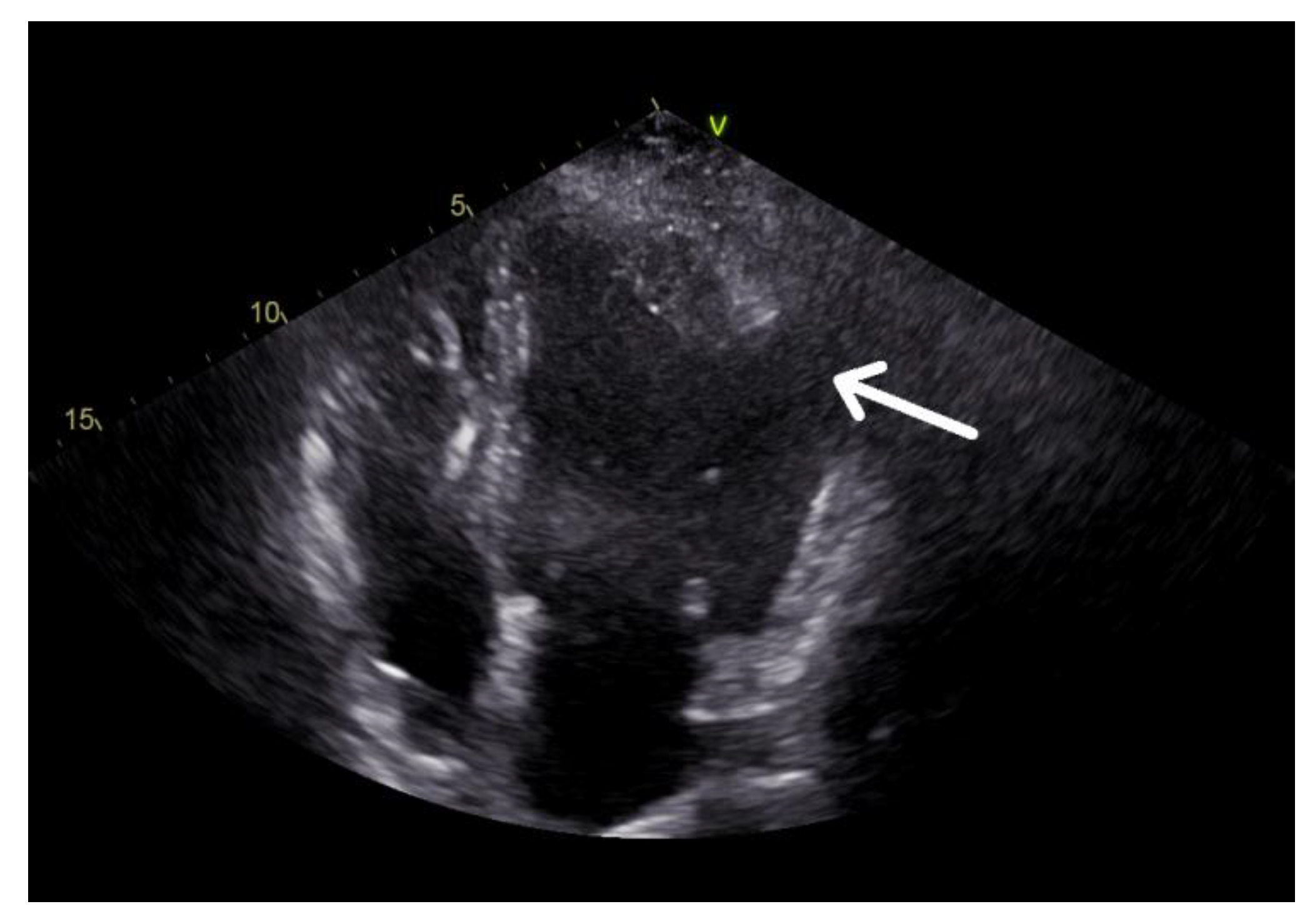
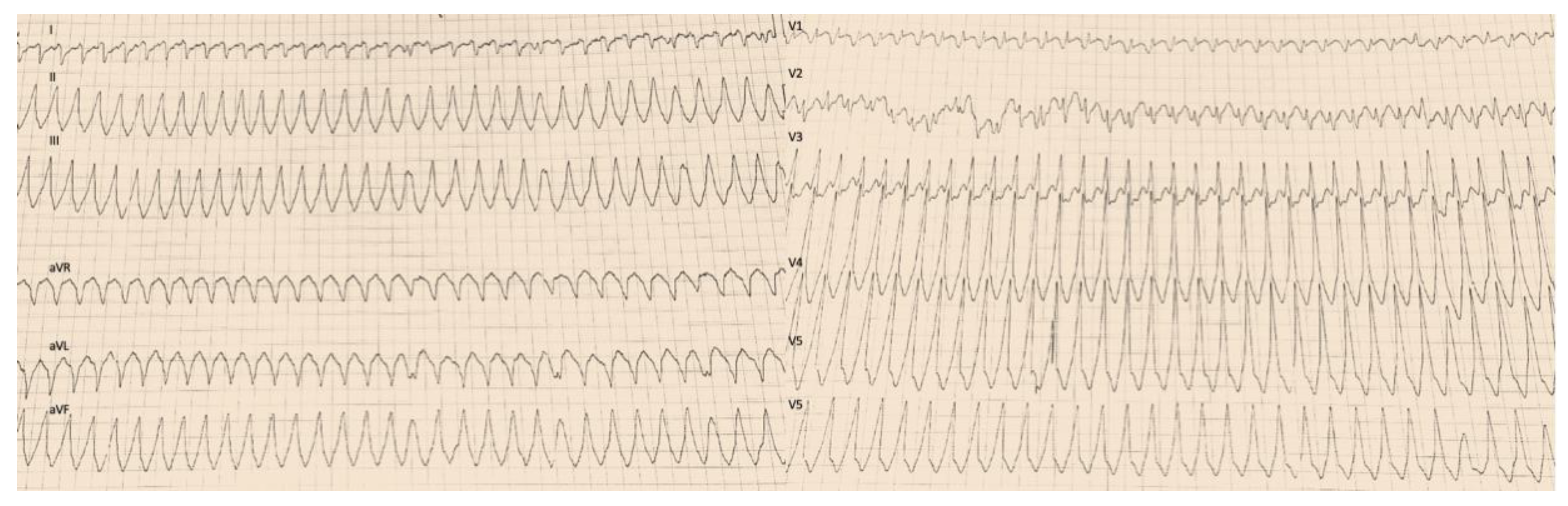
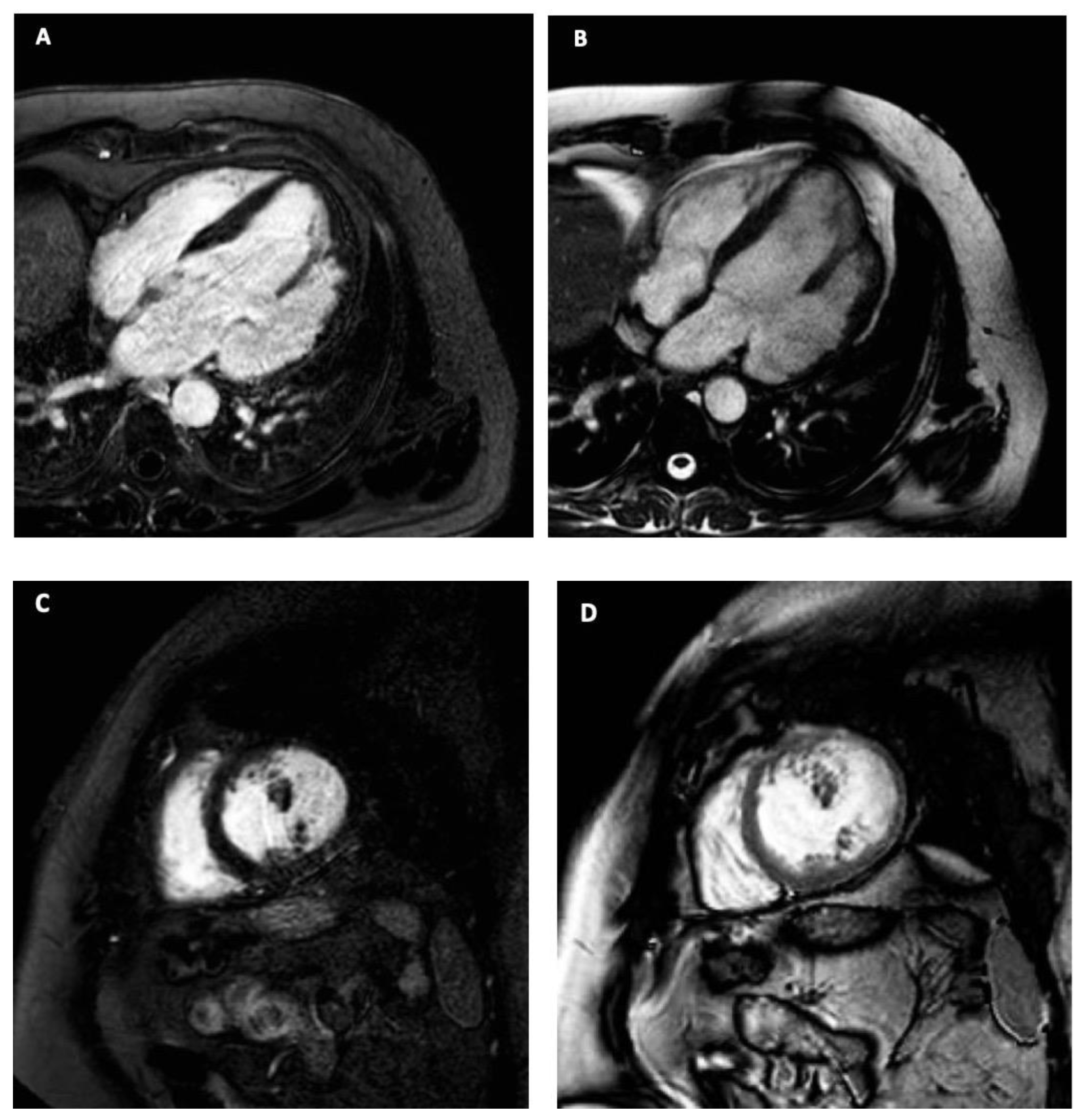
2. Case Description
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Shauq, A.; Agarwal, V.; Crawley, C. Congenital left ventricular diverticulum. Heart Lung Circ. 2006, 15, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Ohlow, M.A. Congenital left ventricular aneurysms and diverticula: Definition, pathophysiology, clinical relevance and treatment. Cardiology 2006, 106, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, J.R.; Haller, J.A.; Ravitch, M. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg. Gynecol. Obstet. 1958, 107, 602–614. [Google Scholar] [PubMed]
- Li, Q.; Qu, H.; Wang, H.; Wang, D.; Li, P.; Liu, T. Ventricular diverticulum: A review of the literature. J. Card. Surg. 2013, 28, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Halbertsma, F.J.; van Oort, A.; van der Staak, F. Cardiac diverticulum and omphalocele: Cantrell’s pentalogy or syndrome. Cardiol. Young 2002, 12, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Mărginean, C.; Mărginean, C.O.; Gozar, L.; Meliţ, L.E.; Suciu, H.; Gozar, H.; Crişan, A.; Cucerea, M. Cantrell Syndrome-A Rare Complex Congenital Anomaly: A Case Report and Literature Review. Front.Pediatr. 2018, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Halpern, L.; Garabedian, C.; Worrall, N.K. Congenital Ventricular Diverticulum or Aneurysm: A Difficult Diagnosis to Make. Case Rep. Cardiol. 2018, 2018, 5839432. [Google Scholar] [CrossRef] [PubMed]
- Marijon, E.; Ou, P.; Fermont, L.; Concordet, S.; Le Bidois, J.; Sidi, D.; Bonnet, D. Diagnosis and outcome in congenital ventricular diverticulum and aneurysm. J. Thorac. Cardiovasc. Surg. 2006, 131, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Gianfaldoni, M.L.; Aquaro, G.D.; Petix, N.R.; Zipoli, A. Left ventricular accessory chamber in an asymptomatic patient. G Ital. Cardiol. 2011, 12, 503–504. [Google Scholar]
- Awad, S.M.; Patel, A.S.; Polimenakos, A.; Braun, R.; Abdulla, R.I. Left ventricular accessory chamber: A case report and review of the literature. Pediatr. Cardiol. 2009, 30, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Gerlis, L.M.; Partridge, J.B.; Fiddler, G.I.; Williams, G.; Scott, O. Two chambered left ventricle. Three new varieties. Br. Heart J. 1981, 46, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Kay, P.H.; Rigby, M.; Mulholland, H.C. Congenital double chambered left ventricle treated by exclusion of accessory chamber. Br. Heart J. 1983, 49, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, S. Overview of left ventricular outpouchings on cardiac magnetic resonance imaging. Cardiovasc. Diagn. Ther. 2015, 5, 464–470. [Google Scholar]
- Yang, C.H.; Wu VC, C.; Hung, K.C.; Lin, F.C. Early Detection of Left Ventricular Diverticulum by Transthoracic Echocardiography. J. Med. Ultrasound 2017, 25, 232–234. [Google Scholar] [CrossRef]
- Cianciulli, T.F.; del Carmen Gonzales Colaso, P.; Saccheri, M.C.; Lax, J.A.; Redruello, H.J.; Guerra, J.E.; Precioso, H.A.; Vidal, L.A. Left ventricular diverticulum, a rare echocardiographic finding: Two adult patients and review of the literature. Cardiol. J. 2009, 16, 76–81. [Google Scholar] [PubMed]
- Hamaoka, K.; Onaka, M.; Tanaka, T.; Onouchi, Z. Congenital ventricular aneurysm and diverticulum in children. Pediatr. Cardiol. 1987, 8, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Mardini, M.K. Congenital diverticulum of the left ventricle. Report of two unusual cases. Br. Heart J. 1984, 51, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wang, R.; Ye, W.; Ren, C. Surgical treatment of congenital left ventricular diverticulum. Surgical treatment of congenital left ventricular diverticulum. J. Thorac. Dis. 2021, 13, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Makkuni, P.; Kotler, M.N.; Figueredo, V.M. Diverticular and Aneurysmal Structures of the Left Ventricle in Adults. Tex. Heart Inst. J. 2010, 37, 699–705. [Google Scholar] [PubMed]
| Left Ventricle | |
|---|---|
| EDV | 221 mL |
| EDVI | 122 mL/m2 |
| ESV | 139 mL |
| ESVI | 77 mL/m2 |
| LVEF | 37% |
| SV | 82 mL |
| SVI | 42 mL/m2 |
| CO | 6.31 L/min |
| CI | 3.5 L/m2/min |
| EDLVST | 10 mm |
| EDLWT | 10 mm |
| LVM | 108 g |
| LVMI | 60 g/m2 |
| Right Ventricle | |
| EDV | 152 mL |
| EDVI | 84 mL/m2 |
| ESV | 65 mL |
| ESVI | 36 mL/m2 |
| LVEF | 57% |
| SV | 87 mL |
| SVI | 48 mL/m2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, C.M.; Lucà, F.; Franzutti, C.; Scappatura, G.; Arcadi, N.; Fratto, P.; Benedetto, F.A.; Gelsomino, S. Congenital Ventricular Diverticulum. J. Clin. Med. 2023, 12, 3153. https://doi.org/10.3390/jcm12093153
Rao CM, Lucà F, Franzutti C, Scappatura G, Arcadi N, Fratto P, Benedetto FA, Gelsomino S. Congenital Ventricular Diverticulum. Journal of Clinical Medicine. 2023; 12(9):3153. https://doi.org/10.3390/jcm12093153
Chicago/Turabian StyleRao, Carmelo Massimiliano, Fabiana Lucà, Claudio Franzutti, Giuseppe Scappatura, Nicola Arcadi, Pasquale Fratto, Francesco Antonio Benedetto, and Sandro Gelsomino. 2023. "Congenital Ventricular Diverticulum" Journal of Clinical Medicine 12, no. 9: 3153. https://doi.org/10.3390/jcm12093153
APA StyleRao, C. M., Lucà, F., Franzutti, C., Scappatura, G., Arcadi, N., Fratto, P., Benedetto, F. A., & Gelsomino, S. (2023). Congenital Ventricular Diverticulum. Journal of Clinical Medicine, 12(9), 3153. https://doi.org/10.3390/jcm12093153






