Electrical Stimulation Exercise for People with Spinal Cord Injury: A Healthcare Provider Perspective
Abstract
1. Introduction
2. Body Composition Assessment
3. Cardiovascular and Metabolism
4. Muscle Spasticity
5. Exercise Adherence
6. Physical Function
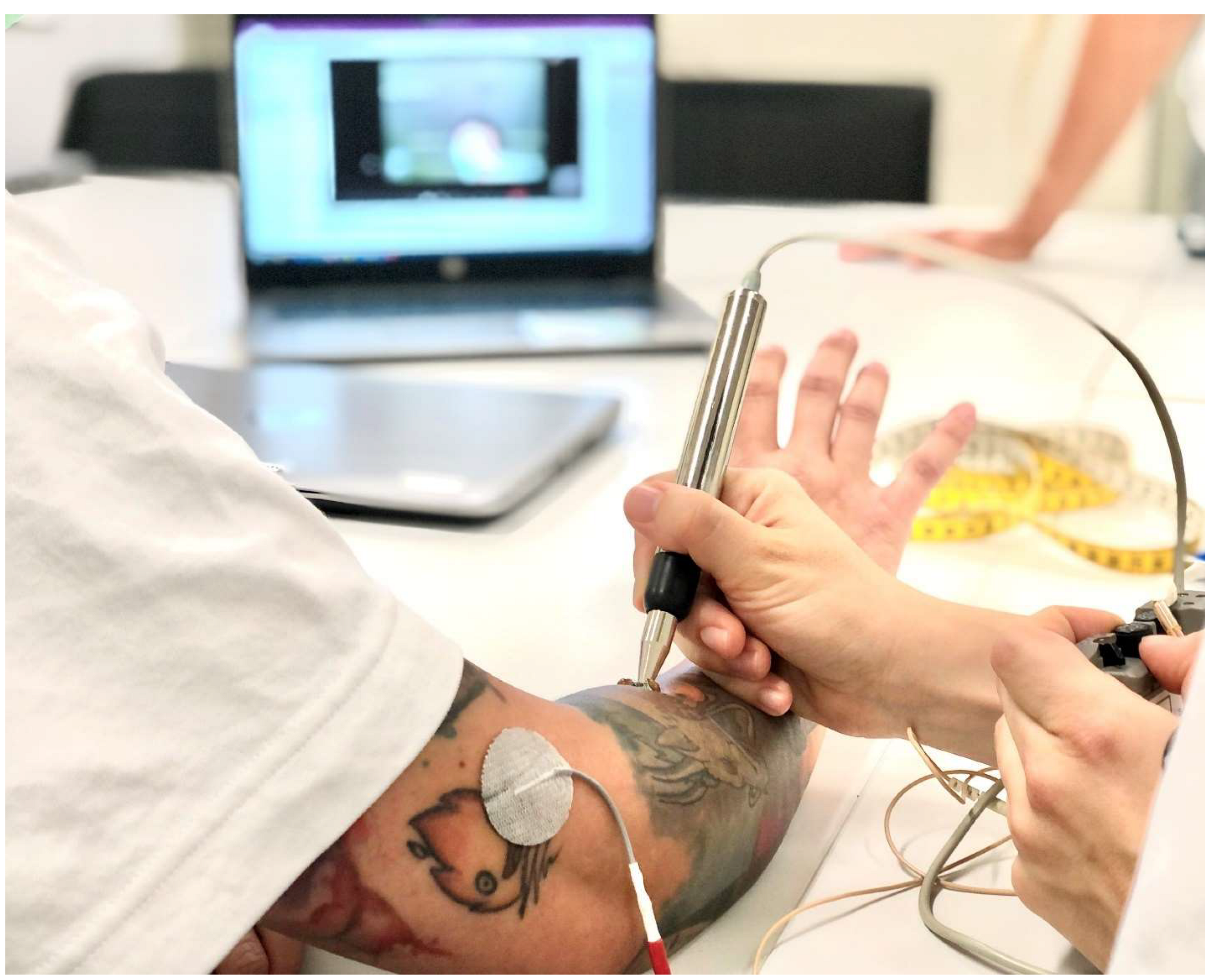
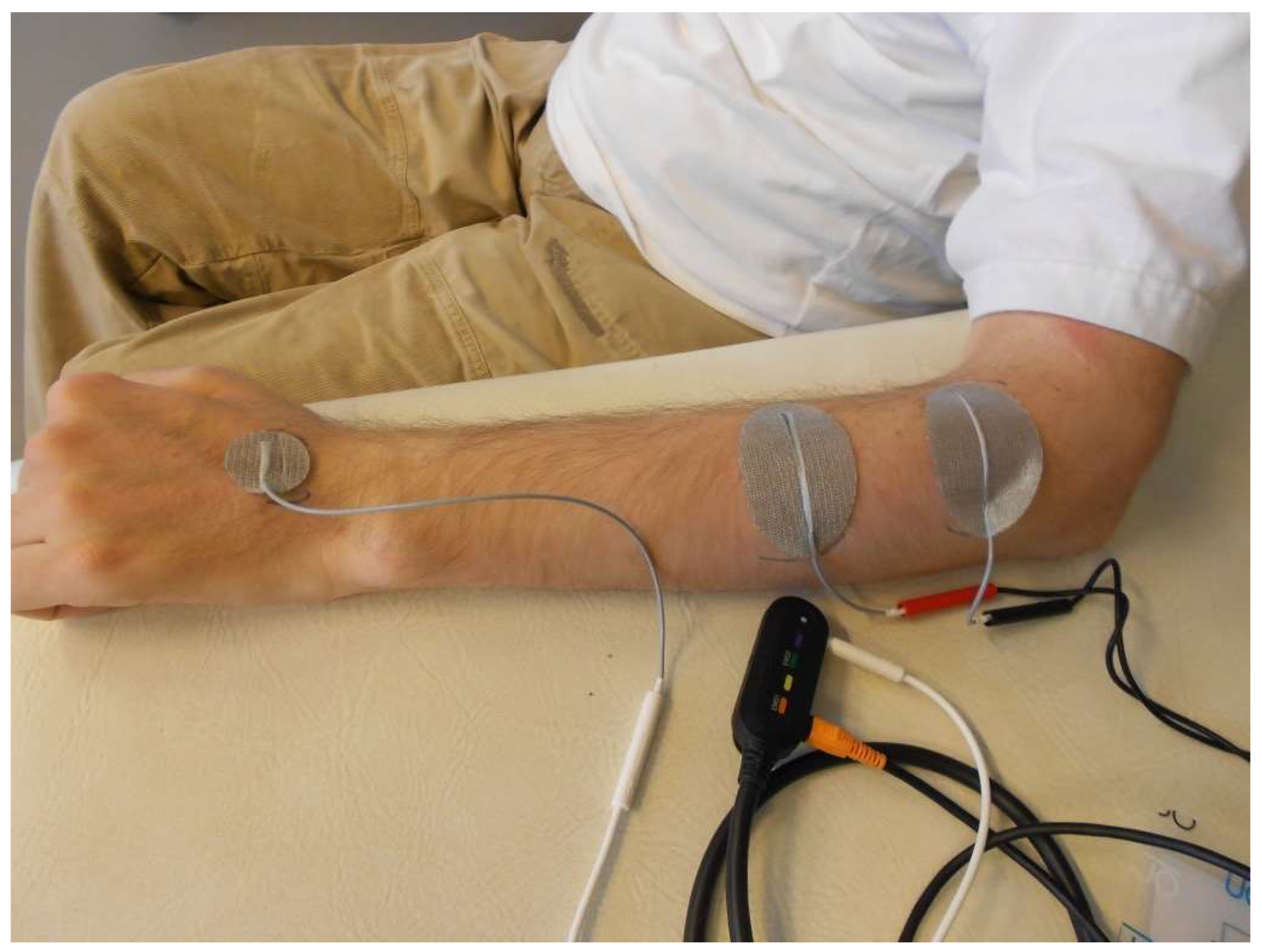
7. Diagnosis and Treatment of Hand Function in Tetraplegia
8. Summary and Conclusions
Funding
Conflicts of Interest
References
- Karamian, B.A.; Siegel, N.; Nourie, B.; Serruya, M.D.; Heary, R.F.; Harrop, J.S.; Vaccaro, A.R. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J. Orthop. Traumatol. 2022, 23, 2. [Google Scholar] [CrossRef]
- Gater, D.R.; Dolbow, D.; Tsui, B.; Gorgey, A.S. Functional Electrical Stimulation Therapies after Spinal Cord Injury. NeuroRehabilitation 2011, 28, 231–248. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Gorgey, A.S.; Sutor, T.W.; Bochkezanian, V.; Musselman, K. Invasive and Non-Invasive Approaches of Electrical Stimulation to Improve Physical Functioning after Spinal Cord Injury. J. Clin. Med. 2021, 10, 5356. [Google Scholar] [CrossRef] [PubMed]
- Duffell, L.D.; Donaldson, N.N. A Comparison of FES and SCS for Neuroplastic Recovery after SCI: Historical Perspectives and Future Directions. Front. Neurol. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Khalil, R.E.; Lester, R.M.; Dudley, G.A.; Gater, D.R. Paradigms of Lower Extremity Electrical Stimulation Training after Spinal Cord Injury. J. Vis. Exp. 2018, 1, 57000. [Google Scholar] [CrossRef]
- Johnston, T.E.; Marino, R.J.; Oleson, C.V.; Schmidt-Read, M.; Leiby, B.E.; Sendecki, J.; Singh, H.; Modlesky, C.M. Musculoskeletal Effects of 2 Functional Electrical Stimulation Cycling Paradigms Conducted at Different Cadences for People with Spinal Cord Injury: A Pilot Study. Arch Phys. Med. Rehabil. 2016, 97, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Hettinga, D.M.; Andrews, B.J. Oxygen consumption during functional electrical stimulation-assisted exercise in persons with spinal cord injury: Implications for fitness and health. Sports Med. 2008, 38, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Bersch, I.; Koch-Borner, S.; Fridén, J. Motor Point Topography of Fundamental Grip Actuators in Tetraplegia: Implications in Nerve Transfer Surgery. J. Neurotrauma 2020, 37, 441–447. [Google Scholar] [CrossRef]
- Gad, P.; Lee, S.; Terrafranca, N.; Zhong, H.; Turner, A.; Gerasimenko, Y.; Edgerton, V.R. Non-Invasive Activation of Cervical Spinal Networks after Severe Paralysis. J. Neurotrauma 2018, 35, 2145–2158. [Google Scholar] [CrossRef]
- Sutor, T.W.; Ghatas, M.P.; Goetz, L.L.; Lavis, T.D.; Gorgey, A.S. Exoskeleton Training and Trans-Spinal Stimulation for Physical Activity Enhancement after Spinal Cord Injury (EXTra-SCI): An Exploratory Study. Front. Rehabil. Sci. 2022, 2, 789422. [Google Scholar] [CrossRef]
- Hachmann, J.T.; Yousak, A.; Wallner, J.J.; Gad, P.N.; Edgerton, V.R.; Gorgey, A.S. Epidural spinal cord stimulation as an intervention for motor recovery after motor complete spinal cord injury. J. Neurophysiol. 2021, 126, 1843–1859. [Google Scholar] [CrossRef]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215. [Google Scholar]
- Bekhet, A.H.; Bochkezanian, V.; Saab, I.M.; Gorgey, A.S. The Effects of Electrical Stimulation Parameters in Managing Spasticity after Spinal Cord Injury: A Systematic Review. Am. J. Phys. Med. Rehabil. 2019, 98, 484–499. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Dolbow, D.R.; Dolbow, J.D.; Gater, D.R. The Effects of Electrical Stimulation on Body Composition and Metabolic Profile after Spinal Cord Injury—Part II. J. Spinal Cord Med. 2015, 38, 23–37. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Gorgey, A.S.; Ketchum, J.M.; Gater, D.R. Home-Based Functional Electrical Stimulation Cycling Enhances Quality of Life in Individuals with Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2013, 19, 324–329. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Sutor, T.W.; Goldsmith, J.A.; Cifu, D.X. Employment of Neuromuscular Electrical Stimulation to Examine Muscle and Bone Qualities after Spinal Cord Injury. J. Clin. Med. 2022, 11, 6681. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Lester, R.M.; Wade, R.C.; Khalil, R.E.; Khan, R.K.; Anderson, M.L.; Castillo, T. A feasibility pilot using telehealth videoconference monitoring of home-based NMES resistance training in persons with spinal cord injury. Spinal Cord Ser. Cases 2017, 3, 17039. [Google Scholar] [CrossRef]
- Martin, R.; Sadowsky, C.; Obst, K.; Meyer, B.; McDonald, J. Functional electrical stimulation in spinal cord injury: From theory to practice. Top. Spinal Cord Inj. Rehabil. 2012, 18, 28–33. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Farkas, G.J.; Berg, A.S.; Welsch, M.A.; Gorgey, A.S.; Gater, D.R. Fat to lean mass ratio in spinal cord injury: Possible interplay of components of body composition that may instigate systemic inflammation and metabolic syndrome. J. Spinal Cord Med. 2022, 45, 833–839. [Google Scholar] [CrossRef]
- Atkins, K.D.; Bickel, C.S. Effects of functional electrical stimulation on muscle health after spinal cord injury. Curr. Opin. Pharmacol. 2021, 60, 226–231. [Google Scholar] [CrossRef]
- Bekhet, A.H.; Jahan, A.M.; Bochkezanian, V.; Musselman, K.E.; Elsareih, A.A.; Gorgey, A.S. Effects of Electrical Stimulation Training on Body Composition Parameters after Spinal Cord Injury: A Systematic Review. Arch Phys. Med. Rehabil. 2022, 103, 1168–1178. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Credeur, D.; Lemacks, J.L.; Stokic, D.S.; Pattanaik, S.; Corbin, G.N.; Courtner, A.S. Electrically Induced Cycling and Nutritional Counseling for Counteracting Obesity after Spinal Cord Injury: A Pilot Study. J. Spinal Cord Med. 2020, 44, 533–540. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Mather, K.J.; Cupp, H.R.; Gater, D.R. Effects of Resistance Training on Adiposity and Metabolism after Spinal Cord Injury. Med. Sci. Sports Exerc. 2012, 44, 165–174. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Shepherd, C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: Case report. J. Spinal Cord Med. 2010, 33, 90–95. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Gill, R.; Gater, D.R.; Lavis, T.D.; Cardozo, C.P.; Adler, R.A. Low-Dose Testosterone and Evoked Resistance Exercise after Spinal Cord Injury on Cardio-Metabolic Risk Factors: An Open-Label Randomized Clinical Trial. J. Neurotrauma 2019, 36, 2631–2645. [Google Scholar] [CrossRef]
- Ye, G.; Grabke, E.P.; Pakosh, M.; Furlan, J.C.; Masani, K. Clinical Benefits and System Design of FES-Rowing Exercise for Rehabilitation of Individuals with Spinal Cord Injury: A Systematic Review. Arch. Phys. Med. Rehabil. 2021, 102, 1595–1605. [Google Scholar] [CrossRef]
- Kim, D.I.; Park, D.S.; Lee, B.S.; Jeon, J.Y. A six-week motor-driven functional electronic stimulation rowing program improves muscle strength and body composition in people with spinal cord injury: A pilot study. Spinal Cord 2014, 52, 621–624. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Hettinga, D.; Steadward, R.D.; Wheeler, G.D.; Bell, G.; Harber, V. Reduced plasma glucose and leptin after 12 weeks of functional electrical stimulation–rowing exercise training in spinal cord injury patients. Arch. Phys. Med. Rehabil. 2010, 91, 1957–1959. [Google Scholar] [CrossRef]
- Wilbanks, S.R.; Bickel, C.S. Scapular stabilization and muscle strength in manual wheelchair users with spinal cord injury and subacromial impingement. Top. Spinal Cord Inj. Rehabil. 2016, 22, 60–70. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Lai, R.E.; Khalil, R.E.; Rivers, J.; Cardozo, C.; Chen, Q.; Lesnefsky, E.J. Neuromuscular electrical stimulation resistance training enhances oxygen uptake and ventilatory efficiency independent of mitochondrial complexes after spinal cord injury: A randomized clinical trial. J. Appl. Physiol. 2021, 131, 265–276. [Google Scholar] [CrossRef]
- Wade, R.C.; Gorgey, A.S. Skeletal muscle conditioning may be an effective rehabilitation intervention preceding functional electrical stimulation cycling. Neural. Regen. Res. 2016, 11, 1232–1233. [Google Scholar] [CrossRef]
- Dolbow, J.D.; Dolbow, D.R.; Gorgey, A.S.; Adler, R.R.; Gater, D.R. The Effects of Aging and Electrical Stimulation Exercise on Bone after Spinal Cord Injury. Aging Dis. 2013, 4, 141–153. [Google Scholar]
- Sutor, T.W.; Kura, J.; Mattingly, A.J.; Otzel, D.M.; Yarrow, J.F. The Effects of Exercise and Activity-Based Physical Therapy on Bone after Spinal Cord Injury. Int. J. Mol. Sci. 2022, 23, 608. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Gorgey, A.S.; Daniels, J.A.; Adler, R.R.; Gater, D.R., Jr. The Effects of Spinal Cord Injury and Exercise on Bone Mass: A Literature Review. NeuroRehabilitation 2011, 29, 261–269. [Google Scholar] [CrossRef]
- Craven, B.C.; Wiest, M.J.; Cervinka, T.; Eng, J.J. Bone Health Following Spinal Cord Injury. In Spinal Cord Injury Rehabilitation Evidence; Version 7.0; Eng, J.J., Teasell, R.W., Miller, W.C., Wolfe, D.L., Townson, A.F., Hsieh, J.T.C., et al., Eds.; SCIRE: Vancouver, BC, Canada, 2020; pp. 1–126. Available online: https://scireproject.com/wp-content/uploads/2022/02/BONE-CHAPTER-FINAL-Nov.20-.20.pdf (accessed on 20 April 2023).
- van der Scheer, J.W.; Goosey-Tolfrey, V.L.; Valentino, S.E.; Davis, G.M.; Ho, C.H. Functional electrical stimulation cycling exercise after spinal cord injury: A systematic review of health and fitness-related outcomes. J. NeuroEngineering Rehabil. 2021, 18, 99. [Google Scholar] [CrossRef]
- Hamzaid, N.A.; Davis, G. Health and Fitness Benefits of Functional Electrical Stimulation-Evoked Leg Exercise for Spinal Cord–Injured Individuals: A Position Review. Top. Spinal Cord Inj. Rehabil. 2009, 14, 88–121. [Google Scholar] [CrossRef]
- Figoni, S.; Dolbow, D.R.; Crawford, C.; White, M.; Pattanaik, S. Does Aerobic Exercise Benefit Persons with Tetraplegia from Spinal Cord Injury? J. Spinal Cord Med. 2021, 44, 690–703. [Google Scholar] [CrossRef]
- Griffin, L.; Decker, M.J.; Hwang, J.Y.; Wang, B.; Kitchen, K.; Ding, Z.; Ivy, J.L. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J. Electromyogr. Kinesiol. 2009, 19, 614–622. [Google Scholar] [CrossRef]
- Farkas, G.J.; Burton, A.M.; McMillan, D.W.; Sneij, A.; Gater, D.R., Jr. The Diagnosis and Management of Cardiometabolic Risk and Cardiometabolic Syndrome after Spinal Cord Injury. J. Pers. Med. 2022, 12, 1088. [Google Scholar] [CrossRef]
- Farkas, G.J.; Gorgey, A.S.; Dolbow, D.R.; Berg, A.S.; Gater, D.R. Energy Expenditure, Cardiorespiratory Fitness, and Body Composition Following Arm Cycling or Functional Electrical Stimulation Exercises in Spinal Cord Injury: A 16-Week Randomized Controlled Trial. Top. Spinal Cord Inj. Rehabil. 2021, 27, 121–134. [Google Scholar] [CrossRef]
- Nash, M.S.; Bilsker, M.S.; Kearney, H.M.; Ramirez, J.N.; Applegate, B.; Green, B.A. Effects of electrically-stimulated exercise and passive motion on echocardiographically-derived wall motion and cardiodynamic functic in tetraplegic persons. Spinal Cord 1995, 33, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Alashram, A.R.; Annino, G.; Mercuri, N.B. Changes in spasticity following functional electrical stimulation cycling in patients with spinal cord injury: A systematic review. J. Spinal Cord Med. 2022, 45, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Lien, A.S.; Tsai, J.L.; Yang, H.C.; Chan, H.L.; Chen, R.S.; Chang, Y.J. The Effect and Dose-Response of Functional Electrical Stimulation Cycling Training on Spasticity in Individuals with Spinal Cord Injury: A Systematic Review with Meta-Analysis. Front. Physiol. 2021, 12, 756200. [Google Scholar] [CrossRef]
- Elgaddal, N.; Kramarow, K.A.; Reuben, C. Physical Activity among Adults Aged 18 and over: United States. 2020. Available online: https://www.cdc.gov/nchs/products/databriefs/db443.htm (accessed on 11 November 2022).
- Soriano, J.E.; Squair, J.W.; Cragg, J.J.; Thompson, J.; Sanguinetti, R.; Vaseghi, B.; Emery, C.A.; Grant, C.; Charbonneau, R.; Larkin-Kaiser, K.A.; et al. A national survey of physical activity after spinal cord injury. Sci. Rep. 2022, 12, 4405. [Google Scholar] [CrossRef] [PubMed]
- Dolbow, D.R.; Gorgey, A.S.; Ketchum, J.M.; Moore, J.R.; Hackett, L.A.; Gater, D.R. Exercise Adherence during Home-Based Functional Electrical Stimulation Cycling by Individuals with Spinal Cord Injury. Am. J. Phys. Med. Rehabil. 2012, 91, 922–930. [Google Scholar] [CrossRef]
- Tiu, C.; Ochoa, C.; Froehlich-Grobe, K. Qualitative analysis of perceived motivators and barriers to exercise in individuals with spinal cord injury enrolled in an exercise study. Spinal Cord Ser. Cases 2022, 8, 74. [Google Scholar] [CrossRef]
- Dolbow, D.R.; Figoni, S.F. Accommodation of Wheelchair-Reliant Individuals by Community Fitness Facilities. Spinal Cord 2015, 53, 515–519. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Gill, R.; Khan, R.; Adler, R.A. Effects of dose de-escalation following testosterone treatment and evoked resistance exercise on body composition, metabolic profile, and neuromuscular parameters in persons with spinal cord injury. Physiol. Rep. 2021, 9, e15089. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Alrubaye, M.; Gill, R.; Rivers, J.; Goetz, L.L.; Cifu, D.X.; Castillo, T.; Caruso, D.; Lavis, T.D.; et al. Testosterone and long pulse width stimulation (TLPS) for denervated muscles after spinal cord injury: A study protocol of andomized clinical trial. BMJ Open 2022, 12, e064748. [Google Scholar] [CrossRef]
- Sadowsky, C.L.; Hammond, E.R.; Strohl, A.B.; Commean, P.K.; Eby, S.A.; Damiano, D.L.; Wingert, J.R.; Bae, K.T.; McDonald, J.W., 3rd. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J. Spinal Cord Med. 2013, 36, 623–631. [Google Scholar] [CrossRef]
- Kuhn, D.; Leichtfried, V.; Schobersberger, W. Four weeks of functional electrical stimulated cycling after spinal cord injury: A clinical cohort study. Int. J. Rehabil. Res. 2014, 37, 243–250. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Battini, E.; Rustici, A.; Stampacchia, G. An integrated gait rehabilitation training based on Functional Electrical Stimulation cycling and overground robotic exoskeleton in complete spinal cord injury patients: Preliminary results. IEEE Int. Conf. Rehabil. Robot. 2017, 2017, 289–293. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Edgerton, V.R. Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Sneed, D.; Sutor, T.W.; Hoenig, H.; Gorgey, A.S. Optimization of Transspinal Stimulation Applications for Motor Recovery after Spinal Cord Injury: Scoping Review. J. Clin. Med. 2023, 12, 854. [Google Scholar] [CrossRef]
- Gad, P.; Gerasimenko, Y.; Zdunowski, S.; Turner, A.; Sayenko, D.; Lu, D.C.; Edgerton, V.R. Weight Bearing over-ground Stepping in an Exoskeleton with Non-invasive Spinal Cord Neuromodulation after Motor Complete Paraplegia. Front. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Shapkova, E.Y.; Pismennaya, E.V.; Emelyannikov, D.V.; Ivanenko, Y. Exoskeleton Walk Training in Paralyzed Individuals Benefits from Transcutaneous Lumbar Cord Tonic Electrical Stimulation. Front. Neurosci. 2020, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Hastings, S.; Zhong, H.; Feinstein, R.; Zelczer, G.; Mitrovich, C.; Gad, P.; Edgerton, V.R. A pilot study combining noninvasive spinal neuromodulation and activity-based neurorehabilitation therapy in children with cerebral palsy. Nat. Commun. 2022, 13, 5660. [Google Scholar] [CrossRef]
- Siu, R.; Brown, E.H.; Mesbah, S.; Gonnelli, F.; Pisolkar, T.; Edgerton, V.R.; Ovechkin, A.V.; Gerasimenko, Y.P. Novel Noninvasive Spinal Neuromodulation Strategy Facilitates Recovery of Stepping after Motor Complete Paraplegia. J. Clin. Med. 2022, 11, 3670. [Google Scholar] [CrossRef] [PubMed]
- Barolat, G.; Myklebust, J.B.; Wenninger, W. Effects of spinal cord stimulation on spasticity and spasms secondary to myelopathy. Appl. Neurophysiol. 1988, 51, 29–44. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R.; Illis, L.S.; Nakajima, K.; Sharkey, P.C.; Sherwood, A.M. Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: II. Neurophysiologic observations. Cent. Nerv. Syst. Trauma 1986, 3, 145–152. [Google Scholar] [CrossRef]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137 Pt 5, 1394–1409. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]
- Wagner, F.B.; Mignardot, J.-B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seanez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Lavrov, I. Spinal Epidural Stimulation Strategies: Clinical Implications of Locomotor Studies in Spinal Rats. Neuroscientist 2017, 23, 664–680. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Gill, S.; Holman, M.E.; Davis, J.C.; Atri, R.; Bai, O.; Goetz, L.; Lester, D.L.; Trainer, R.; Lavis, T.D. The feasibility of using exoskeletal-assisted walking with epidural stimulation: A case report study. Ann. Clin. Transl. Neurol. 2020, 7, 259–265. [Google Scholar] [CrossRef]
- Formento, E.; Minassian, K.; Wagner, F.; Mignardot, J.B.; Le Goff, C.G.; Rowald, A.; Bloch, J.; Micera, S.; Capogrosso, M.; Courtine, G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci. 2018, 21, 1728–1741. [Google Scholar] [CrossRef]
- Anderson, K.D. Targeting Recovery: Priorities of the Spinal Cord-Injured Population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef]
- Snoek, G.J.; IJzerman, M.J.; Hermens, H.J.; Maxwell, D.; Biering-Sørensen, F. Survey of the needs of patients with spinal cord injury: Impact and priority for improvement in hand function in tetraplegics. Spinal Cord 2004, 42, 526–532. [Google Scholar] [CrossRef]
- Anderson, K.D.; Fridén, J.; Lieber, R.L. Acceptable benefits and risks associated with surgically improving arm function in individuals living with cervical spinal cord injury. Spinal Cord 2008, 47, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.A.; Eng, J.J.; Hsieh, J.T.C.; Wolfe, D.L.; Team SCIRESR. The Health and Life Priorities of Individuals with Spinal Cord Injury: A Systematic Review. J. Neurotrauma 2012, 29, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Lee, J.; Shin, H.I. The natural course of passive tenodesis grip in individuals with spinal cord injury with preserved wrist extension power but paralyzed fingers and thumbs. Spinal Cord 2018, 56, 900–906. [Google Scholar] [CrossRef]
- Harvey, L.; Baillie, R.; Ritchie, B.; Simpson, D.; Pironello, D.; Glinsky, J. Does three months of nightly splinting reduce the extensibility of the flexor pollicis longus muscle in people with tetraplegia? Physiother. Res. Int. 2007, 12, 5–13. [Google Scholar] [CrossRef]
- Bersch, I.; Fridén, J. Role of Functional Electrical Stimulation in Tetraplegia Hand Surgery. Arch. Phys. Med. Rehabil. 2016, 97 (Suppl. S6), S154–S159. [Google Scholar] [CrossRef]
- Bersch, I.; Koch-Borner, S.; Fridén, J. Electrical stimulation-a mapping system for hand dysfunction in tetraplegia. Spinal Cord 2018, 56, 516–522. [Google Scholar] [CrossRef]
- Bersch, I.; Krebs, J.; Fridén, J. A Prediction Model for Various Treatment Pathways of Upper Extremity in Tetraplegia. Front. Rehabil. Sci. 2022, 3, 889577. [Google Scholar] [CrossRef]
- Bersch, I.; Fridén, J. Upper and lower motor neuron lesions in tetraplegia: Implications for surgical nerve transfer to restore hand function. J. Appl. Physiol. 2020, 129, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Davis, J.; Bersch, I.D.A.; Goldberg, G.; Gorgey, A.S. Electrical stimulation and denervated muscles after spinal cord injury. Neural Regen. Res. 2020, 15, 1397–1407. [Google Scholar]
- Zijdewind, I.; Gant, K.; Bakels, R.; Thomas, C.K. Do Additional Inputs Change Maximal Voluntary Motor Unit Firing Rates after Spinal Cord Injury? Neurorehabilit. Neural Repair 2012, 26, 58–67. [Google Scholar] [CrossRef]
- Thomas, C.K.; Häger, C.K.; Klein, C.S. Increases in human motoneuron excitability after cervical spinal cord injury depend on the level of injury. J. Neurophysiol. 2017, 117, 684–691. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolbow, D.R.; Gorgey, A.S.; Johnston, T.E.; Bersch, I. Electrical Stimulation Exercise for People with Spinal Cord Injury: A Healthcare Provider Perspective. J. Clin. Med. 2023, 12, 3150. https://doi.org/10.3390/jcm12093150
Dolbow DR, Gorgey AS, Johnston TE, Bersch I. Electrical Stimulation Exercise for People with Spinal Cord Injury: A Healthcare Provider Perspective. Journal of Clinical Medicine. 2023; 12(9):3150. https://doi.org/10.3390/jcm12093150
Chicago/Turabian StyleDolbow, David R., Ashraf S. Gorgey, Therese E. Johnston, and Ines Bersch. 2023. "Electrical Stimulation Exercise for People with Spinal Cord Injury: A Healthcare Provider Perspective" Journal of Clinical Medicine 12, no. 9: 3150. https://doi.org/10.3390/jcm12093150
APA StyleDolbow, D. R., Gorgey, A. S., Johnston, T. E., & Bersch, I. (2023). Electrical Stimulation Exercise for People with Spinal Cord Injury: A Healthcare Provider Perspective. Journal of Clinical Medicine, 12(9), 3150. https://doi.org/10.3390/jcm12093150






