Diagnostic Value of the Sentinel Lymph Node Technique in Patients with Muscle-Invasive Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Radiocolloid Injection and SPECT/CT Imaging
2.3. Surgical Procedure
2.4. Histopathological Evaluation
2.5. Definitions and Statistical Analysis
3. Results
3.1. Patients and Disease Characteristics
3.2. Sentinel Lymph Node Detection
3.3. Lymph Node Characteristics
3.4. SLN Technique’s Diagnostic Values
3.5. Morbidity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global Cancer Statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Fung, F.D.H.; Leung, C.; Cheung, W.W.L.; Goggins, W.B.; Ng, C.F. The Global Epidemiology of Bladder Cancer: A Joinpoint Regression Analysis of Its Incidence and Mortality Trends and Projection. Sci. Rep. 2018, 8, 1129–1135. [Google Scholar] [CrossRef]
- Sinha, A.; West, A.; Hayes, J.; Teoh, J.; Decaestecker, K.; Vasdev, N. Methods of Sentinel Lymph Node Detection and Management in Urinary Bladder Cancer—A Narrative Review. Curr. Oncol. 2022, 29, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Bassi, P.; Ferrante, G.D.; Piazza, N.; Spinadin, R.; Carando, R.; Pappagallo, G.; Pagano, F. Prognostic Factors of Outcome after Radical Cystectomy for Bladder Cancer: A Retrospective Study of a Homogeneous Patient Cohort. J. Urol. 1999, 161, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Liss, M.A.; Noguchi, J.; Lee, H.J.; Vera, D.R.; Kader, A.K. Sentinel Lymph Node Biopsy in Bladder Cancer: Systematic Review and Technology Update. Indian J. Urol. 2015, 31, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Dorin, R.P.; Daneshmand, S.; Eisenberg, M.S.; Chandrasoma, S.; Cai, J.; Miranda, G.; Nichols, P.W.; Skinner, D.G.; Skinner, E.C. Lymph Node Dissection Technique Is More Important than Lymph Node Count in Identifying Nodal Metastases in Radical Cystectomy Patients: A Comparative Mapping Study. Eur. Urol. 2011, 60, 946–952. [Google Scholar] [CrossRef]
- Małkiewicz, B.; Kiełb, P.; Gurwin, A.; Knecht, K.; Wilk, K.; Dobruch, J.; Zdrojowy, R. The Usefulness of Lymphadenectomy in Bladder Cancer-Current Status. Medicina 2021, 57, 415–432. [Google Scholar] [CrossRef]
- Tanis, P.J.; Nieweg, O.E.; Valdés Olmos, R.A.; Rutgers, E.J.T.; Kroon, B.B.R. History of Sentinel Node and Validation of the Technique. Breast Cancer Res. 2001, 3, 109–112. [Google Scholar] [CrossRef]
- Hood, J.L.; San Roman, S.; Wickline, S.A. Exosomes Released by Melanoma Cells Prepare Sentinel Lymph Nodes for Tumor Metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Perera, M.; Manning, T.; Bozin, M.; Cabarkapa, S.; Chen, E.; Lawrentschuk, N. Penile Cancer: Contemporary Lymph Node Management. J. Urol. 2017, 197, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Diab, Y. Sentinel Lymph Nodes Mapping in Cervical Cancer a Comprehensive Review. Int. J. Gynecol. Cancer 2017, 27, 154–158. [Google Scholar] [CrossRef]
- Montes, H.Z. TNM Classification of Malignant Tumors, 7th Edition. Int. J. Radiat. Oncol. Biol. Phys. 2010, 35, 270–272. [Google Scholar] [CrossRef]
- Sherif, A.; de La Torre, M.; Malmström, P.U.; Thörn, M. Lymphatic Mapping and Detection of Sentinel Nodes in Patients with Bladder Cancer. J. Urol. 2001, 166, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, R.; Johansson, M.; Alamdari, F.; Sidiki, A.; Holmström, B.; Hansson, J.; Vasko, J.; Marits, P.; Gabrielsson, S.; Riklund, K.; et al. Sentinel Node Detection in Muscle-Invasive Urothelial Bladder Cancer Is Feasible after Neoadjuvant Chemotherapy in All PT Stages, a Prospective Multicenter Report. World J. Urol. 2017, 35, 921–927. [Google Scholar] [CrossRef]
- Aljabery, F.; Shabo, I.; Olsson, H.; Gimm, O.; Jahnson, S. Radio-Guided Sentinel Lymph Node Detection and Lymph Node Mapping in Invasive Urinary Bladder Cancer: A Prospective Clinical Study. BJU Int. 2017, 120, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Liedberg, F.; Chebil, G.; Davidsson, T.; Gudjonsson, S.; Månsson, W. Intraoperative Sentinel Node Detection Improves Nodal Staging in Invasive Bladder Cancer. J. Urol. 2006, 175, 84–88. [Google Scholar] [CrossRef]
- Polom, W.; Markuszewski, M.; Cytawa, W.; Czapiewski, P.; Lass, P.; Matuszewski, M. Fluorescent Versus Radioguided Lymph Node Mapping in Bladder Cancer. Clin. Genitourin. Cancer 2017, 15, 405–409. [Google Scholar] [CrossRef]
- Zarifmahmoudi, L.; Ghorbani, H.; Sadri, K.; Tavakkoli, M.; Keshvari, M.; Salehi, M.; Sadeghi, R. Sentinel Node Biopsy in Urothelial Carcinoma of the Bladder: Systematic Review and Meta-Analysis. Urol. Int. 2019, 103, 373–382. [Google Scholar] [CrossRef]
- Sadeghi, R.; Forghani, M.N.; Memar, B.; Rajabi Mashhadi, M.T.; Dabbagh Kakhki, V.R.; Abdollahi, A.; Zakavi, S.R. How Long the Lymphoscintigraphy Imaging Should Be Continued for Sentinel Lymph Node Mapping? Ann. Nucl. Med. 2009, 23, 507–510. [Google Scholar] [CrossRef]
- Jangjoo, A.; Forghani, M.N.; Mehrabibahar, M.; Rezapanah, A.; Kakhki, V.R.D.; Zakavi, S.R.; Ghavamnasiri, M.R.; Kashani, I.; Hashemian, F.; Sadeghi, R. Comparison of Early and Delayed Lymphoscintigraphy Images of Early Breast Cancer Patients Undergoing Sentinel Node Mapping. Nucl. Med. Commun. 2010, 31, 285–291. [Google Scholar] [CrossRef]
- Połom, W.; Markuszewski, M.; Cytawa, W.; Lass, P.; Matuszewski, M. Radio-Guided Lymph Node Mapping in Bladder Cancer Using SPECT/CT and Intraoperative γ-Probe Methods. Clin. Nucl. Med. 2016, 41, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Manny, T.B.; Hemal, A.K. Fluorescence-Enhanced Robotic Radical Cystectomy Using Unconjugated Indocyanine Green for Pelvic Lymphangiography, Tumor Marking, and Mesenteric Angiography: The Initial Clinical Experience. Urology 2014, 83, 824–829. [Google Scholar] [CrossRef]
- Nathanson, S.D. Insights into the Mechanisms of Lymph Node Metastasis. Cancer 2003, 98, 413–423. [Google Scholar] [CrossRef]
- Sadeghi, R.; Hasanzadeh, M. Sentinel Lymph Node Biopsy Algorithm: Can It Be a Universal Method for Midline Tumors? Gynecol. Oncol. 2014, 132, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Nieweg, O.E.; Tanis, P.J.; Kroon, B.B.R. The Definition of a Sentinel Node. Ann. Surg. Oncol. 2001, 8, 538–541. [Google Scholar] [CrossRef]
- Leissner, J.; Ghoneim, M.A.; Abol-Enein, H.; Thüroff, J.W.; Franzaring, L.; Fisch, M.; Schulze, H.; Managadze, G.; Allhoff, E.P.; El-Baz, M.A.; et al. Extended Radical Lymphadenectomy in Patients with Urothelial Bladder Cancer: Results of a Prospective Multicenter Study. J. Urol. 2004, 171, 139–144. [Google Scholar] [CrossRef]
- Sadeghi, R.; Gholami, H.; Zakavi, S.R.; Kakhki, V.R.D.; Tabasi, K.T.; Horenblas, S. Accuracy of Sentinel Lymph Node Biopsy for Inguinal Lymph Node Staging of Penile Squamous Cell Carcinoma: Systematic Review and Meta-Analysis of the Literature. J. Urol. 2012, 187, 25–31. [Google Scholar] [CrossRef]
- Roth, B.; Zehnder, P.; Birkhuser, F.D.; Burkhard, F.C.; Thalmann, G.N.; Studer, U.E. Is Bilateral Extended Pelvic Lymphadenectomy Necessary for Strictly Unilateral Invasive Bladder Cancer? J. Urol. 2012, 187, 1577–1582. [Google Scholar] [CrossRef]
- Tehranian, S.; Treglia, G.; Krag, D.N.; Dabbagh Kakhki, V.R.; Zakavi, S.R.; Sadeghi, R.; Keshtgar, M. Sentinel Node Mapping in Anal Canal Cancer: Systematic Review and Meta-Analysis. J. Gastrointest. Liver Dis. 2013, 22, 321–328. [Google Scholar]
- Sherif, A.; Garske, U.; de la Torre, M.; Thörn, M. Hybrid SPECT-CT: An Additional Technique for Sentinel Node Detection of Patients with Invasive Bladder Cancer. Eur. Urol. 2006, 50, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodayan, S.; Hasanzadeh, M.; Treglia, G.; Azad, A.; Yousefi, Z.; Zarifmahmoudi, L.; Sadeghi, R. Sentinel Node Biopsy for Lymph Nodal Staging of Uterine Cervix Cancer: A Systematic Review and Meta-Analysis of the Pertinent Literature. Eur. J. Surg. Oncol. 2015, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Jangjoo, A.; Dabbagh Kakhki, V.R.; Rasoul Zakavi, S.; Memar, B.; Naser Forghani, M.; Mehrabibahar, M.; Sadeghi, R. Factors Affecting Sentinel Lymph Node Detection Failure in Breast Cancer Patients Using Intradermal Injection of the Tracer. Rev. Esp. Med. Nucl. 2010, 29, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh Kakhki, V.R.; Bagheri, R.; Tehranian, S.; Shojaei, P.; Gholami, H.; Sadeghi, R.; Krag, D.N. Accuracy of Sentinel Node Biopsy in Esophageal Carcinoma: A Systematic Review and Meta-Analysis of the Pertinent Literature. Surg. Today 2014, 44, 607–619. [Google Scholar] [CrossRef]
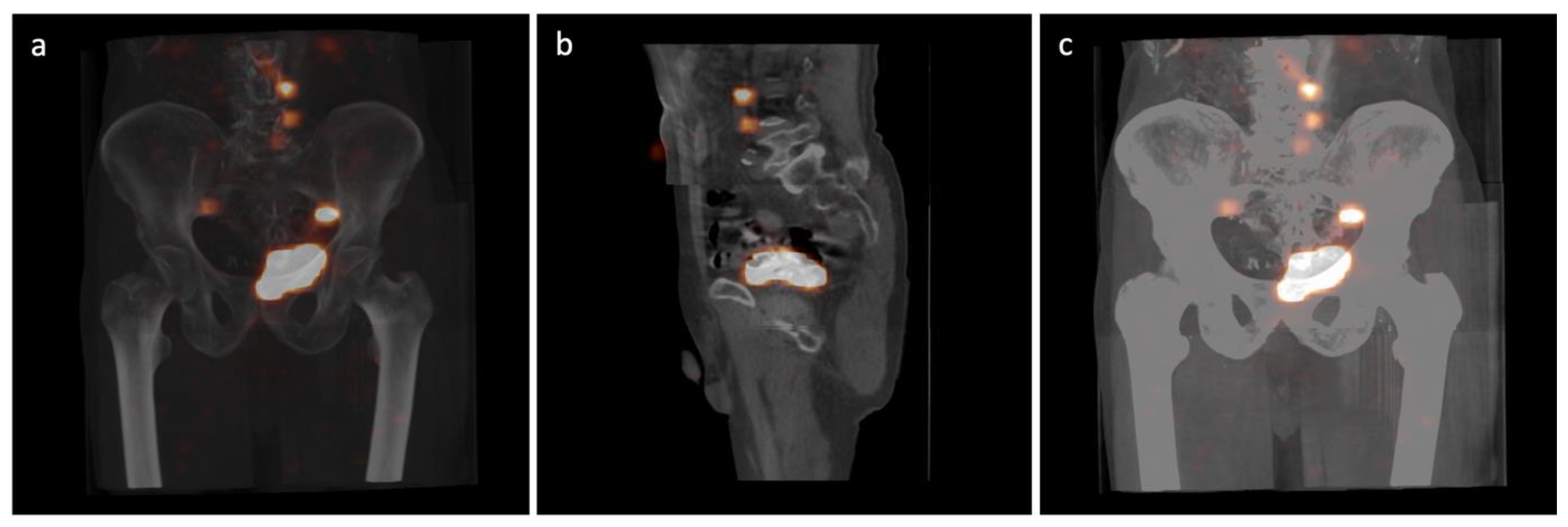

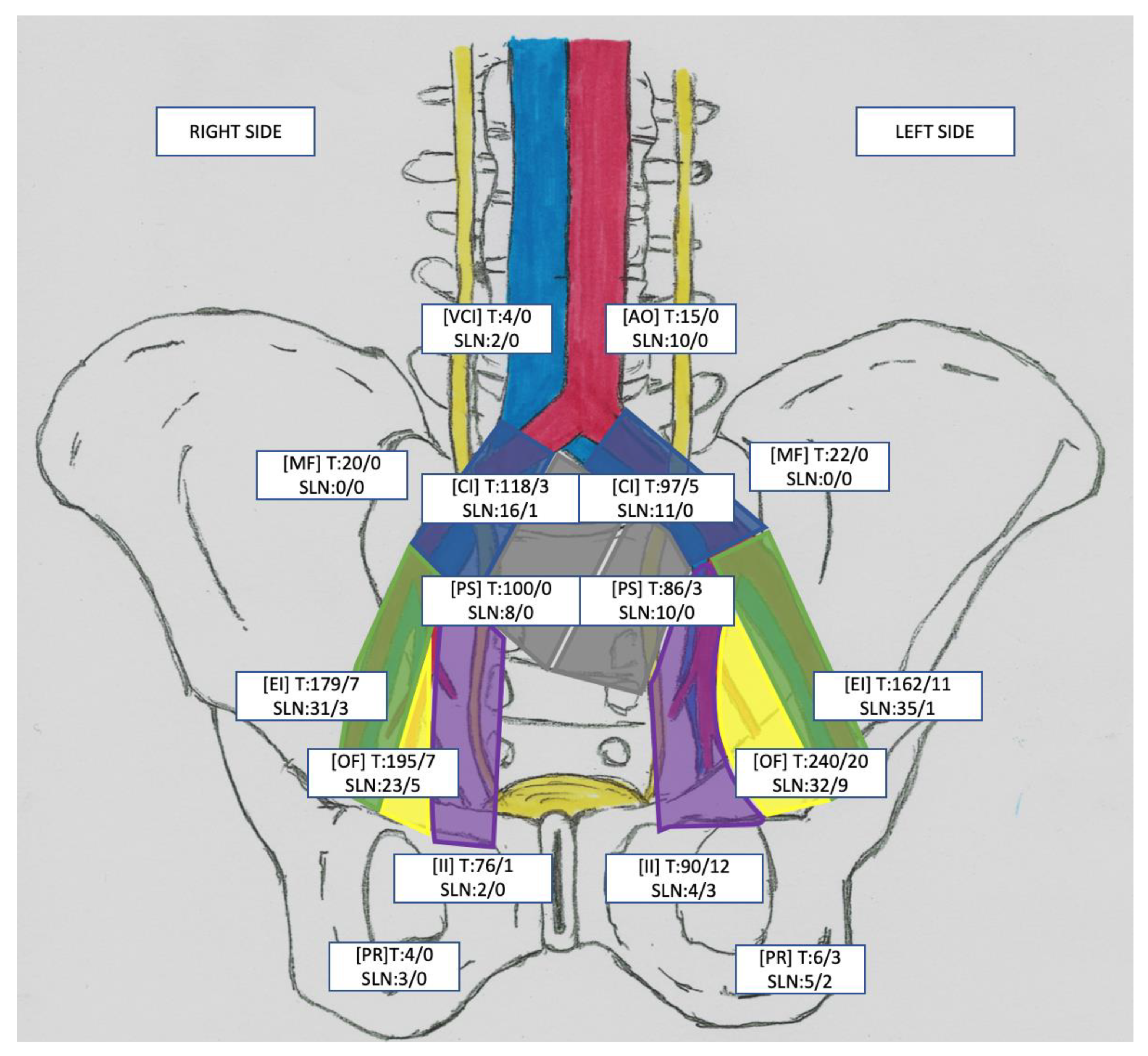
| Variable | Total N = 54 | |
|---|---|---|
| Age (years): | ||
| Mean ± SD | 66.9 ± 8.0 | |
| Median (Q1, Q3) | 68 (61, 72) | |
| Min–Max | 44–82 | |
| Gender: | n | % |
| Male | 43 | 79.6 |
| Female | 11 | 20.4 |
| ACCI: | ||
| Mean ± SD | 6.6 ± 1.7 | |
| Median (Q1, Q3) | 7 (5, 8) | |
| Min–Max | 3–10 | |
| BMI (kg/m2): | ||
| Mean ± SD | 26.0 ± 4.7 | |
| Median (Q1, Q3) | 26 (23, 28) | |
| Min–Max | 17–43 | |
| Neoadjuvant chemotherapy: | ||
| Yes | 24 | 44.4 |
| No | 30 | 55.6 |
| pT: | n | % |
| pT2 | 18 | 33.3 |
| pT3 | 19 | 35.2 |
| pT4 | 17 | 31.5 |
| pN: | n | % |
| pN0 | 32 | 59.3 |
| pN1 | 7 | 13.0 |
| pN > 1 | 15 | 27.8 |
| Number of lymph nodes removed (LNs): | ||
| Total Mean ± SD | 1414 26.2 ± 9.2 | |
| Median (Q1, Q3) | 23 (20, 32) | |
| Min–Max | 11–50 | |
| Number of lymph nodes involved (LN+): | ||
| Mean ± SD | 1.3 ± 2.8 | |
| Median (Q1, Q3) | 0 (0, 2) | |
| Min–Max | 0–14 | |
| Scope of LN Dissection | LN+ Patients with Correct Staging (%, 95% CI) | LN+ Patients with All Positive LNs Removed (%, 95% CI) | LN+ Removed (%, 95% CI) | LNs That Need to Be Removed (%, 95% CI) |
|---|---|---|---|---|
| ePLND | 20/22 90.9 (78.9–100.0) | 19/22 86.4 (72.0–100.0) | 68/72 94.4 (89.2–99.7 | 1385/1414 97.9 (97.2–98.7) |
| SLN | 14/22 63.6 (43.6–83.7) | 6/22 27.3 (8.7–45.9) | 24/72 33.3 (22.4–44.2) | 192/1414 13.6 (11.8–15.4) |
| ePLND + SLN | 22/22 100.0 (100–100) | 22/22 100.0 (100–100) | 72/72 100.0 (100–100) | 1414/1414 100 (100–100) |
| Diagnostic Parameter * | γ-Probe | SPECT/CT |
|---|---|---|
| Sensitivity | 63.64% | 66.67% |
| Specificity | 6.25% | 9.09% |
| Positive predictive value (PPV) | 31.82% | 31.82% |
| Negative predictive value (NPV) | 20.00% | 30.00% |
| Accuracy (ACC) | 29.63% | 31.48% |
| False-negative rate | 36.36% | 33.33% |
| Relative risk (RR) | 39.77% | 45.45% |
| Likelihood ratio + (LR+) | 0.68 | 0.73 |
| Likelihood ratio − (LR−) | 5.82 | 3.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małkiewicz, B.; Jędrzejuk, D.; Gurwin, A.; Wilk, K.; Knecht-Gurwin, K.; Kiełb, P.; Krajewski, W.; Bolanowski, M.; Hałoń, A.; Szydełko, T. Diagnostic Value of the Sentinel Lymph Node Technique in Patients with Muscle-Invasive Bladder Cancer. J. Clin. Med. 2023, 12, 3092. https://doi.org/10.3390/jcm12093092
Małkiewicz B, Jędrzejuk D, Gurwin A, Wilk K, Knecht-Gurwin K, Kiełb P, Krajewski W, Bolanowski M, Hałoń A, Szydełko T. Diagnostic Value of the Sentinel Lymph Node Technique in Patients with Muscle-Invasive Bladder Cancer. Journal of Clinical Medicine. 2023; 12(9):3092. https://doi.org/10.3390/jcm12093092
Chicago/Turabian StyleMałkiewicz, Bartosz, Diana Jędrzejuk, Adam Gurwin, Karol Wilk, Klaudia Knecht-Gurwin, Paweł Kiełb, Wojciech Krajewski, Marek Bolanowski, Agnieszka Hałoń, and Tomasz Szydełko. 2023. "Diagnostic Value of the Sentinel Lymph Node Technique in Patients with Muscle-Invasive Bladder Cancer" Journal of Clinical Medicine 12, no. 9: 3092. https://doi.org/10.3390/jcm12093092
APA StyleMałkiewicz, B., Jędrzejuk, D., Gurwin, A., Wilk, K., Knecht-Gurwin, K., Kiełb, P., Krajewski, W., Bolanowski, M., Hałoń, A., & Szydełko, T. (2023). Diagnostic Value of the Sentinel Lymph Node Technique in Patients with Muscle-Invasive Bladder Cancer. Journal of Clinical Medicine, 12(9), 3092. https://doi.org/10.3390/jcm12093092







